Novel Ga-doped, self-supported, independent aligned ZnO nanorods: one-pot hydrothermal synthesis and structurally enhanced photocatalytic performance†
Siyuan
Yang
a,
Chunyu
Ge
a,
Zuotao
Liu
a,
Yueping
Fang
*a,
Zesheng
Li
b,
Daibin
Kuang
*b and
Chengyong
Su
b
aInstitute of Biomaterial, College of Science, South China Agricultural University, Guangzhou, 510642, China. E-mail: ypfang@scau.edu.cn (Yueping Fang); Tel: +86-20-85285565; Fax: +86-20-85285565
bMOE Key Laboratory of Bioinorganic and Synthetic Chemistry, School of Chemistry and Chemical Engineering, Sun Yat-Sen University, Guangzhou, 510275, P. R. China. E-mail: kuangdb@mail.sysu.edu.cn (Daibin Kuang); Tel: +86-20-84113015; Fax: +86-20-84113015
First published on 28th October 2011
Abstract
A simple solution-liquid-solid method under hydrothermal conditions has been developed for the one-pot synthesis of Ga-doped/self-supported aligned ZnO 1-D nanostructures, which exhibits enhanced activity for the photocatalytic degradation of rhodamine B.
As an important functional oxide semiconductor, ZnO with a bang gap of about 3.37 eV and a large excitonic binding energy of about 60 meV at room temperature, has attracted extensive attention for multi-purpose applications involving UV photodetectors, photocatalysis, field emission, solar cells, light-emitting diodes, etc.1 Due to their unique structures and surface properties, one dimensional (1-D) ZnO nanostructures such as nanowires, nanorods, nanotubes, and nanobelts have been widely reported in these fields.2 Recently, massive aligned ZnO 1-D nanostructures onto various planar substrates have been synthesized by chemical vapor deposition, electrochemical deposition, hydrothermal, and solvothermal methods.3 However, it is still a challenge to develop a facile synthesis method to prepare independent aligned 1-D nanostructures,4 especially for those who are advantaged in substrate-free/self-supported concept.5
The solution-liquid-solid (SLS) growth of 1-D nanostructures was first reported by Buhro and co-workers, who used nanoparticles of a low melting point metal (e.g., In and Ga, etc.) in an organic solution to induce the growth of GaAs nanowires with a narrow diameter distribution.6 Subsequently, the Korgel group and the Buhro group have reported a lot of related works on the SLS growth of semiconductor nanofibres.7 Recently, we have further developed the SLS method under hydrothermal conditions for the growth of Sn-filled In(OH)3 nanotubes8 and periodically twinned nanotowers and nanodendrites9 of HgSe in an aqueous solution. In this communication, we demonstrate the first SLS growth of a new type of Ga-doped/self-supported aligned ZnO nanorods (GSAN) existing independently, by a one-pot Ga-mediated hydrothermal synthesis using low-cost starting materials of Zinc stearate (ZnSt2) (see ESI for details)†.
Typical scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images of the as-prepared products are shown in Fig. 1. It indicates that the GSAN sample has a large-area dandelion-like morphology with relatively homogeneous and independent distribution (Fig. 1 A and B). From two higher magnification images (Fig. 1 C and D), it can be seen that one GSAN unit comprises of numerous aligned 1-D nanorods with highly close-packed structures. In comparison to routine substrate-supported 1-D aligned models,3 our products are obviously distinguished by their close-packed, self-supported and independent features. Furthermore, the diameter (Fig. 1E) and length (Fig. 1 C and D) of these nanorods can be determined in the ranges of 20–60 nm and 2–4 μm, respectively. Individual nanorod has the nature of ZnO single crystalline, as implicated by high-resolution TEM (HRTEM) image (Fig. 1 F) and their corresponding fast Fourier transform pattern (inset in Fig. 1 F). Representative HRTEM image taken from the white square in Fig. 1 E shows the wurtzite structure and highly inter-oriented lattices. The fringe spacing in the [001] direction measures 0.515 nm, which agrees well with the interplanar spacing of the (001) planes.
 | ||
| Fig. 1 SEM images of as-prepared GSAN products: (A) a low magnification image; (B) a higher magnification image; (C) and (D) a single GSAN. TEM images of as-prepared products: (E) close-packed aligned nanorods; (F) HRTEM image and the corresponding FFT patterns (inset), taken in the white square in E. | ||
The structure and composition of the as-prepared product have been determined by powder-X-ray diffraction (XRD) and energy dispersive X-ray (EDX) analyses and the results are shown in Fig. 2. From the XRD pattern (Fig. 2A), it can be readily indexed the GSAN sample into the wurtzite ZnO crystal structure (JCPDS 36-1451). The sharp and clear diffraction peaks clearly indicate a good crystallization of the product. It suggests the nice ZnO structure has been obtained via the hydrolization of ZnSt2 under hydrothermal treatment. Furthermore, EDX measurement (Fig. 2B) affirms that the GSAN sample contains Zn, O and Ga with Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O
O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga ≈ 23
Ga ≈ 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28.8
28.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 in atomic ratio. In addition, the ICP-AES result demonstrates that the Ga content in the composite materials is as high as 6.74 wt.%. Fig. 2C shows the corresponding EDX elemental mapping of Zn, O and Ga. It is found that Ga is homogeneously spreading on the ZnO dandelions consisting of nanorods. All these result demonstrate that the Ga doped ZnO nanorod heterostructures has been achieved via the present hydrothermal process.
1.2 in atomic ratio. In addition, the ICP-AES result demonstrates that the Ga content in the composite materials is as high as 6.74 wt.%. Fig. 2C shows the corresponding EDX elemental mapping of Zn, O and Ga. It is found that Ga is homogeneously spreading on the ZnO dandelions consisting of nanorods. All these result demonstrate that the Ga doped ZnO nanorod heterostructures has been achieved via the present hydrothermal process.
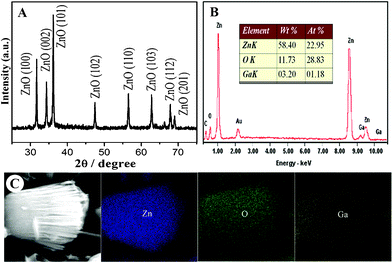 | ||
| Fig. 2 XRD pattern (A), EDX spectra (B) and element mappings (C) of as-prepared GSAN product. | ||
To show further insight into the detailed nanostructure and growth process of the GSAN architectures, typical SEM images of GSAN acquired from multiple viewpoints are presented in Fig. 3. Evidently, the GSAN shows a highly self-supported, independent aligned ZnO 1-D nanostructures, where the ZnO nanorods are vertically and well aligned assembled without extraneous supporting substrates from a side (Fig. 3A) and front (Fig. 3C) view. And from a cross-sectional morphology (Fig. 3B), it can be seen that two sections are well linked by their bottom despite that the scaffold branches have been detached. This evince that these independent structures are of very high stability. More interestingly, a back view of the GSAN (Fig. 3D) reveals its bottom is a flat surface, hinting at the original sites where the nanorod growth got started and assembled into the resulted independent aligned nanostructures.
 | ||
| Fig. 3 Typical SEM images of GSAN from multiple viewpoints: (A) side, (B) section, (C) front and (D) back view. | ||
Control experiments are further performed to reveal the influence of Ga on the final morphology of the GSAN (see Fig. S1)†. In the absence of Ga during the hydrothermal process, the hexagonal plates of ZnO (plate sample) were obtained (A and B). It shows the Ga plays an important role in the formation of GSAN framework. Second, as the excessive amount of Ga was used, the product show that numerous short nanorods grown vertically on a micro-substrate (C and D). This suggests that the aligned ZnO nanorods of GSAN might originate and grow from Ga liquid droplets through a SLS growth mode.8,9
Scheme 1 illustrates a formation process of the Ga-doped ZnO nanorods with the present synthesis route. In brief, this process maybe consists of four main steps:10 i) formation of the liquid Ga droplets in hydrothermal solution (A); ii) production of ZnO by ZnSt2 hydrolization and its diffusion to liquid Ga droplet to form liquid-solid interface (B); iii) 1-D growth of the Ga-doped ZnO nanorods at the liquid-solid interface (C); iv) continuous 1-D growth of ZnO consumes the liquid Ga droplet, thus completing the SLS assembly of the Ga-doped ZnO nanorods (D). As a result, self-supported, independent aligned ZnO nanorods were obtained by this simple substrate-free Ga-mediated hydrothermal synthesis. It is noteworthy that this should be a new and general mechanism for the growth of Ga-doped aligned ZnO nanorods. With a judicious choice of metal droplet, reactant and experimental conditions, one should be able to fabricate new nanomaterials else with different compositions, sizes, morphologies, and heterostructures.
 | ||
| Scheme 1 A formation process of the Ga-doped ZnO nanorods. | ||
Recent advances in the vertically aligned nanostructures of ZnO on large-area substrates have attracted much attention in various technology applications.2b,3,11 Particularly, this type of architectures is of great importance for solar cells, since these nano-films made of aligned 1-D nanostructures provides a higher interfacial area between the donor and the acceptor material with highly-efficient electron transport pathways.2b However, for another important environmental application of photocatalytic degradation, design and synthesis of monodispersed ZnO nanostructures would be more favorable strategy to achieve an optimized utilization efficiency,12 because such independent nanostructures are highly dispersible in the solution reaction system. Obviously, our strategy has successfully demonstrated the independent aligned ZnO 1-D nanostructures, which integrates both the aforementioned design principles.
The room-temperature photoluminescence (PL) spectrum of the GSAN sample has been acquired to learn its emission properties (see Fig. S2A)†. One can see two weaker UV emission band centered at 383 nm (3.25 eV) and 370 nm (3.35 eV), respectively, and a broad and stronger visible emission band between 400 and 600 nm. The visible emission appears to consist of five main components between 400 and 600 nm. It is generally believed that the visible emission of ZnO is due to transitions involving defect states, in particular oxygen vacancies.13 The emission at 3.35 eV is attributed to the neutral donor-bound exciton of ZnO, and the other emission at 3.25 eV is probably caused by the free-electron-neutral accepter transition. PL spectrum of the ZnO hexagonal plates obtained under the same conditions in the absence of Ga is shown in Fig. S2B. One can see a relatively sharp and strong UV emission band centered at 385 nm and a broad visible emission band between 450 nm and 700 nm. The near band edge UV emission is unambiguously attributed to free excitonic emission of ZnO. The visible emission appears to consist of two main components at ∼540 and ∼610 nm. It is generally believed that the visible emission of ZnO is due to transitions involving defect states, in particular oxygen vacancies. The PL properties of the samples suggest that there are more intrinsic defects, such as oxygen vacancies, for the Ga-doped aligned ZnO nanorods than for the ZnO hexagonal plates.14
To demonstrate the potential environmental application, the photocatalytic activity of the GSAN sample was investigated by evaluating the degradation of rhodamine B (RhB) (see ESI for details†). Fig. 4 shows the change of absorption spectra of RhB solution when exposed to UV light at different times in presence of the photocatalyst of the GSAN sample. The spectrum clearly shows that the absorption peak of RhB drops gradually with an increase of UV-visible exposure time for 120 min (A). The decay profile of GSAN sample indicates that the photodegradation rate of RhB is more than 90% after UV-visible exposure time for 120 min (B). It is worth noting that the GSAN sample shows higher photocatalytic activity than the Ga-free plate sample under the same testing condition. Structurally, there are four aspects interpret to the enhanced performance of GSAN: i) unique 1-D nanostructures allow higher surface properties relative to the bulk morphologies, ii) the self-supported mode gives a high structural stability, iii) the independent existence should make for a high availability, and iv) the Ga-doped modified structures specially capacitate its improved emission properties.15
 | ||
| Fig. 4 (A) The variation of adsorption spectra of aqueous RhB solution in the presence of the catalyst of GSAN sample under UV-light irradiation; (B) the comparison of photocatalytic degradation curves (C/C0vs. Time, C0 and C are the initial and actual concentration of RhB). | ||
In conclusion, we reported here a novel Ga-doped, self-supported, independent aligned ZnO nanorods, by a facile one-pot Ga-mediated hydrothermal synthesis. The mediated agent, Ga, not only plays an important role in the formation of aligned 1-D nanostructures with self-supported feature, but also gives the meaningful Ga-doped modified structures. We suggest this unique 1-D nanostructure allows a higher photocatalytic activity relative to the Ga-free plate-like counterpart due to its structurally enhanced properties. This product has the potential applications in environmental science.
Acknowledgements
This research was supported by the key Academic Program of the 3rd phase “211 Project” of South China Agricultural University, NSF of China (20963002), Ministry of Education key project (208108), Guangxi Natural Science Foundation (0832099).References
- (a) Q. F. Zhang, T. P. Chou, B. Russo, S. A. Jenekhe and G. Z. Cao, Angew. Chem. Int. Ed., 2008, 47, 2402 CrossRef CAS; (b) C. Soci, A. Zhang, B. Xiang, S. A. Dayeh, D. P. R. Aplin, J. Park, X. Y. Bao, Y. H. Lo and D. Wang, Nano Lett., 2007, 7, 1003 CrossRef CAS; (c) J. M. Bao, M. A. Zimmler, F. Capasso, X. W. Wang and Z. F. Ren, Nano Lett., 2006, 6, 1719 CrossRef CAS; (d) C. X. He, B. X. Lei, Y. F. Wang, C. Y. Su, Y. P. Fang and D. B. Kuang, Chem. Eur. J., 2010, 16(29), 8757 CrossRef CAS.
- (a) B. Cheng and E. T. Samulski, Chem. Commun., 2004, 986 RSC; (b) I. Gonzalez-Valls and M. Lira-Cantu, Energy Environ. Sci., 2009, 2, 19 RSC.
- (a) X. Wang, J. Song, P. Li, J. H. Ryou, R. D. Dupuis, C. J. Summers and Z. L. Wang, J. Am. Chem. Soc., 2005, 127, 7920 CrossRef CAS; (b) H. Yu, Z. Zhang, M. Han, X. Hao and F. Zhu, J. Am. Chem. Soc., 2005, 127, 2378 CrossRef CAS; (c) J. Liu, X. Huang, Y. Li, X. Ji, Z. Li, X. He and F. Sun, J. Phys. Chem. C, 2007, 111, 4990 CrossRef CAS; (d) S. Kar, A. Dev and S. Chaudhuri, J. Phys. Chem. B, 2006, 110, 17848 CrossRef CAS.
- (a) X. Han, G. Wang, L. Zhou and J. G. Hou, Chem. Commun., 2006, 212 RSC; (b) L. Zhang, X. Cao, Y. Ma, X. Chen and Z. Xue, CrystEngComm, 2010, 12, 3201 RSC; (c) Y. Zhang, W. Liu, L. Jiang, L. Fan, C. Wang, W. Hu, H. Zhong, Y. Li and S. Yang, J. Mater. Chem., 2010, 20, 953 RSC.
- (a) Y. Liu, H. Wang, Y. Wang, H. Xu, M. Li and H. Shen, Chem. Commun., 2011, 47, 3790 RSC; (b) X. Xia, J. Tu, Y. Mai, X. Wang, C. Gu and X. Zhao, J. Mater. Chem., 2011, 21, 9319–9325 RSC.
- H. Yu and W. E. Buhro, Adv. Mater., 2003, 15, 416 CrossRef CAS.
- (a) D. C. Lee, F. V. Mikulec and B. A. Korgel, J. Am. Chem. Soc., 2004, 126, 4951 CrossRef CAS; (b) C. K. Chan, R. N. Patel, M. J. Connell, B. A. Korgel and Y. Cui, A. C. S. Nano, 2010, 4, 1443 CrossRef CAS; (c) H. Yu, J. B. Li, R. A. Loomis, L. W. Wang and W. E. Buhro, Nat. Mater., 2003, 2, 517 CrossRef CAS; (d) J. W. Sun, L. W. Wang and W. E. Buhro, J. Am. Chem. Soc., 2008, 130, 7997 CrossRef CAS.
- Y. P. Fang, X. G. Wen and S. H. Yang, Angew. Chem. Int. Ed., 2006, 45, 4655 CrossRef CAS.
- A. M. Qin, X. S. Zhou, Y. F. Qiu, Y. P. Fang, C. Y. Su and S. H. Yang, Adv. Mater., 2008, 20, 768 CrossRef.
- (a) H. Y. Wang and G. S. Fischman, J. Appl. Phys., 1994, 76, 1557 CrossRef CAS; (b) D. T. J. Hurle, J. Cryst. Growth, 1995, 147, 239 CrossRef CAS.
- (a) X. Wang, C. J. Summers and Z. Wang, Nano Lett., 2004, 4, 423 CrossRef CAS; (b) H. Zeng, X. Xu, Y. Bando, U. K. Gautam, T. Zhai, X. Fang, B. Liu and D. Golberg, Adv. Funct. Mater., 2009, 19, 3165 CrossRef CAS; (c) I. Gonzalez-Valls and M. Lira-Cantu, Energy Environ. Sci., 2010, 3, 789 RSC.
- (a) P. Li, Z. Wei, T. Wu, Q. Peng and Y. Li, J. Am. Chem. Soc., 2011, 133, 5660 CrossRef CAS; (b) F. Barka-Bouaifel, B. Sieber, N. Bezzi, J. Benner, P. Roussel, L. Boussekey, S. Szunerits and R. Boukherrou, J. Mater. Chem., 2011, 21, 10982 RSC.
- (a) L. Guo and S. H. Yang, Chem. Mater., 2000, 12, 2268 CrossRef CAS; (b) S. Maensiri, P. Laokul and V. Promarak, J. Cryst. Growth, 2006, 289, 102 CrossRef CAS.
- L. W. Chang, J. W. Yeh, C. L. Cheng, F. S. Shieu and H. C. Shih, App. Sur. Sci., 2011, 257, 3145 Search PubMed.
- (a) S. W. Kang, S. K. Mohanta, Y. Y. Kim and H. K. Cho, Crystal Growth & Design, 2008, 8, 1458 CrossRef; (b) W. Lin, K. Ding, Z. Lin, J. Zhang, J. Huang and F. Huang, CrystEngComm, 2011, 13, 3338 RSC.
Footnote |
| † Electronic Supplementary Information (ESI) available. See DOI: 10.1039/c1ra00555c/ |
| This journal is © The Royal Society of Chemistry 2011 |
