19F MRI detection of β-galactosidase activity for imaging of gene expression†
Shin
Mizukami
ab,
Hisashi
Matsushita
a,
Rika
Takikawa
a,
Fuminori
Sugihara
c,
Masahiro
Shirakawa
d and
Kazuya
Kikuchi
*ab
aDivision of Advanced Science and Biotechnology, Graduate School of Engineering, Osaka University, 2-1 Yamadaoka, Suita, Osaka 565-0871, Japan. E-mail: kkikuchi@mls.eng.osaka-u.ac.jp; Fax: (+81) 6-6879-7875
bImmunology Frontier Research Center (IFReC), Osaka University, Osaka, 565-0871, Japan
cInternational Graduate School of Arts and Sciences, Yokohama City University, Kanagawa, 230-0045, Japan
dGraduate School of Engineering, Kyoto University, Kyoto, 615-8510, Japan
First published on 7th April 2011
Abstract
Imaging of gene expression by magnetic resonance imaging (MRI) yields direct information regarding living systems that cannot be obtained via other methods. In this study, we report the rational design and synthesis of a novel 19F MRI probe that detects β-galactosidase (β-gal) activity, enabling the imaging of gene expression in cells. The 19F MRI signal of the probe was quenched by the intramolecular paramagnetic resonance enhancement from a Gd3+ ion. A contrivance was made in the probe structure to recover the 19F MRI signal after hydrolysis by β-gal with a following self-immolative reaction. This 19F MRI signal change was observed in the physiological aqueous condition. The probe could also detect β-gal activity in fixed HEK293T cells. In conclusion, this new probe enables the 19F MRI detection of cellular gene expression. The probe design strategy is also expected to lead to the development of MRI probes for a wide variety of hydrolase activities.
Introduction
Imaging of gene expression gives us various information such as the expression timing of target proteins, gene transfer efficiency, and detection of a disease-related gene expression. To monitor gene expression by various methods, reporter proteins1–3 are useful. Fluorescence detection of gene expression by using fluorescent proteins is particularly important because fluorescence measurement has several advantages including sensitivity, convenience, spatiotemporal resolution, etc. However, the poor transmission of fluorescence is one of the limitations for the in vivo application. Use of magnetic resonance imaging (MRI)4 is one way to solve the problem, because MRI yields high-resolution images of deep regions of living animal bodies. Therefore, MRI is currently considered to be one of the most promising techniques for in vivo investigation of physiological events.5Recently, several smart 1H MRI probes for visualizing gene expressionvia β-galactosidase activity have been reported.6 In principle, however, such 1H MRI signal enhancement needs to be discriminated from the background 1H MRI signals of water, fatty acids, and other biomolecules. To avoid this limitation, we have focused on the use of 19F MRI. 19F, as well as 1H, is one of the most highly sensitive nuclei for NMR spectroscopy and MRI,7 and almost no intrinsic 19F MRI signals are observed in animal bodies. Thus, 19F MRI probes that can visualize biological events have been increasingly reported.8 We have also developed off-on switching 19F MRI probes to detect protease activity9 on the basis of paramagnetic relaxation enhancement (PRE),10 a phenomenon in which the relaxation of nuclei is enhanced near paramagnetic molecules.
By expanding this probe principle, we here report a novel 19F MRI probe that detects cellular gene expression. β-galactosidase (β-gal) was chosen as the reporter protein for gene expression, because it has several advantages where reporter proteins are concerned.3,11 The advantages are as follows: (1) induction of β-gal synthesis occurs over a large dynamic range, (2) β-gal is tolerated and functional in many organisms including mammals, (3) various substrates of β-gal are available or easily synthesized, (4) many assay methods that use β-D-galactopyranoside-coupled aglycones are available, and (5) there is almost no intrinsic β-gal activity in mammalian cells. Therefore, β-gal is one of the most widely used reporter proteins for imaging of gene expression. Through the detection of β-gal activity, we tried 19F MRI detection of cellular gene expression.
Results
Probe design concept, synthesis and physical properties
Several probes have been developed that can detect β-gal activity.3,12 X-gal is one of the most widely used probes among them. Such β-D-galactopyranoside-coupled aromatic compounds are known to be the substrates of β-gal, and several fluorescent probes for β-gal have been developed. Taking this substrate recognition property of β-gal into consideration, we designed a 19F MRI probe Gd-DFP-Gal for detecting β-gal activity by combining the paramagnetic relaxation enhancement (PRE) based probe design principle that we previously developed9 with the structures of conventional β-gal probes (Fig. 1). The transverse relaxation time T2 of the 19F nucleus near Gd3+ is expected to be reduced by the PRE from Gd3+, which has seven unpaired electrons in its 4f orbital. Thus, the T2 of the trifluoromethyl (CF3) group of Gd-DFP-Gal was expected to be strongly reduced.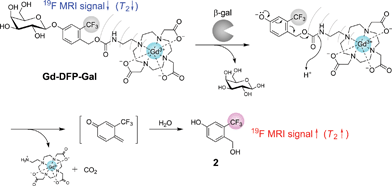 | ||
| Fig. 1 Structure of Gd-DFP-Gal and the principle for the 19F MRI detection of β-gal activity. | ||
Another designed function of Gd-DFP-Gal is the self-immolative property that can be induced by enzymatic cleavage. When Gd-DFP-Gal is hydrolyzed by β-gal, the probe is expected to be automatically converted to the corresponding quinone methide by the successive elimination of the substituent at the benzyl position.13 Thus, the T2 of the trifluoromethyl group extends after the β-galactoside bond is cleaved because of the cancellation of the intramolecular PRE. MRI signal intensity (i.e., the peak height of the NMR signal) is proportional to exp(−t/T2), where t is the echo time in the spin-echo method. Thus, the T2 extension leads to an increase in the MRI signal. On the basis of this principle, we expected that the originally quenched 19F MRI signal of Gd-DFP-Gal would emerge upon the enzyme reaction.
Gd-DFP-Gal was synthesized in five steps (Scheme S1, ESI†). Details of the synthetic procedure are described in the Supporting Information.† As we expected, the NMR peak of Gd-DFP-Gal was not observed, although that of the Gd-free probe DFP-Gal was a sharp single peak (Fig. S2, ESI†). Disappearance of the 19F NMR peak of Gd-DFP-Gal indicates that the T2 was markedly reduced because of the strong intramolecular PRE.
In vitro detection of β-gal activity by 19F NMR and 19F MRI
Because the relaxation times of Gd-DFP-Gal were dramatically reduced, it was expected that the enzymatic degradation of Gd-DFP-Gal would induce the recovery of the disappeared 19F NMR peak. Gd-DFP-Gal was incubated with β-gal at 37 °C in the reaction buffer (pH 7.3) containing 5% D2O, and the time course of the 19F NMR peak was monitored (Fig. 2a). A single peak appeared at around 16 ppm (internal standard: sodium trifluoroacetate) and increased in a time-dependent manner. As the progress of the enzyme reaction was confirmed by RP-HPLC, the peak of Gd-DFP-Gal disappeared and a new peak appeared (Fig. 2b). The new peak was identified to be 4-hydroxymethyl-3-trifluoromethylphenol (compound 2) from the retention time. In addition, the ESI-MS of the HPLC peak fraction gave a molecular weight identical to 2 (m/z = 192). No other noticeable peaks in the reaction solution HPLC diagram suggest that Gd-DFP-Gal was converted to 2 by β-galactosidase activity with nearly complete efficiency.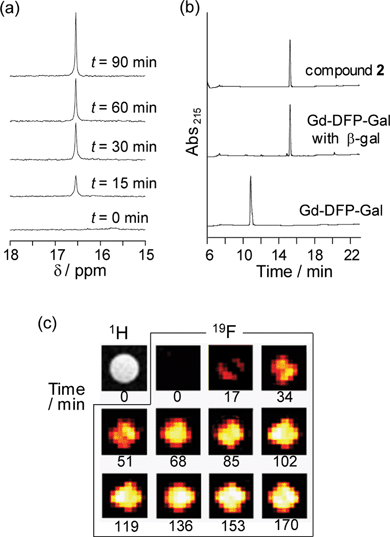 | ||
| Fig. 2 Detection of β-gal activity by Gd-DFP-Gal. (a) Time-dependent 19F NMR spectral change of Gd-DFP-Gal (1 mM) under incubation with β-gal. Sodium trifluoroacetate was used as the internal standard (0 ppm). (b) Confirmation of the enzymatic cleavage by RP-HPLC (eluent: H2O–acetonitrile containing 0.1% TFA). (c) Time course of the density-weighted 19F MR phantom images of Gd-DFP-Gal (1 mM) at 37 °C after β-gal was added. | ||
The relaxation times T1 and T2 of the reaction sample became 0.306 s and 0.086 s, respectively, after the enzyme reaction. Both of them showed considerable extension compared to those of Gd-DFP-Gal, probably due to the cancellation of the intramolecular PRE from Gd3+. These values are still less than those of the Gd3+-free probe DFP-Gal: 1.293 s for T1 and 0.271 s for T2. When the relaxation times of Gd-DFP-Gal were measured at various probe concentrations after the enzymatic cleavage, both T1 and T2 extended as the concentration decreased (Fig. S3 and Table S1, ESI†). This concentration dependency of the relaxation times indicates that the intermolecular PRE is effective under the experimental condition even after the enzyme reaction is complete. To confirm the probe specificity, Gd-DFP-gal was incubated with other similar enzymes, α-galactosidase and β-glucuronidase. However, 19F NMR signals of Gd-DFP-gal were not recovered by incubation with such enzymes (Fig. S4, ESI†).
To demonstrate the possibility of further application, 19F MRI detection of β-gal activity was performed using Gd-DFP-Gal. 19F MRI phantom images were measured using an 11.7 T MRI instrument. Gd-DFP-Gal was mixed with Escherichia coli β-gal before being poured into a 1-mm-inner radius capillary. The density-weighted MR images were then captured by the fast spin-echo method. As expected from the 19F NMR results, Gd-DFP-Gal showed no 19F MRI signals in the absence of β-gal. After the probe was mixed with β-gal, however, the 19F MRI signals gradually increased in a time-dependent manner (Fig. 2c). Without addition of the enzyme, the MRI image did not show any signals for several hours (data not shown). These results demonstrate that this novel mechanism-based probe Gd-DFP-Gal enables the specific 19F MRI detection of β-gal activity.
19F NMR and 19F MRI detection of β-gal expression in fixed HEK293T cells
Next, the applicability of Gd-DFP-Gal to the detection of intracellular gene expression was confirmed. β-gal was expressed in HEK293T cells, and the cells were fixed with formaldehyde and detergent. Then, Gd-DFP-Gal was incubated with the cells, and β-gal activity in the medium supernatant was analyzed by 19F NMR (Fig. 3a). As a result, incubation of Gd-DFP-Gal only with the cells expressing β-gal induced the clear increase of a single 19F NMR peak (Fig. 3b).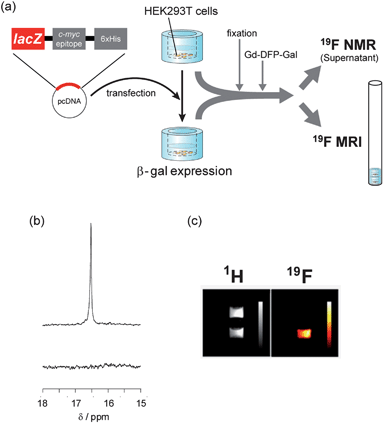 | ||
| Fig. 3 19F NMR and 19F MRI detection of gene expression in HEK293T cells. (a) Illustration of the experimental procedures for the 19F NMR and 19F MRI measurements. (b) 19F NMR spectra of the culture medium containing 1 mM Gd-DFP-Gal incubated with fixed cells expressing (top) or not expressing (bottom) β-gal. (c) 1H (left) and 19F (right) MR images of culture vessels containing 1 mM Gd-DFP-Gal fixed cells. Color scale bars were inserted in the images. | ||
Then, the 19F MRI detection of β-gal gene expression was attempted. HEK293T cells expressing or not expressing β-gal were cultured on 7-mm-diameter glass vessels. After the fixation of the cells, Gd-DFP-Gal (final conc.: 1 mM) was added into the glass vessels, and the cells were incubated at 37 °C for 2 h. The vessels were stacked in an 8 mm NMR tube, as shown in Fig. 3b, and the 1H and 19F MR images were captured. Although both vessels showed indistinguishable signal intensity in 1H MRI (Fig.3c left), only the vessel that included HEK293T cells expressing β-gal showed remarkable 19F MRI signals (Fig. 3c right). These results indicate that Gd-DFP-Gal can specifically detect gene expression in fixed HEK293T cells by means of reporter β-gal activity.
Discussion
From the point of probe design strategy, development of Gd-DFP-Gal is an important step forward in molecular imaging studies. Our previous 19F MRI probe that detects protease activity also utilized the cancellation of the intramolecular PRE for signal switching.9 In these cases, the 19F atoms and the paramagnetic ions were conjugated to each other at the opposite end of the probes (Fig. 4a). The MRI signals were enhanced by enzymatic cleavage of the substrate linker. Although this strategy works for a wide variety of hydrolases such as other proteases, endonucleases and phosphodiesterases, it could not be applied to several hydrolases such as phosphatases that have a substrate-binding pocket covering one end of the substrate. In order to detect these enzyme activities by MRI, we expanded the probe design concept by exploiting a self-immolative reaction, as presented in this study (Fig. 1). We now have the ability to design 19F MRI probes for a broader range of hydrolases by choosing either of the ways illustrated in Fig. 4.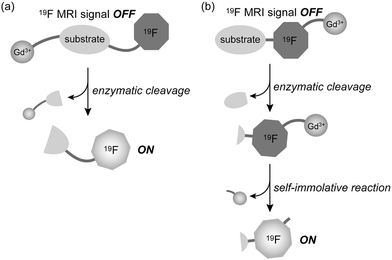 | ||
| Fig. 4 Two 19F MRI probe design strategies using PRE cancellation by (a) enzymatic cleavage of the substrate linker, and (b) enzyme activity-induced self-immolative reaction. | ||
Although imaging of gene expression in fixed cells by using Gd-DFP-Gal, there are two obstacles for future perspective to in vivo imaging. One is the membrane permeability of the probes. Since the new probe did not permeate cell membrane, the cells needed to be fixed with formaldehyde for imaging. However, use of cell-penetrating peptides,14 which enabled the incorporation of proteins into live cells, may dissolve the problem.
The other is the sensitivity. Generally, the sensitivity of 19F MRI probes is worse than 1H MRI probes. This is because 1H MRI visualizes many water molecules around the probe molecules, although 19F MRI probes give only the probe signals. Concerning the problem, improvement of both probes and instruments will contribute to solve it. About the probe sensitization, we are under the development and would report elsewhere in future.
Conclusions
In summary, we succeeded in the imaging of gene expression in mammalian cells using a novel 19F MRI probe that detects β-gal activity. The probe design concept is based on the MRI signal quenching by PRE. Also, by exploiting a self-immolative organic reaction in the probe design, we could overcome the limitation of our previous probe design, in which the 19F atoms and the paramagnetic ions should be located at the opposite end of the probes. New design strategy will lead to the development of the probes for a wider variety of hydrolases such as phosphatases. Although in vivo imaging of gene expression by 19F MRI is still challenging, the further progress of the probe properties will contribute to the solution of the difficult and significant subject.Experimental section
19F NMR relaxation time measurements
Samples were prepared at 500 μM concentration in 10 mM Tris buffer (pH 7.3) containing 10 mM magnesium chloride and 5% D2O. The longitudinal relaxation time T1 was measured by an inversion recovery method and the transverse relaxation time T2 was measured by the spin-echo method.Enzyme reaction
Gd-DFP-Gal was dissolved at 500 μM in 10 mM Tris buffer (pH 7.3) containing 10 mM magnesium chloride and 5% D2O. Samples (500 μL) were incubated with β-gal (5.03 U) at 37 °C for 2 h. The reaction progress was monitored by 19F NMR and RP-HPLC using an octadecyl silane (ODS) column. For the 19F MRI experiment, 1 mM Gd-DFP-Gal was dissolved in 10 mM Tris buffer solution (pH 7.3) containing 10 mM magnesium chloride and 5% D2O. Samples with or without β-gal (1.2 mU) were filled into glass capillaries (inner diameter: approximately 1 mm; Hischmann Laborgerate). The capillaries were then inserted into an 8 mm NMR tube and the 19F MRI were measured.Cellular experiments
HEK293T cells were grown at 37 °C in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U mL−1penicillin G, and 100 mg mL−1streptomycin in a humidified atmosphere with 5% CO2. The cells were plated at 1.2 × 106cells in 60 mm dishes or 1.2 × 105cells cm−2 on 24-well plates. Next, the cells were transfected with pcDNA™4/TO/myc-His/lacZ©plasmid using Lipofectamine 2000, and the cells were incubated for 24 h at 37 °C in a CO2 incubator. After the cells were washed three times with phosphate-buffered solution (PBS), they were incubated with trypsin-EDTA at 37 °C for 5 min under 5% CO2.For 19F NMR analysis, the cells were cultured with 1 mM Gd-DFP-Gal for 2 h at 37 °C in the reaction buffer (10 mM Tris-sodium buffer (pH 7.3) and 10 mM magnesium chloride) on 24-well plates. Then, the supernatants were moved into NMR tubes and the 19F NMR spectra were measured.
For 19F MRI analysis, the cells were moved onto 7 mm (outer diameter) glass vessels (Hilgenberg GmbH), and were incubated for 7 h at 37 °C in DMEM with 10% FBS. After the cells were washed three times with PBS, they were incubated with 3.7% formaldehyde for 10 min at room temperature. Then, cells were washed three times with PBS and incubated with 1 mM Gd-DFP-Gal for 2 h at 37 °C in the reaction buffer (Tris-sodium buffer (pH 7.3) and 10 mM magnesium chloride). The vessels were put into an 8 mm NMR tube, and the 1H and 19F MRI were measured.
Acknowledgements
We thank Dr Tetsuro Kokubo at Yokohama City University for the use of the MRI instrument, and Dr Haruhiko Bito (University of Tokyo), Dr Hiroyuki Okuno (University of Tokyo) and Dr Shin-ichi Muramatsu (Jichi Medical University) for the helpful discussion. This research is supported by Ministry of Education, Culture, Sports, Science and Technology–Japan (Grant No. 21685019 and 20675004), by the Japan Society for the Promotion of Science (JSPS) through its Funding Program for World-Leading Innovative R&D on Science and Technology (FIRST Program), by Ministry of Health, Labour and Welfare–Japan, and by the New Energy and Industrial Technology Development Organization (NEDO) of Japan. S.M. acknowledges the Inamori Foundation.Notes and references
- R. Y. Tsien, Annu. Rev. Biochem., 1998, 67, 509 CrossRef CAS.
- C. H. Contag and M. H. Bachman, Annu. Rev. Biomed. Eng., 2002, 4, 235 CrossRef CAS.
- (a) G. P. Nolan, S. Fiering, J.-F. Nicholas and L. A. Herzenberg, Proc. Natl. Acad. Sci. U. S. A., 1988, 85, 2603 CrossRef CAS; (b) I. G. Serebriiskii and E. A. Golemis, Anal. Biochem., 2000, 285, 1 CrossRef CAS.
- (a) A. Jasanoff, Trends Neurosci., 2005, 28, 120 CrossRef CAS; (b) D. E. Sosnovik and R. Weissleder, Curr. Opin. Biotechnol., 2007, 18, 4 CrossRef CAS.
- (a) R. Weissleder and M. J. Pittet, Nature, 2008, 452, 580 CrossRef CAS; (b) J. L. Major and T. J. Meade, Acc. Chem. Res., 2009, 42, 893 CrossRef CAS.
- (a) A. Y. Louie, M. M. Hüber, E. T. Ahrens, U. Rothbächer, R. Moats, R. E. Jacobs, S. E. Fraser and T. J. Meade, Nat. Biotechnol., 2000, 18, 321 CrossRef CAS; (b) Y. T. Chang, C. M. Cheng, Y. Z. Su, W. T. Lee, J. S. Hsu, G. C. Liu, T. L. Cheng and Y. M. Wang, Bioconjugate Chem., 2007, 18, 1716 CrossRef CAS; (c) E. L. Que, D. W. Domaille and C. J. Chang, Chem. Rev., 2008, 108, 1517 CrossRef CAS; (d) W. Cui, L. Liu, V. D. Kodibagkar and R. P. Mason, Magnetic Resonance in Medicine, 2010, 64, 65 Search PubMed.
- (a) J. Yu, V. D. Kodibagkar, W. Cui and R. P. Mason, Curr. Med. Chem., 2005, 12, 819 CrossRef CAS; (b) C. Belle, C. Beguin, S. Hamman and J.-L. Pierre, Coord. Chem. Rev., 2009, 253, 963 CrossRef CAS.
- (a) W. Cui, P. Otten, Y. Li, K. S. Koeneman, J. Yu and R. P. Mason, Magn. Reson. Med., 2004, 51, 616 CrossRef CAS; (b) M. Higuchi, N. Iwata, Y. Matsuba, K. Sato, K. Sasamoto and T. C. Saido, Nat. Neurosci., 2005, 8, 527 CrossRef CAS; (c) V. D. Kodibagkar, J. Yu, L. Liu, H. P. Hetherington and R. P. Mason, Magn. Reson. Imaging, 2006, 24, 959 CrossRef; (d) K. Tanaka, N. Kitamura, K. Naka and Y. Chujo, Chem. Commun., 2008, 6176 RSC; (e) P. Porcari, S. Capuani, E. D'Amore, M. Lecce, A. La Bella, F. Fasano, R. Campanella, L. M. Migneco, F. S. Pastore and B. Maraviglia, Phys. Med. Biol., 2008, 53, 6979 CrossRef CAS; (f) K. Tanaka, N. Kitamura, Y. Takahashi and Y. Chujo, Bioorg. Med. Chem., 2009, 17, 3818 CrossRef CAS; (g) Y. Takaoka, T. Sakamoto, S. Tsukiji, M. Narazaki, T. Matsuda, H. Tochio, M. Shirakawa and I. Hamachi, Nat. Chem., 2009, 1, 557 CrossRef CAS; (h) K. Tanabe, H. Harada, M. Narazaki, K. Tanaka, K. Inafuku, H. Komatsu, T. Ito, H. Yamada, Y. Chujo, T. Matsuda, M. Hiraoka and S. Nishimoto, J. Am. Chem. Soc., 2009, 131, 15982 CrossRef CAS.
- (a) S. Mizukami, R. Takikawa, F. Sugihara, Y. Hori, H. Tochio, M. Wälchli, M. Shirakawa and K. Kikuchi, J. Am. Chem. Soc., 2008, 130, 794 CrossRef CAS; (b) S. Mizukami, R. Takikawa, F. Sugihara, M. Shirakawa and K. Kikuchi, Angew. Chem., Int. Ed., 2009, 48, 3641 CrossRef CAS.
- L. Helm, Prog. Nucl. Magn. Reson. Spectrosc., 2006, 49, 45 CrossRef.
- C. V. Hall, P. E. Jacob, G. M. Ringold and F. Lee, J. Mol. Appl. Genet., 1983, 2, 101 Search PubMed.
- (a) J. P. Horwitz, J. Chua, R. J. Curby, A. J. Tomson, M. A. DaRooge, B. E. Fisher, J. Mauricio and I. Klundt, J. Med. Chem., 1964, 7, 574 CrossRef CAS; (b) A. B. Pardee, F. Jacob and J. Monod, J. Mol. Biol., 1959, 1, 165 CrossRef CAS.
- (a) D. Shabat, R. J. Amir, A. Gopin, N. Pessah, M. Shamis and W.-M. Dai, Chem.–Eur. J., 2004, 10, 2626 CrossRef CAS; (b) J. A. Duimstra, F. J. Femia and T. J. Meade, J. Am. Chem. Soc., 2005, 127, 12847 CrossRef CAS; (c) T. Komatsu, K. Kikuchi, H. Takakusa, K. Hanaoka, T. Ueno, M. Kamiya, Y. Urano and T. Nagano, J. Am. Chem. Soc., 2006, 128, 15946 CrossRef CAS; (d) N.-H. Ho, R. Weissleder and C.-H. Tung, ChemBioChem, 2007, 8, 560 CrossRef CAS.
- E. Vives, J. Schmidt and A. Pelegrin, Biochim. Biophys. Acta, 2008, 1786, 126 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis of compounds, representative HPLC chromatograms and 19F NMR. See DOI: 10.1039/c1sc00071c |
| This journal is © The Royal Society of Chemistry 2011 |
