SiO2-supported RuCl3/3-(dichloroiodo)benzoic acid: green catalytic system for the oxidation of alcohols and sulfides in water
Xiao-Mei
Zeng
b,
Jiang-Min
Chen
*ab,
Akira
Yoshimura
b,
Kyle
Middleton
b and
Viktor V.
Zhdankin
*b
aCollege of Biological and Chemical Engineering, Jiaxing University, 56 South Yuexiu Rd, Jiaxing, 314001, China. E-mail: chemcjm@yahoo.com.cn
bDepartment of Chemistry and Biochemistry, University of Minnesota Duluth, 1039 University Dr, Duluth, MN 55812, USA. E-mail: vzhdanki@d.umn.edu; Fax: +1-218-726-7394
First published on 27th September 2011
Abstract
A green, recyclable and efficient catalytic oxidative system based on SiO2-supported RuCl3 and 3-(dichloroiodo)benzoic acid for the oxidation of alcohols and sulfides in water is developed. This catalytic oxidative system effects clean and efficient oxidation of a wide range of alcohols to the corresponding aldehydes and ketones, or sulfides to sulfoxides in high conversions with excellent chemoselectivity, under mild conditions and with an easy-work-up procedure. Furthermore, the SiO2–RuCl3 catalyst can be recovered by simple filtration and recycled in up to six consecutive runs without significant loss of activity. The reduced form of 3-(dichloroiodo)benzoic acid, 3-iodobenzoic acid, can be easily separated from reaction mixtures and converted back to 3-(dichloroiodo)benzoic acid by treatment with bleach and aqueous HCl in about 90% overall yield.
The selective oxidation of alcohols and sulfides into the corresponding carbonyl compounds or sulfoxides is one of the most important processes for the production of fine and speciality chemicals and could be of interest to many scientists.1,2 However, many traditional methods rely upon environmentally damaging oxidants, or harmful organic solvents,3–5 or require vigorous reaction conditions.6,7 This represents a major drawback from the green chemistry viewpoint.8 Thus, there is increasing demand for oxidation processes that are catalytic and use green, economic and efficient oxidants with special emphasis on benign solvents.9–11
As highly efficient oxygen transfer agents, hypervalent iodine compounds have been widely used in organic synthesis for various synthetically useful oxidative transformations12–28 with the advantages of easy handling, mild reaction conditions and low toxicity. However, their low atom economy remains a major drawback, as stoichiometric amounts of aryliodides or similar waste products are produced, which complicates isolation and purification of the products.29–30 To overcome these disadvantages, recyclable and reusable hypervalent iodine reagents have been developed in recent years, involving both polymeric31–42 and molecular43–50 species.
The other approach is to combine a catalyst and a hypervalent iodine reagent as an efficient catalytic oxidation system. Homogeneous ruthenium catalysts are of particular interest for the oxidation of alcohols and other substrates using hypervalent iodine reagents as oxidants. Mueller and Godoy51 reported in 1981 that several ruthenium complexes can catalyze the oxidation of alcohols to carbonyl compounds and carboxylic acids using excess iodosylbenzene as the oxidant in dichloromethane solution at room temperature. Our group has also reported a highly efficient RuCl3-catalyzed selective oxidation of alcohols to carbonyl compounds by (diacetoxyiodo)benzene (DIB)52 in aqueous acetonitrile. However, these reactions still occur in harmful organic solvents, while the use of water as a reaction solvent has attracted great attention and has become an active area of research in green chemistry.53–55 Moreover, homogeneously catalyzed organic reactions commonly suffer from disadvantages, such as difficult separation of the product and recovery of the catalyst, low regioselectivity and long reaction times, etc. These problems can be addressed by the immobilization of homogeneous catalysts onto solid supports. Recently, silica-supported catalytic metallic species have been successfully employed in aqueous media,56–58 since silica displays many advantageous properties—excellent stability (both chemical and thermal), high surface area, good accessibility, and organic groups can be robustly anchored to the surface to provide catalytic centers.59–62 Furthermore, water has been found to be a promising medium for heterogeneous catalysis. Heterogeneous catalysts possess increased advantages over their homogeneous counterparts, however, a heterogeneous catalytic oxidative system, such as a silica-supported ruthenium catalyst, is still unknown.
Therefore, as a part of our studies33–47 towards the development of practical and environmentally benign oxidation reactions, we herein report the design and synthesis of a recyclable SiO2-supported RuCl3 (SiO2–RuCl3) catalyst (Scheme 1) and develop a mild, simple, cost-effective and green procedure for the catalytic oxidation of alcohols and sulfides using the recyclable SiO2–RuCl3 catalyst and 3-(dichloroiodo)benzoic acid as a catalytic oxidative system in water under mild conditions (Scheme 2). 3-(Dichloroiodo)benzoic acid is a readily available and stable hypervalent iodine oxidant that can be easily regenerated and reused.47,63
Our approach to the preparation of SiO2-supported RuCl3 (SiO2–RuCl3, 3) consists of building a suitable ligand structure 2 on the surface of commercial aminopropyl silica (AMPS, 1), followed by complexation with RuCl3 (Scheme 1). The synthesized SiO2–RuCl3 was characterized by FT-IR and elemental analysis. The amount of ruthenium loaded on the surface of the silica gel was determined by elemental analysis (loading 0.047 mmol g−1 based on Cl analysis).
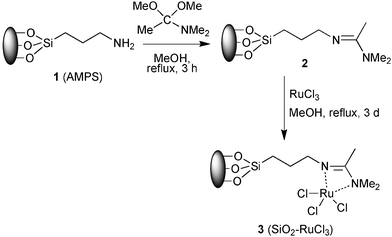 | ||
| Scheme 1 Synthesis of SiO2-supported RuCl3. | ||
Various alcohols 4 were smoothly oxidized into corresponding carbonyl compounds 8 using 1.5 equiv. 3-(dichloroiodo)benzoic acid 6 as the oxidant in the presence of 0.23 mol% of SiO2–RuCl33 in water at room temperature (Scheme 2). Conversions were measured by GC-MS with a prior column calibration using authentic samples of reactants and products. The reaction products were isolated by removal of the solid resin followed by aqueous work-up of the organic solution; products 8 were identified by direct comparison of the retention times and MS data with those obtained for authentic samples or by 1H NMR. In the oxidation protocol (Scheme 2), the by-product, 3-iodobenzoic acid 7 was conveniently separated from the organic solution by treatment with aqueous NaHCO3;47 it can be recovered by filtration after acidification of the aqueous solution and converted to reagent 6 by reaction with commercial bleach and HCl.63 The SiO2-supported catalyst 3 was easily separated by filtration and directly reused several times without loss of its activity and selectivity.
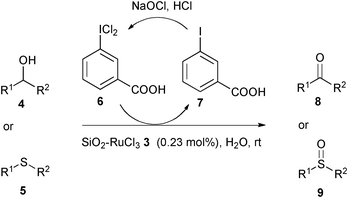 | ||
| Scheme 2 SiO2–RuCl3 catalyzed oxidation of organic substrates 4 and 5 using 3-(dichloroiodo)benzoic acid 6 as a recyclable oxidant. | ||
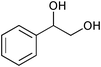
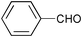
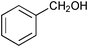
The results of the SiO2–RuCl3 catalyzed oxidation of alcohols are summarized in Table 1. In most cases, the combination of SiO2–RuCl3 and 3-(dichloroiodo)benzoic acid resulted in the efficient oxidation and high conversion of alcohols. Primary benzylic alcohols were selectively oxidized to the corresponding aldehydes with nearly 100% conversion and in excellent yields (90–99%) after 2–3 h (Table 1, entries 1–3) or 12 h (entry 4). Secondary benzylic alcohols and alicyclic alcohols were converted to the respective ketones (entries 5–9). Simple primary alcohols were less reactive, e.g., phenyl acetaldehyde was obtained in 17% (entry 10), while the reaction of 1-octanol did not show any measurable conversion even after 24 h (entry 11). The formation of undesired side-products (e.g., carboxylic acids) was not observed. The oxidation of a glycol, 1-phenyl-1,2-ethanediol (entry 12), resulted in a partial C–C bond cleavage and afforded a mixture of benzaldehyde (74% yield) and 2-hydroxy-1-phenylethan-1-one (26% yield).
| Entry | Substrate | Product | Time/h | Conversion (%)b | Yield (%)c |
|---|---|---|---|---|---|
| a General oxidation procedure: to a suspension of alcohol 4 or sulfide 5 (0.2 mmol) and 3-(dichloroiodo)benzoic acid 6 (96 mg, 0.3 mmol) in 2 mL of water, SiO2–RuCl33 (10 mg, 0.23 mol%) was added whilst stirring at room temperature. The reaction mixture was stirred until the complete consumption of starting material 4 or 5 (monitored by TLC and GC-MS), then 2 mL of saturated aqueous NaHCO3 was added and the mixture stirred for 15–30 min; the solid (SiO2–RuCl3) was filtered, washed with EtOAc (1 mL × 3) and collected for the next run. The filtrate was extracted with EtOAc (3 mL × 3) and dried over Na2SO4. The aqueous layer was acidified with 10% HCl to pH = 3 and the 3-iodobenzoic acid precipitated, it was then filtered and collected for regenerating 3-(dichloroiodo)benzoic acid. The organic layer was concentrated to afford the respective product of oxidation 8 or 9. b Based on GC-MS analysis. c Yield of isolated product. | |||||
| 1 |
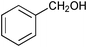
|
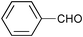
|
0.75 | 100 | 99 |
| 2 |
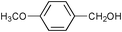
|
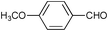
|
2.5 | 100 | 95 |
| 3 |
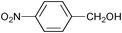
|
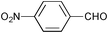
|
2.5 | 100 | 99 |
| 4 |
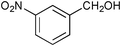
|
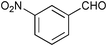
|
12 | >99 | 93 |
| 5 |
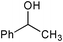
|
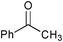
|
2 | 100 | 99 |
| 6 |
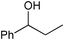
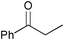
|
|
3.5 | >99 | 96 |
| 7 |
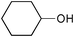
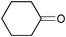
|
|
2.5 | 100 | 99 |
| 8 |
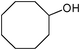
|
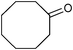
|
5 | 100 | 99 |
| 9 |
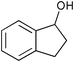
|
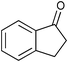
|
2.5 | 100 | 86 |
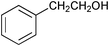
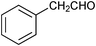
| 10 |
|
|
18 | 17 | 17 |
| 11 | n-C7H15CH2OH | n-C7H15CHO | 24 | 0 | - |
| 12 |
|
|
3 | >99 | 74 |
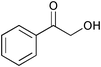
|
26 | ||||
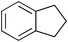
| 13 |
|
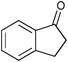
|
4.5 | 100 | 99 |
| 14 |
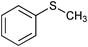
|
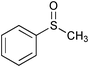
|
1.5 | 100 | 98 |
| 15 |
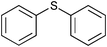
|
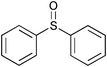
|
0.75 | 100 | 99 |
The SiO2–RuCl3/3-(dichloroiodo)benzoic acid catalytic system can also be used for the oxidation of sulfides 5, as illustrated by the conversion of diaryl, and aryl alkyl sulfides to sulfoxides in excellent yields (entries 14 and 15). Interestingly, the SiO2–RuCl3/3-(dichloroiodo)benzoic acid system can oxidize indane to indanone in good yield after 4.5 h (entry 13).
A comparison of reactivity of the SiO2–RuCl3/3-(dichloroiodo)benzoic acid catalytic oxidative system with other oxidative systems based on RuCl3 or oxidants in the absence of catalyst is summarized in Table 2. In particular, a comparison of the catalytic activity of the SiO2-supported catalyst 3 with the common catalyst RuCl3 showed a generally higher activity of catalyst 3. For example, the reaction of primary benzylic alcohols in the presence of 0.23 mol% RuCl3 (Table 2, entry 2) required on average twice as long a time to reach 99% conversion of the alcohol to the respective aldehyde. In the absence of any ruthenium catalyst, the similar oxidation was extremely slow (Table 2, entry 1).
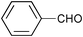
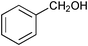
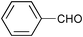
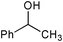
| Entry | Substrate | Catalyst | Oxidant (equiv.) | Product | Time/hb | Conversion (%)c | Yield (%)d |
|---|---|---|---|---|---|---|---|
| a Reactions in entries 1–8 use 0.23 mol% catalyst or no catalyst unless otherwise noted. b All reactions of organic substrates (0.2 mmol) were performed at room temperature in H2O (2 mL). c Based on GC-MS analysis. d Isolated yield. e Isolated PhSO2Me f Using a SiO2–RuCl3/3-iodobenzoic acid tandem catalytic system composed of 0.1 equiv. 3-iodobenzoic acid and 0.23 mol% SiO2–RuCl3. | |||||||
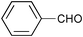
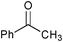
| 1 |
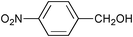
|
None | 3-(Dichloroiodo)benzoic acid (1.5) |
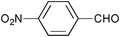
|
12 | 9 | 8 |
| 2 |
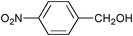
|
RuCl3 | 3-(Dichloroiodo)benzoic acid (1.5) |
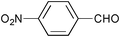
|
5 | 99 | 95 |
| 3 |
|
None | NaOCl (3) |
|
24 | < 2 | 0 |
| 4 |
|
SiO2–RuCl3 | NaOCl (3) |
|
0.75 | 100 | 84 |
| 5 |
|
None | NaOCl (3) |
|
24 | 0 | 0 |
| 6 |
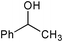
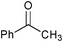
|
SiO2–RuCl3 | NaOCl (3) |
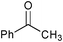
|
2 | 100 | 43 |
| 7 |
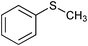
|
None | NaOCl (3) |
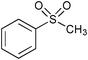
|
17 | 100 | 90e |
| 8 |
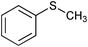
|
SiO2–RuCl3 | NaOCl (3) |
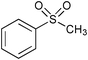
|
1.5 | 100 | 94e |
| 9 |
|
SiO2–RuCl3/3-iodobenzoic acid | NaOCl (3) |
|
0.75 | 100 | 39f |
| 10 |
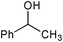
|
SiO2–RuCl3/3-iodobenzoic acid | NaOCl (3) |
|
2 | 39 | 27f |
| 11 |
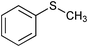
|
SiO2–RuCl3/3-iodobenzoic acid | NaOCl (3) |
|
1.5 | 100 | 80e,f |
Similar to other oxidation procedures for alcohols and sulfides, organic solvents and/or bases,64–69 even in the presence of a ruthenium complex as a catalyst,69 are necessary when sodium hypochlorite (NaOCl) is employed as an oxidant. In the absence of any catalyst in water under mild conditions, the oxidative reactions of alcohols did not occur or did not show any measurable conversion after 24 h (Table 2, entries 3 and 5). Moreover, yields of target product in the presence of catalyst 3 and NaOCl (Table 2, entries 4 and 6) were much lower than those obtained using 3-(dichloroiodo)benzoic acid as an oxidant (Table 1, entries 1 and 5). Interestingly, thioanisole (Table 2, entries 7, 8 and 11) was converted to phenyl methyl sulfone using NaOCl as an oxidant with high yields in the absence of any catalyst or in the presence of catalyst 3 in water; however, catalyst 3 dramatically accelerated the reaction under milder conditions. In contrast, thioanisole gave exclusively phenyl methyl sulfoxide using 3-(dichloroiodo)benzoic acid as an oxidant (Table 1, entry 14), which indicated that 3-(dichloroiodo)benzoic acid showed excellent chemoselectivity.
A one-pot procedure for the oxidation of alcohols and sulfides based on the SiO2–RuCl3/3-iodobenzoic acid tandem catalytic system is especially attractive since catalyst 3 shows high catalytic activity and hypervalent iodine reagents in stoichiometric amounts are relatively expensive. We have carried out the oxidative reactions with catalytic amounts of both catalyst 3 and 3-iodobenzoic acid and using sodium hypochlorite as the stoichiometric oxidant (Table 2, entries 9–11). A one-pot procedure showed noticeably lower yields in the oxidation of alcohols and sulfide compared to the procedure employing the recyclable SiO2–RuCl3/3-(dichloroiodo)benzoic acid catalytic oxidative system in water (Table 1, entries 1, 5 and 14).
When using a heterogeneous catalyst, the important point is the deactivation and recyclability of the catalyst. To test this, a series of six consecutive runs in the case of entry 1 (Table 1) were carried out. After the first alcohol oxidation, the catalyst, SiO2–RuCl3, was separated from the reaction mixture by filtration, thoroughly washed with water, and then reused as the catalyst for the next run under the same conditions. As shown in Fig. 1, the benzaldehyde yields remained essentially constant for the six successive cycles, which demonstrates that there is no significant change in the activity of the catalyst, reflecting the high stability and reusability of the SiO2–RuCl3 catalyst. Moreover, it was experimentally confirmed that no Ru was present in the filtrate.
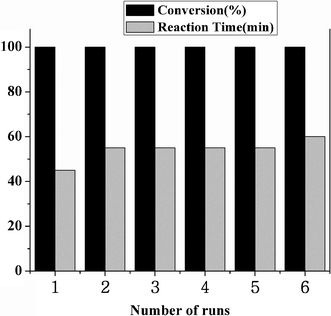 | ||
| Fig. 1 Recyclability of the SiO2–RuCl3 catalyst for the oxidation of benzyl alcohol in water at room temperature (0.2 mmol substrate, 0.23 mol% SiO2–RuCl3 catalyst, 0.3 mmol 3-(dichloroiodo)benzoic acid, and 2 mL H2O). | ||
The reduced form of 3-(dichloroiodo)benzoic acid, 3-iodobenzoic acid, can be easily separated from the reaction mixtures and converted back to 3-(dichloroiodo)benzoic acid by treatment with bleach and aqueous HCl in about 90% overall yield. Thus, the recovery and recyclability of the catalyst, as well as the oxidant, make the process even more cost-effective.
In summary, we have developed a mild, simple, cost-effective and green procedure for the oxidation of alcohols and sulfides using a recyclable SiO2–RuCl3/3-(dichloroiodo)benzoic acid catalytic oxidative system in water under mild conditions. Moreover, the mild reaction conditions, high yields of products, ease of work-up, clean procedure, and excellent chemoselectivity make this method potentially attractive for the oxidation of organic compounds in the industrial setting.
Experimental
Preparation of SiO 2 –RuCl3: aminopropyl silica (AMPS, 5.0 g, 5 mmol) was added to a solution of dimethylacetamide (6.65 g, 50 mmol) in methanol (50 mL) and the reaction mixture was refluxed for 3 h. The mixture was cooled to room temperature, then RuCl3 (0.41 g, 2 mmol) was added, and the resulting mixture was refluxed with stirring for 3 days. The solid product was filtered, washed with methanol, water and Et2O until the washings became colorless. The catalyst, isolated as a dark yellow solid, was dried under vacuum for 5 h before use. Elemental analysis: C, 8.03%; H, 1.64%; N, 1.83%; Cl, 0.50%. The loading of Ru was 0.047 mmol g−1 based on Cl analysis. FT-IR (KBr): 3432, 2950, 1648, 1400, 1098, 800, 564, and 470 cm−1.Acknowledgements
Jiang-Min Chen thanks the financial Foundation of Jiaxing University for supporting his visit to the University of Minnesota Duluth. This work was supported by a research grant from the National Science Foundation (CHE-1009038).References
- R. A. Sheldon and J. K. Kochi, Metal-Catalyzed Oxidations of Organic Compounds, Academic Press, New York, 1981 Search PubMed.
- M. Hudlicky, Oxidations in Organic Chemistry, American Chemical Society, Washington, DC, 1990 Search PubMed.
- Z. Hou, N. Theyssen, A. Brinkmann and W. Leitner, Angew. Chem., Int. Ed., 2005, 44, 1346–1349 CrossRef CAS.
- B. Z. Zhan, M. A. White, T. K. Sham, J. A. Pincock, R. J. Doucet, K. V. R. Rao, K. N. Robertson and T. S. Cameron, J. Am. Chem. Soc., 2003, 125, 2195–2199 CrossRef CAS.
- B. T. Guan, D. Xing, G. X. Cai, X. B. Wan, N. Yu, Z. Fang and Z. J. Shi, J. Am. Chem. Soc., 2005, 127, 18004–18005 CrossRef CAS.
- C. X. Zhang, P. Chen, J. Liu, Y. H. Zhang, W. Shen, H. L. Xu and Y. Tang, Chem. Commun., 2008, 3290–3292 RSC.
- J. Shen, W. Shan, Y. H. Zhang, J. M. Du, H. L. Xu, K. N. Fan, W. Shen and Y. Tang, Chem. Commun., 2004, 2880–2881 RSC.
- P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, Inc., New York, 1998 Search PubMed.
- K. Yamaguchi and N. Mizuno, Angew. Chem., Int. Ed., 2002, 41, 4538–4542 CrossRef CAS.
- D. R. Jensen, J. S. Pugsley and M. S. Signam, J. Am. Chem. Soc., 2001, 123, 7475–7476 CrossRef CAS.
- R. A. Sheldon and J. K. Kochi, Metal-Catalyzed Oxidation of Organic Compounds, Academic Press, New York, 1984 Search PubMed.
- A. Varvoglis, Hypervalent Iodine in Organic Synthesis, Academic Press, London, 1997 Search PubMed.
- Hypervalent Iodine Chemistry, ed. Wirth, T., Springer-Verlag, Berlin, 2003 Search PubMed.
- G. F. Koser, Aldrichim. Acta, 2001, 34, 89–102 CAS.
- G. F. Koser, Adv. Heterocycl. Chem., 2004, 86, 225–292 CrossRef CAS.
- R. M. Moriarty, J. Org. Chem., 2005, 70, 2893–2903 CrossRef CAS.
- V. V. Zhdankin and P. J. Stang, Chem. Rev., 2008, 108, 5299–5358 CrossRef CAS.
- U. Ladziata and V. V. Zhdankin, ARKIVOC, 2006,(ix), 26–58 CAS.
- M. A. Ciufolini, N. A. Braun, S. Canesi, M. Ousmer, J. Chang and D. Chai, Synthesis, 2007, 3759–3772 CrossRef CAS.
- V. V. Zhdankin, Science of Synthesis, 2007, vol. 31a, ch. 31.4.1, pp. 161–234 Search PubMed.
- M. Ochiai and K. Miyamoto, Eur. J. Org. Chem., 2008, 4229–4239 CrossRef CAS.
- T. Dohi and Y. Kita, Chem. Commun., 2009, 2073–2085 RSC.
- U. Ladziata and V. V. Zhdankin, Synlett, 2007, 527–537 CAS.
- V. V. Zhdankin, J. Org. Chem., 2011, 76, 1185–1197 CrossRef CAS.
- V. V. Zhdankin, ARKIVOC, 2009,(i), 1–62 CAS.
- M. Uyanik and K. Ishihara, Chem. Commun., 2009, 2086–2099 RSC.
- M. Ngatimin and D. W. Lupton, Aust. J. Chem., 2010, 63, 653–658 CrossRef CAS.
- M. S. Yusubov, V. N. Nemykin and V. V. Zhdankin, Tetrahedron, 2010, 66, 5745–5752 CrossRef CAS.
- D. Lenoir, Angew. Chem., Int. Ed., 2006, 45, 3206–3210 CrossRef CAS.
- D. J. C. Constable and V. L. Cunningham, Green Chem., 2002, 4, 521 RSC.
- W. Qian, W. Bao and Y. M. Zhang, Angew. Chem., Int. Ed., 2005, 44, 952–955 CrossRef CAS.
- H. Togo and K. Sakuratani, Synlett, 2002, 1966–1975 CrossRef CAS.
- J. M. Chen, X. M. Zeng, K. Middleton and V. V. Zhdankin, Tetrahedron Lett., 2011, 52, 1952–1955 CrossRef CAS.
- J. M. Chen, X. M. Zeng and V. V. Zhdankin, Synlett, 2010, 2771–2774 CrossRef CAS.
- M. S. Yusubov and V. V. Zhdankin, Mendeleev Commun., 2010, 20, 185–181 CrossRef CAS.
- J. M. Chen and X. Huang, Synthesis, 2004, 2459–2462 CAS.
- J. M. Chen and X. Huang, Synthesis, 2004, 1577–1580 CAS.
- J. M. Chen, L. L. Wu and X. Huang, Chin. Chem. Lett., 2004, 15, 1387–1388 CAS.
- J. M. Chen, X. M. Zeng, K. Middleton, M. S. Yusubov and V. V. Zhdankin, Synlett, 2011, 1613–1617 CrossRef CAS.
- J. M. Chen and X. Huang, Synlett, 2004, 552–554 CAS.
- U. Ladziata, J. Willging and V. V. Zhdankin, Org. Lett., 2006, 8, 167–170 CrossRef CAS.
- R. R. Karimov, M. Kazhkenov Z.-G, M. J. Modjewski, E. M. Peterson and V. V. Zhdankin, J. Org. Chem., 2007, 72, 8149–8151 CrossRef CAS.
- M. S. Yusubov, R. Y. Yusubova, T. V. Funk, K.-W. Chi, A. Kirschning and V. V. Zhdankin, Synthesis, 2010, 3681–3685 CrossRef CAS.
- A. A. Zagulyaeva, M. S. Yusubov and V. V. Zhdankin, J. Org. Chem., 2010, 75, 2119–2122 CrossRef CAS.
- A. Yoshimura, C. T. Banek, M. S. Yusubov, V. N. Nemykin and V. V. Zhdankin, J. Org. Chem., 2011, 76, 3812–3819 CrossRef CAS.
- M. S. Yusubov, T. V. Funk, K.-W. Chi, E.-H. Cha, G. H. Kim, A. Kirschning and V. V. Zhdankin, J. Org. Chem., 2008, 73, 295–297 CrossRef CAS.
- M. S. Yusubov, L. A. Drygunova and V. V. Zhdankin, Synthesis, 2004, 2289–2292 CrossRef CAS.
- H. Tohma, A. Maruyama, A. Maeda, T. Maegawa, T. Dohi, M. Shiro, T. Morita and Y. Kita, Angew. Chem., Int. Ed., 2004, 43, 3595–3598 CrossRef CAS.
- P. J. Stang and V. V. Zhdankin, J. Am. Chem. Soc., 1993, 115, 9808–9809 CrossRef CAS.
- M. S. Yusubov, M. P. Gilmkhanova, V. V. Zhdankin and A. Kirschning, Synlett, 2007, 563–565 CAS.
- P. Mueller and J. Godoy, Tetrahedron Lett., 1981, 22, 2361–2364 CrossRef CAS.
- M. S. Yusubov, W. K. Chi, J. Y. Park, R. Karimov and V. V. Zhdankin, Tetrahedron Lett., 2006, 47, 6305–6308 CrossRef CAS.
- P. A. Grieco, Organic Synthesis in Water, Blackie Academic and Professional, London, 1998 Search PubMed.
- P. J. Figiel, M. Leskela and T. Repo, Adv. Synth. Catal., 2007, 349, 1173–1179 CrossRef CAS.
- V. N. Telvekar and N. C. Jadhav, Synth. Commun., 2008, 38, 3107–3111 CrossRef CAS.
- S. Minakata and M. Komatsu, Chem. Rev., 2009, 109, 711–724 CrossRef CAS.
- T. Shamim and S. Paul, Catal. Lett., 2010, 136, 260–265 CrossRef CAS.
- Y. Gu, C. Ogawa, J. Kobayashi, Y. Mori and S. Kobayashi, Angew. Chem., Int. Ed., 2006, 45, 7217–7220 CrossRef CAS.
- S. Paul and J. H. Clark, J. Mol. Catal. A: Chem., 2004, 215, 107–111 CrossRef CAS.
- D. Choudhary, S. Paul, R. Gupta and J. H. Clark, Green Chem., 2006, 8, 479–482 RSC.
- T. Shamim, M. Gupta and S. Paul, J. Mol. Catal. A: Chem., 2009, 302, 15–19 CrossRef CAS.
- T. Shamim, D. Choudhary, S. Mahajan, R. Gupta and S. Paul, Catal. Commun., 2009, 10, 1931–1935 CrossRef CAS.
- A. Podgoršek, M. Jurisch, S. Stavber, M. Zupan, J. Iskra and J. A. Gladysz, J. Org. Chem., 2009, 74, 3133–3140 CrossRef.
- N. Fukuda and T. Ikemoto, J. Org. Chem., 2010, 75, 4629–4631 CrossRef CAS.
- P. Kowalski, K. Mitka, K. Ossowska and Z. Kolarska, Tetrahedron, 2005, 61, 1933–1953 CrossRef CAS.
- P. D. Hampton, M. D. Whealon, L. M. Roberts, A. A. Yaeger and R. Boydson, Org. Process Res. Dev., 2008, 12, 946–949 CrossRef CAS.
- G. Pozzi, M. Cavazzini, S. Quici, M. Benaglia and G. D. Anna, Org. Lett., 2004, 6, 441–443 CrossRef CAS.
- J. H. A. Michiel, C. J. F. Castellana, J. Hayley, D. J. Peter and S. A. Roger, Green Chem., 2011, 13, 905–912 RSC.
- G. Luca, A. W. C. E. Isabel, M. Pasi and S. A. Roger, Adv. Synth. Catal., 2003, 345, 1321–1328 CrossRef.
| This journal is © The Royal Society of Chemistry 2011 |
