Optimization of distyryl-Bodipy chromophores for efficient panchromatic sensitization in dye sensitized solar cells†
Safacan
Kolemen
a,
O. Altan
Bozdemir
a,
Yusuf
Cakmak
a,
Gokhan
Barin
a,
Sule
Erten-Ela
b,
Magdalena
Marszalek
c,
Jun-Ho
Yum
c,
Shaik M.
Zakeeruddin
c,
Mohammad K.
Nazeeruddin
c,
Michael
Grätzel
c and
Engin U.
Akkaya
*ad
aUNAM-Institute of Materials Science and Nanotechnology, Bilkent University, 06800, Ankara, Turkey. E-mail: eua@fen.bilkent.edu.tr; Fax: +90 312-266-4068; Tel: +90 312-290-2450
bInstitute of Solar Energy, Ege University, Bornova, Izmir 35100, Turkey
cLaboratory of Photonics and Interfaces, Institute of Chemical Sciences and Engineering, Ecole Polytechnique Fédérale de Lausanne (EPFL), Station 6, CH-1015, Lausanne, Switzerland
dDepartment of Chemistry, Bilkent University, 06800, Ankara, Turkey
First published on 1st March 2011
Abstract
Versatility of Bodipy (4,4-difluoro-4-bora-3a,4a-diaza-s-indacene) dyes was further expanded in recent dye-sensitized solar cell applications. Here we report a series of derivatives designed to address earlier problems in Bodipy sensitized solar cells. In the best case example, an overall efficiency of a modest 2.46% was achieved, but panchromatic nature of the dyes is quite impressive. This is the best reported efficiency in liquid electrolyte solar cells with Bodipy dyes as photosensitizers.
Introduction
The dye sensitized solar cell (DSSC) concept is a viable alternative to traditional semiconductor based photovoltaic constructs.1 Practical potential is also being realized in the form of rapid commercialization.2 One of the primary issues, which may benefit from rational design, is the choice of the sensitizer dye. Near IR sensitizability and panchromaticity are important and challenging goals and these can be attained in principle by designing appropriate organic dyes.3 We are particularly interested in a class of dyes known as Bodipy dyes.4Bodipy dyes are important fluorophores with a multitude of potential applications.5 Recent developments in Bodipy chemistry6 allow diverse modification on the core structure. Through these modifications, many characteristics of the parent chromophore can be altered in the desired direction, for example, it is possible to shift absorption wavelength from 500 nm to 800 nm with simple chemical transformations.7 In addition, strong electron donor and acceptor groups can be placed on the chromophore. Solubility and aggregation characteristics of the dyes can also be modulated as needed.8Design and synthesis
The first rationally designed example of a Bodipy based sensitizer was reported a few years ago,9 followed by a few more recent articles including both liquid electrolyte8 and solid state10 based DSSCs. It is clear that optimal solar cell performance of a photosensitizer is dependent on a large number of parameters; however, absorption range, anchoring groups and the direction of electronic reorganization on excitation should be among the most important ones. Based on earlier data8a,10a and with the above considerations, we set out to synthesize different Bodipy-based photosensitizers for use in liquid electrolyte dye sensitized solar cells (Fig. 1 and Fig. 3). In each one of them, we tried to investigate the role of at least one parameter in solar cell performance. In our first report8a of a Bodipy based photosensitizer, central chromophoric core was derived from 1,3,5,7-tetramethyl Bodipy. As a result, in that construct the meso-phenyl subsitutent was forced to an orthogonal arrangement in relation to the Bodipy pseudo-plane. The orthogonality of the anchor bearing phenyl group is very likely to limit conjugation and reduce electron flow from the donor to the anchor/acceptor group. With this perspective, it seemed highly reasonable to synthesize a new distyryl Bodipy where two protruding methyl groups at positions 1 and 7 were removed (PS-1).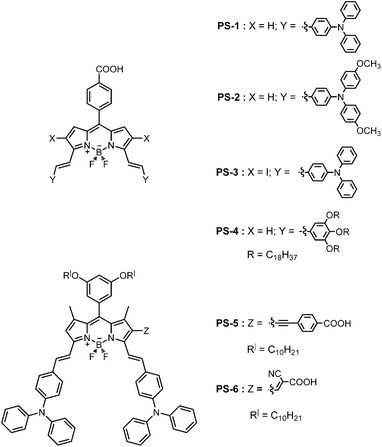 | ||
| Fig. 1 Sensitizers PS-1 through PS-6. | ||
In the second dye (PS-2), we wanted to study the effect of a stronger donor group on the overall solar cell efficiency. To that end, we replaced diphenylaminophenyl group with bis-(p-methoxyphenyl)aminophenyl groups. In designing PS-3, our aim was to find any correlation between the efficiency and the relative ease of populating triplet states. In that compound, placement of two iodo substituents ensure efficient inter system crossing through heavy atom effect related spin–orbit coupling. During the course of this study, we realized that panchromatic absorption, while highly desired, is a consequence of aggregation on titania for the Bodipy derivatives that we studied.
Thus a reasonable goal was to introduce long alkyl chains on the chromophore, either on the donor side or on the meso-substituent. In PS-4, we replaced diphenylaminophenyl groups with gallic aldehyde derived donor groups. In PS-5, in addition placing these long alkyl groups on the meso substituent, the anchor group is attached directly to the Bodipy core through an ethynyl linkage. PS-6 resembles PS-5 in that aggregation limiting alkyl groups are on the meso substituent and the anchor group is attached directly to the Bodipy core. But here, instead of benzoic acid moiety the anchor group is also a strong acceptor which happens to be a common feature in most photosensitizers for DSSC,11 a cyanoacetic acid derived group.
On the other hand, PS-7 (Fig. 3) should be considered separately because with that compound, we wanted to study the effect of excitation energy transfer (mostly through space) in a photosensitizer. To that end, two Bodipy chromophores were tethered onto the 2 and 6 positions of the core chromophore, which happens to be a longer wavelength absorbing (hence, energy acceptor) distyryl Bodipy dye. Our expectations were; stronger absorption in the entire visible spectrum, enhanced panchromaticity and improved overall photovoltaic yield.
In the synthesis, apart from the well-known Bodipy reaction using the appropriate aldehyde and pyrrole, Knoevenagel and Sonogashira reactions have also been employed (Fig. 2). In PS-3, iodination protocol has been employed with quantitative yield.12 The yields are acceptable in all cases, except the final coupling reaction for PS-7. The details of the reactions have been given in the Supporting Information†.
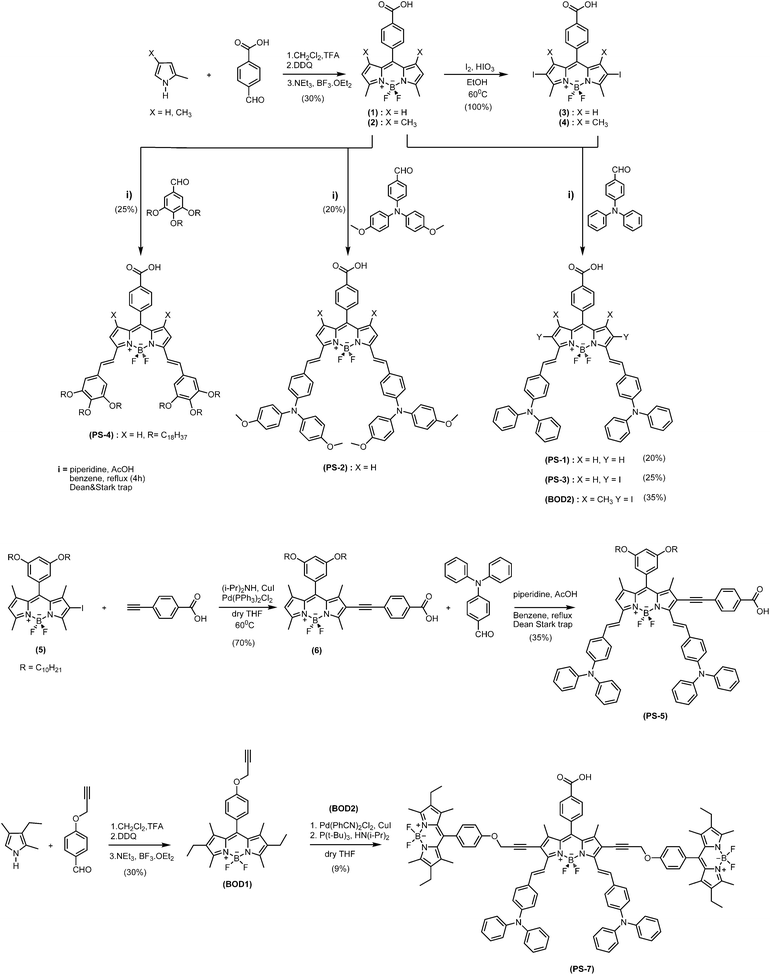 | ||
| Fig. 2 Reaction scheme for the target sensitizers. | ||
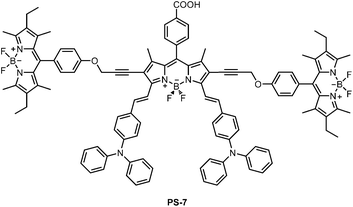 | ||
| Fig. 3 The molecular structure of the sensitizer PS-7. | ||
Results and discussion
Photophysical properties
In the characterization of the photosensitizers (Table 1), our first goal was to acquire absorption spectra of the sensitizers in solution and on titania and also to demonstrate efficient energy transfer in PS-7. Absorption spectra in solution (Fig. 4) show typical disytryl Bodipy bands (S0 → S1) in the long wavelength region (650–760 nm). The absorption peak is somewhat broad, which is not a disadvantage in solar cell applications.| Dye | λ abs a/nm | ε max a/M−1 cm−1 | λ ems a/nm | ϕ f a , b/% | τ f a/ns | Eoxc/V | Eredc/V | EHOMOc/eV | ELUMOc/eV | Eband gapc |
|---|---|---|---|---|---|---|---|---|---|---|
| a Data were collected in CHCl3. b PS-4 was determined relative to tetrastyryl dye 10 and others were to tetrasyryl dye 3 according to ref. 7b. c Electrochemical data were collected in CH2Cl2. Potentials were quoted with reference to the internal reference electrode. d not determined. | ||||||||||
| PS-1 | 724 | 60000 | 799 | 4.5 | 2.1 | 0.78 | −0.74 | 5.08 | 3.56 | 1.52 |
| PS-2 | 746 | 66000 | 835 | 0.7 | 0.7 | 0.56 | −0.87 | 5.05 | 3.62 | 1.43 |
| PS-3 | 761 | 68000 | 824 | <0.001 | n.d.d | 0.86 | −0.51 | 5.17 | 3.79 | 1.38 |
| PS-4 | 668 | 74000 | 699 | 32 | 3.7 | 0.92 | −0.74 | 5.25 | 3.60 | 1.65 |
| PS-5 | 707 | 71000 | 758 | 12 | 2.8 | 0.68 | −0.87 | 5.03 | 3.48 | 1.55 |
| PS-6 | 695 | 79000 | 742 | 11 | 2.9 | 0.72 | −0.94 | 5.21 | 3.55 | 1.66 |
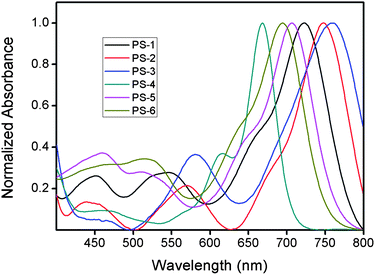 | ||
| Fig. 4 Normalized absorbance spectra of sensitizers PS-1 through PS-6 in CHCl3. | ||
PS-7 shows two distinct absorption bands, one centered around 527 nm and one 735 nm (Fig. 5-top). In PS-7, we expect energy transfer from two side chain linked Bodipys to the near IR absorbing Bodipy core. Model compounds of these two Bodipy units have been synthesized in order to investigate the energy transfer; BOD1 resembles the side chain Bodipy and BOD2 resembles the core unit (Fig. 7). In Fig. 5-top, equal absorbance solutions of the two model compounds and PS-7 were studied, and the efficiency of energy transfer was clearly demonstrated though a comparative depiction of the emission spectra.
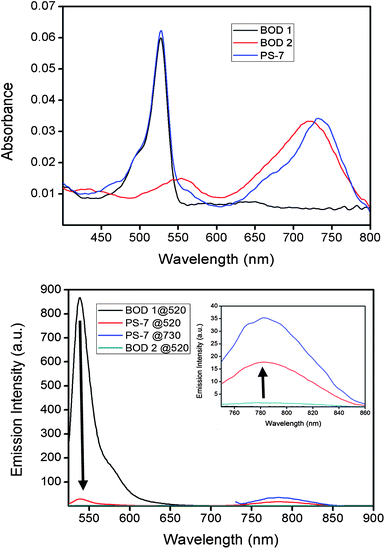 | ||
| Fig. 5 The absorption spectra of compounds BOD1, BOD2, and PS-7 at equal absorbance values at 527 nm (for BOD1 and PS-7) and at 725 nm (BOD2 and PS-7) (top) and emission spectra of the energy transfer cassette (PS-7) in CHCl3 (bottom) in comparison to the selected reference compounds. | ||
Low fluorescence in PS-7 compared to BOD1 at 540 nm and fluorescence enhancement at 781 nm compared to BOD2 is a clear confirmation of the energy transfer (Fig. 5-bottom). Quantum yield of BOD1 at 540 nm is 0.75, and decreased to 0.05 when employed as an antenna module in PS-7, suggesting a 96% energy transfer efficiency. Also, the excitation spectrum of PS-7 in Fig. 6 clearly demonstrates energy transfer from the donor to acceptor chromophores, when emission data is collected at 781 nm. Two peaks correspond to the donor (529 nm) and acceptor (737 nm) moieties.
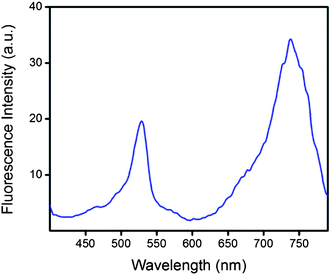 | ||
| Fig. 6 Excitation spectrum of PS-7. Emission data were collected at 781 nm with an optically dilute solution in CHCl3. | ||
 | ||
| Fig. 7 Structures of model compounds; BOD1 and BOD2. | ||
Electrochemistry of the sensitizers
Cyclic voltammetry (CV) results of the Bodipy dyes are given in Table 1. In our previous8a and present CV results, Bodipy derivatives showed both reversible reduction and oxidation potentials (Fig. 8). The double oxidation peaks near 0.65 and 0.8 V are likely to be associated with the oxidation of the Bodipy unit and the DPAP (diphenylaminophenyl) moiety, somewhat perturbed13 by the electron withdrawing Bodipy core, respectively. It is clear that the LUMO energy levels are appropriate (higher than the conduction band of TiO2) for the efficient charge injection from the LUMO level of the sensitizers to the conduction band of the nanocrsytalline titania (4.2 eV). In order to have a continuous electron cycle within the cell, the oxidized dye has to be reduced by the electron donation from the liquid electrolyte redox couple (4.9 eV) which is also an energetically favorable process with Bodipy dyes. Charge separation (wide band gap) is strongly needed between the acceptor and donor moieties of the sensitizers for efficient electron transfer. While PS-4 and PS-6 have the wider band gaps, PS-3 has the lowest value. This low charge separation associated with the PS-3 should be considered as one of the reasons for its low overall conversion efficiency.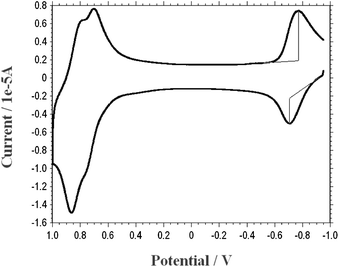 | ||
| Fig. 8 Cyclic voltammogram of PS-1. | ||
Photovoltaic characterization
Typical cell characterization data are presented in Table 2. The IPCE (incident photon to current efficiency) plot as a function of wavelength is essentially flat between the 400–850 nm region, showing a maximum value (30%) around 750 nm (Fig. 9). PS-1 gave an open circuit voltage (Voc) of 0.47 V, a short-circuit photocurrent density (Jsc) of 5.95 mA cm−2, and a fill factor (ff) of 0.67, corresponding to an overall conversion efficiency (η) of 1.88%. Concluding from the data acquired, removal of methyl groups enhances overall efficiency.| Dye | V oc a/V | J sc a/mA cm−2 | ff a | η a/% |
|---|---|---|---|---|
a
V
oc is the open-circuit potential, Jsc, short circuit current, ff is the fill factor, and η is the overall efficiency of the cell under standard conditions.
b Dipping: 4 h in 0.1 mM THF, TiO2: 7 + 4, Electrolyte: A6986 [0.6 M 1-butyl-3-methyl imidazolium iodide (BMII), 0.1 M LiI, 0.05 M I2, 0.05 M tert-butylpyridine (TBP) in Acetonitrile/Valeronitrile (85/15 v/v)].
c Dipping: 24 h in CB/EtOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) + 2 mM cheno (chenodeoxycholic acid), TiO2: 8 + 5 + TiCl4, Electrolyte: Z1040 [1 M LiI, 0.044 M I2, 0.25 M TBP in Acetonitrile/Valeronitrile (85/15 v/v)]. 1) + 2 mM cheno (chenodeoxycholic acid), TiO2: 8 + 5 + TiCl4, Electrolyte: Z1040 [1 M LiI, 0.044 M I2, 0.25 M TBP in Acetonitrile/Valeronitrile (85/15 v/v)].
|
||||
| PS-1 b | 0.47 | 5.95 | 0.67 | 1.88 |
| PS-1 c | 0.43 | 9.17 | 0.62 | 2.46 |
| PS-2 b | 0.46 | 4.52 | 0.63 | 1.32 |
| PS-3 b | 0.35 | 1.05 | 0.61 | 0.23 |
| PS-4 b | 0.47 | 5.45 | 0.71 | 1.81 |
| PS-5 b | 0.52 | 3.74 | 0.71 | 1.40 |
| PS-6 b | 0.42 | 2.55 | 0.70 | 0.75 |
| PS-7 b | 0.40 | 0.69 | 0.72 | 0.20 |
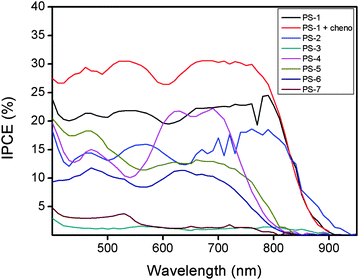 | ||
| Fig. 9 Incident photon to current conversion efficiencies as a function of wavelength for the liquid electrolyte based DSSCs. | ||
In PS-2, addition of p-methoxy groups on the diphenylamino phenyl moiety seems to provide no positive effect on overall conversion efficiency. Compared to PS-1, PS-2 has a lower short-circuit photocurrent density value. Methoxy substitution just shifted the absorption wavelength and increased the extinction coefficient.
The iodinated Bodipy (PS-3) and the energy transfer cassette sensitizers (PS-7) performed poorly. We suspect easy access to the triplet manifold may open the path to degradative chemical reactions in the diiodo compound and the flexibility of the energy transfer cassette might have led to vibrational losses in energy. PS-3 and PS-7 have the lowest short-circuit photocurrent densities among the sensitizers, which suggests poor electron transfer from the excited dyes to the conduction band of the semiconductor.
Photovoltaic parameters of PS-4 are similar to PS-1. In fact, electron donation from the 3,4,5-tris(octadecyloxy) group is less than the diphenylamino phenyl moiety, suppressed aggregate formation on TiO2 with the help of long alkyl chains gave compatible overall efficiency value.
The orientation of the anchoring groups is also important. Based on our earlier theoretical calculations,8a it appears that the meso-position (8-position) is better suited for efficient electron injection compared to the 2-position of the Bodipy core. For example, comparing PS-1 and PS-5 would be highly relevant in providing experimental insight into this argument. When the anchoring group is substituted in the meso-position (PS-1), overall conversion is 1.88%. On the other hand, it is 1.40% for the PS-5 when the anchor group is attached to the 2-position. Further proof for the effectiveness of substitution through the meso-position is the comparison of PS-6 and the Bodipy derivative that we published earlier.8a Both have a cyanoacetic acid derived group as an anchor, but the positions are different. Overall conversion efficiencies are 0.75% (2-position substituted) and 1.66% (meso substituted, Voc: 0.56 V, Jsc: 4.03 mA cm−2, ff: 0.73) respectively.
In our most efficient sensitizer, PS-1, we used chenodeoxycholic acid (cheno) as an additive, which seems to control aggregation and cause an additional boost in the efficiency by improving short circuit current. DSSC with cheno additive gave 9.174 mA cm−2 (Fig. 10) short circuit photocurrent density (Jsc), corresponding to an overall efficiency of 2.46%. This is the best reported efficiency in liquid electrolyte solar cells with Bodipy dyes as photosensitizers. Panchromacity remains as impressive as ever. As a matter of fact, the distyryl-Bodipy dyes especially PS-1, performs better than most other organic dyes in the near IR region in terms of photon to current conversion efficiency.
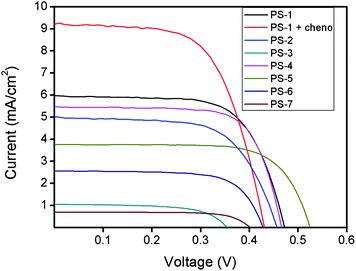 | ||
| Fig. 10 Current vs. voltage graphs of the photosensitizers. | ||
Conclusions
In conclusion, essentially flat curves in the IPCE plot between the wavelengths of 400–800 nm are highly noteworthy, highlighting the panchromatic nature of these dyes. Bodipy dyes show remarkable properties especially as near IR sensitizers. Modifications for improving the performance of these dyes in other parts of the solar spectrum will make them highly promising as sensitizer dyes for solar cells. Fortunately, it is reasonable to expect the rich chemistry of the Bodipy dyes to yield such derivatives. Our work along this line is in progress.Acknowledgements
The authors gratefully acknowledge support from TUBITAK (Grant No. 108T388) and partial support from the Turkish Academy of Sciences (TUBA).Notes and references
- (a) B. O'Regan and M. Grätzel, Nature, 1991, 353, 737 CrossRef CAS; (b) M. Grätzel, Nature, 2001, 414, 338 CrossRef CAS; (c) M. K. Nazeeruddin, Coord. Chem. Rev., 2004, 248, 1161 CrossRef CAS; (d) M. K. Nazeeruddin, A. Kay, I. Rodicio, R. Humphry-Baker, E. Muller, P. Liska, N. Vlachopoulos and M. Grätzel, J. Am. Chem. Soc., 1993, 115, 6382 CrossRef CAS.
- A. Jager-Waldau, Renewable Sustainable Energy Rev., 2007, 11, 1414 CrossRef.
- (a) A. Mishra, M. K. R. Fischer and P. Bauerle, Angew. Chem., Int. Ed., 2009, 48, 2474 CrossRef CAS; (b) Y. Ooyama and Y. Harima, Eur. J. Org. Chem., 2009, 2903 CrossRef CAS; (c) N. Robertson, Angew. Chem., Int. Ed., 2008, 47, 1012 CrossRef CAS; (d) S. Ito, H. Miura, S. Uchida, M. Takata, K. Sumioka, P. Liska, P. Comte, P. Pechy and M. Grätzel, Chem. Commun., 2008, 5194 RSC; (e) J. He, G. Benko, F. Korodi, T. Polivka, R. Lomoth, B. Akermark, L. Sun, A. Hagfeldt and V. Sundstrom, J. Am. Chem. Soc., 2002, 124, 4922 CrossRef CAS; (f) H. Imahori, T. Umeyama and S. Ito, Acc. Chem. Res., 2009, 42, 1809 CrossRef CAS; (g) N. Koumura, Z.-S. Wang, S. Mori, M. Miyashita, E. Suzuki and K. Hara, J. Am. Chem. Soc., 2006, 128, 14256 CrossRef CAS; (h) A. Ajayaghosh, V. K. Praveen and C. Vijayakumar, Chem. Soc. Rev., 2008, 37, 109 RSC; (i) S. Sreejith, P. Carol, P. Chithra and A. Ajayaghosh, J. Mater. Chem., 2008, 18, 264 RSC.
- (a) G. Ulrich, R. Ziessel and A. Harriman, Angew. Chem., Int. Ed., 2008, 47, 1184 CrossRef CAS; (b) R. Ziessel, G. Ulrich and A. Harriman, New J. Chem., 2007, 31, 496 RSC; (c) A. Loudet and K. Burgess, Chem. Rev., 2007, 107, 4891 CrossRef CAS.
- (a) O. A. Bozdemir, Y. Cakmak, F. Sozmen, T. Ozdemir, A. Siemiarczuk and E. U. Akkaya, Chem.–Eur. J., 2010, 16, 6346 CrossRef CAS; (b) S. Erbas, A. Gorgulu, M. Kocakusakogullari and E. U. Akkaya, Chem. Commun., 2009, 4956 RSC; (c) O. A. Bozdemir, R. Guliyev, O. Buyukcakir, S. Selcuk, S. Kolemen, G. Gulseren, T. Nalbantoglu, H. Boyaci and E. U. Akkaya, J. Am. Chem. Soc., 2010, 132, 8029 CrossRef CAS; (d) T. Bura, P. Retailleau and R. Ziessel, Angew. Chem., Int. Ed., 2010, 49, 6659 CrossRef CAS; (e) T. Ozdemir, S. Atilgan, I. Kutuk, L. T. Yildirim, A. Tulek, M. Bayindir and E. U. Akkaya, Org. Lett., 2009, 11, 2105 CrossRef CAS; (f) R. Guliyev, O. Buyukcakir, F. Sozmen and O. A. Bozdemir, Tetrahedron Lett., 2009, 50, 5139 CrossRef CAS; (g) L. L. Li, J. Y. Han, B. Nguyen and K. Burgess, J. Org. Chem., 2008, 73, 1963 CrossRef CAS; (h) J. L. Fan, K. X. Guo, X. J. Peng, J. J. Du, J. Y. Wang, S. G. Sun and H. L. Li, Sens. Actuators, B, 2009, 142, 191 CrossRef; (i) X. Qi, E. J. Jun, L. Xu, S.-J. Kim, J. S. J. Hong, Y. J. Yoon and J. Yoon, J. Org. Chem., 2006, 71, 2881 CrossRef CAS; (j) X. Qi, S. K. Kim, S. J. Han, L. Xu, A. Y. Jee, H. N. Kim, C. Lee, Y. Kim, M. Lee, S.-J. Kim and J. Yoon, Tetrahedron Lett., 2008, 49, 261 CrossRef CAS; (k) S. Ozlem and E. U. Akkaya, J. Am. Chem. Soc., 2009, 11, 48 CrossRef.
- (a) K. Umezawa, Y. Nakamura, H. Makino, D. Citterio and K. Suzuki, J. Am. Chem. Soc., 2008, 130, 1550 CrossRef CAS; (b) K. Rurack, M. Kollmannsberger and J. Daub, Angew. Chem., Int. Ed., 2001, 40, 385 CrossRef CAS; (c) A. Haefele, C. Zedde, P. Retailleau, G. Ulrich and R. Ziessel, Org. Lett., 2010, 12, 1672 CrossRef CAS; (d) D. C. Wang, J. L. Fan, X. Q. Gao, B. S. Wang, S. G. Sung and X. J. Peng, J. Org. Chem., 2009, 74, 7675 CrossRef CAS.
- (a) Z. Dost, S. Atilgan and E. U. Akkaya, Tetrahedron, 2006, 62, 8484 CrossRef CAS; (b) O. Buyukcakir, O. A. Bozdemir, S. Kolemen, S. Erbas and E. U. Akkaya, Org. Lett., 2009, 11, 4644 CrossRef CAS; (c) T. Rohand, W. Qin, N. Boens and W. Dehaen, Eur. J. Org. Chem., 2006, 4658 CrossRef CAS.
- (a) S. Erten-Ela, D. Yilmaz, B. Icli, Y. Dede, S. Icli and E. U. Akkaya, Org. Lett., 2008, 10, 3299 CrossRef CAS; (b) D. Kumaresan, R. P. Thummel, T. Bura, G. Ulrich and R. Ziessel, Chem.–Eur. J., 2009, 15, 6335 CrossRef CAS; (c) C. Y. Lee and J. T. Hupp, Langmuir, 2010, 26, 3760 CrossRef CAS.
- S. Hattori, K. Ohkubo, Y. Urano, H. Sunahara, T. Nagano, Y. Wada, N. V. Tkachenko, H. Lemmetyinen and S. Fukuzumi, J. Phys. Chem. B, 2005, 109, 15368 CrossRef CAS.
- (a) S. Kolemen, Y. Cakmak, S. Erten-Ela, Y. Altay, J. Brendel, M. Thelakkat and E. U. Akkaya, Org. Lett., 2010, 12, 3812 CrossRef CAS; (b) T. Rousseau, A. Cravino, T. Bura, G. Ulrich, R. Ziessel and J. Roncali, Chem. Commun., 2009, 1673 RSC; (c) T. Rousseau, A. Cravino, T. Bura, G. Ulrich and R. Ziessel, J. Mater. Chem., 2009, 19, 2298 RSC; (d) B. Kim, B. Ma, V. R. Donuru, H. Liu and J. M. J. Frèchet, Chem. Commun., 2010, 46, 4148 RSC; (e) T. Rousseau, A. Cravino, E. Ripaud, P. Leriche, S. Rihn, A. D. Nicola, R. Ziessel and J. Roncali, Chem. Commun., 2010, 46, 5082 RSC.
- (a) J. R. Durrant, S. A. Haque and E. Palomares, Coord. Chem. Rev., 2004, 248, 1247 CrossRef; (b) G. Benko, J. Kallioinen, J. E. I. Korppi-Tommola, A. P. Yartsev and V. Sundstrom, J. Am. Chem. Soc., 2002, 124, 489 CrossRef.
- T. Yogo, Y. Urano, Y. Ishitsuka, F. Maniwa and T. Nagano, J. Am. Chem. Soc., 2005, 127, 12162 CrossRef CAS.
- D. P. Hagberg, T. Edvinsson, T. Marinado, G. Boschloo, A. Hagfeldt and L. Sun, Chem. Commun., 2006, 2245 RSC.
Footnote |
| † Electronic Supplementary Information (ESI) available: Experimental Section; synthetic routes, 1H, 13C NMR, and mass spectra of sensitizers (PS-1, PS-2, PS-3, PS-4, PS-5, PS-7); absorption spectra of sensitizers PS-1, PS-2, PS-3, PS-4, PS-5, PS-7 on titania; Cyclic voltammograms of the sensitizers PS-3, PS-4, and PS-5; photophysical parameters for PS-7; device fabrication. See DOI: 10.1039/c0sc00649a/ |
| This journal is © The Royal Society of Chemistry 2011 |
