Single nanoparticle spectroscopy for real-time in vivo quantitative analysis of transport and toxicity of single nanoparticles in single embryos†‡
Kerry J.
Lee
,
Prakash D.
Nallathamby
,
Lauren M.
Browning
,
Tanvi
Desai
,
Pavan K.
Cherukuri
and
Xiao-Hong Nancy
Xu
*
Department of Chemistry and Biochemistry, Old Dominion University, Norfolk, VA 23529, USA. E-mail: xhxu@odu.edu; Fax: +1 (757) 683-5698; Tel: +1 (757) 683-5698 Web: www.odu.edu/sci/xu/xu.htm
First published on 12th April 2012
Abstract
Nanomaterials exhibit distinctive physicochemical properties and promise a wide range of applications from nanotechnology to nanomedicine, which raise serious concerns about their potential environmental impacts on ecosystems. Unlike any conventional chemicals, nanomaterials are highly heterogeneous, and their properties can alter over time. These unique characteristics underscore the importance of study of their properties and effects on living organisms in real time at single nanoparticle (NP) resolution. Here we report the development of single-NP plasmonic microscopy and spectroscopy (dark-field optical microscopy and spectroscopy, DFOMS) and ultrasensitive in vivo assay (cleavage-stage zebrafish embryos, critical aquatic species) to study transport and toxicity of single silver nanoparticles (Ag NPs, 95.4 ± 16.0 nm) on embryonic developments. We synthesized and characterized purified and stable (non-aggregation) Ag NPs, determined their sizes and doses (number), and their transport mechanisms and effects on embryonic development in vivo in real time at single-NP resolution. We found that single Ag NPs passively entered the embryos through their chorionic pores via random Brownian diffusion and stayed inside the embryos throughout their entire development (120 h), suggesting that the embryos can bio-concentrate trace NPs from their environment. Our studies show that higher doses and larger sizes of Ag NPs cause higher toxic effects on embryonic development, demonstrating that the embryos can serve as ultrasensitive in vivo assays to screen biocompatibility and toxicity of the NPs and monitor their potential release into aquatic ecosystems.
Introduction
Advances in nanotechnology demand rational design of biocompatible nanomaterials and development of sensitive methods to effectively and rapidly characterize and screen biocompatibility and toxicity of nanomaterials. Engineered nanomaterials possess distinctive physicochemical properties and promise wide ranges of applications from consumer products to health care.1–4 Owing to their small sizes, nanomaterials can enter living organisms and enable sub-cellular imaging and effective drug delivery.1–3,5–8 Due to their high surface area-to-volume ratios, nanomaterials can potentially serve as ultrasensitive molecular sensors for early disease diagnosis and as targeted vehicles to carry a larger payload of therapeutic molecules for effective drug delivery.2,5,6,9–12 However, their unusually high surface area-to-volume ratios also lead to their high reactivity, which can potentially cause adverse effects. The possible release of engineered nanomaterials to ecosystems raises serious concerns about their potential environmental impacts and prompts a wide range of studies on nanotoxicity.11,13–17Unlike any conventional chemicals and materials, nanomaterials are highly heterogeneous and dynamic, and they can alter over time. Individual NPs can have their own distinctive physicochemical properties and effects on living organisms, which underscore the importance of characterization of their properties and effects in real time at single-NP resolution. Nanomaterials need special care in order to maintain their stability (non-aggregation), fully suspend and homogenously distribute them in a population of living organisms.11,16,18 Engineered nanomaterials contain various residual chemicals, which depend highly upon their preparation protocols. Therefore, to effectively characterize nanotoxicities and better understand their dependence upon physicochemical properties of nanomaterials, it is essential to prepare stable and purified nanomaterials, and to develop in vivo assays and imaging tools that can quantitatively determine their doses, sizes and surface properties in situ in real time at single-NP resolution with both spatial and temporal resolutions.
Noble metal nanoparticles (e.g., Ag NPs) display distinctive optical, electronic and therapeutic properties, and have been increasingly used in consumer products, ranging from socks, home appliances, water treatment, to disinfection.4,9 Their potential release into aquatic environments would create direct impacts on human health, due in part to the inability of effectively removing them from drinking water, and human consumption of fish. Fish are renowned for their ability to bioconcentrate trace contaminants from the environment. Thus, it is crucial to study the potential toxic effects of the NPs on such aquatic organisms.
Zebrafish (Danio rerio) can be an effective aquatic model organism to monitor potential release of nanomaterials into ecosystems (e.g., rivers, ocean). Notably, zebrafish embryos are a superior in vivo model organism over other model organisms (e.g., mouse, rat, human).19–21 For example, zebrafish embryos complete their early development rapidly (within 120 h) and each developmental stage is well defined. A massive amount of embryos can be produced over night at very low cost. The embryos are transparent, which enables direct visualization of pathological and mal-development phenotypes and direct study of transport and effects of NPs on embryonic development in vivo in real time. Zebrafish share similar genetic phenotypes and drug binding sites with humans, and they have been used as in vivo model organisms to screen efficacies of therapeutic agents and toxicities of conventional chemicals.22,23 However, zebrafish embryos have not yet been widely used as standard in vivo assays to systematically study transport and toxicity of nanomaterials.16–18
Even though several studies have reported the observation of effects of Ag NPs on embryonic development, systematic characterization of embryonic toxicity of a library of well-design nanomaterials has not yet been carried out to validate the effectiveness of the embryos as in vivo assays. Many studies did not characterize physicochemical properties of individual NPs in vivo in situ in real time.24–26 As described above, physicochemical properties (e.g., sizes, shapes, and surface properties) of individual Ag NPs are not identical and they can alter as they are incubated with living organisms (e.g., zebrafish embryos). Thus, it is insufficient for one to characterize the physicochemical properties of the NPs at the initial point of the experiment. In fact, it is critical to develop and utilize single-NP detection (SND) to monitor and characterize sizes of individual Ag NPs in vivo in situ in real time, in order to quantitatively study dose- and size-dependent nanotoxicity.
In our previous studies, we used the cleavage-stage embryos to study small spherical Ag NPs (11.6 ± 3.5 nm) and Au NPs (11.6 ± 0.9 nm), and characterized chemical dependent biocompatibility and toxicity of stable and purified NPs in vivo in real time at single-NP resolution.16–18 The NPs exhibit distinctive plasmonic optical properties and their localized surface plasmonic resonance (LSPR) spectra depend highly upon their sizes, shapes, dielectric constants, and surrounding environments.27–33 We have demonstrated that single Ag NPs as small as 2 nm can be imaged and characterized using DFOMS with a halogen lamp as an illuminator.7,8,34,35 We have found that single Ag NPs resist photobleaching and photoblinking and they can serve as photostable optical probes to image single molecules on/in single live cells for any desired period of time in real time at nanometer (nm) spatial and millisecond (ms) temporal resolutions.5,7,8,18,34,35
In this study, we synthesized and purified larger Ag NPs with average diameters of 95.4 ± 16.0 nm (57–133 nm) and used single nanoparticle optical rulers to characterize the sizes of single NPs in vivo in real time to study their transportation and effects on embryonic development. We compared the results from this study with those from our previous studies of transport and effects of smaller Ag (11.6 ± 3.5 nm) and Au NPs (11.6 ± 0.9 nm) on embryonic development,16,17 aiming to better understand size-dependent transport and toxicity of Ag NPs, and to determine the effectiveness of the embryos as ultrasensitive in vivo assays for characterization of nanotoxicity.
Experimental
Reagents and supplies
All chemicals (except noted) were purchased from Sigma-Aldrich, and used as received. Deionized (DI) water (18 MΩ water, Barnstead) was used to prepare solutions and rinse glassware.Synthesis and characterization of stable and purified Ag NPs (95.4 ± 16.0 nm)
We synthesized Ag NPs (95.4 ± 16.0 nm) as we described previously.31,32 Briefly, sodium citrate (20 mL, 34 mM in DI water) was rapidly added into AgNO3 (500 mL, 1.06 mM in DI water) under stirring and refluxing. The mixture was continuously refluxed and stirred for 95 min, while the colors of the mixture turned from colorless to straw yellow, then opaque yellow, and finally muddy yellow. We then turned off the heating mantle and continued refluxing and stirring the solution until it was cooled to room temperature.The NP solution was immediately filtered using 0.22 μm filters and washed three times with DI water using centrifugation (Beckman, JA-20) to remove any possible residual chemicals from NP synthesis to prepare purified Ag NPs. The supernatants of the NP solution after the third wash were collected and used for control experiments to study potential effects of any residual chemicals on embryonic developments.
The purified NPs in the pellet were resuspended in egg water (1.0 mM NaCl, embryonic medium) and characterized over time for 120 h using high-resolution transmission electron microscopy (HR-TEM) (JEOL, JEM-2100 F), dynamic light scattering (DLS) (Nicomp 380 ZLS particle sizing system), UV-vis spectroscopy (Hitachi U2010), and DFOMS (Fig. 1 and 2). The concentrations of NPs suspended in egg water were calculated and determined using the approaches as we described previously.16,36
We have fully described the designs and applications of DFOMS for real-time imaging and spectroscopic characterization of single NPs in solutions, single live cells and embryos, and for single molecule detection (SMD).7,8,34,35 In this study, a dark-field microscope coupled with a CCD camera (EMCCD or Coolsnap HQ2) and a spectrograph (SpectraPro-150, Roper Scientific) or Multispectral Imaging System (MSIS, N-MSI-VIS-FLEX, CRi, Hopkiton, MA) are used to image and characterize LSPR spectra of single Ag NPs. The conventional spectrograph acquires the spectra of single NPs one at a time. In contrast, the MSIS is an integrated system of a CCD camera (SonyICX 285) and liquid-crystal-tunable-filter (LCTF).6,37–39 DFOMS-MSIS can simultaneously acquire dark-field plasmonic optical images and spectra of massive amounts of single NPs with spectral resolution of 1 nm, and enable high-throughput spectral analysis and characterization of single NPs. The dark-field microscope is equipped with a dark-field condenser (oil, 1.43–1.20), a microscope illuminator (Halogen lamp, 100 W), and a 100× objective (Plan fluor 100×, N.A. 0.5–1.3, oil).
We determined the potential release of Ag+ from the NPs over time by measuring Ag+ concentrations in the supernatant of the NPs using atomic absorption spectroscopy (AAS, Hitachi, Z-1800), as the NPs were incubated in egg water for 120 h. We collected the supernatants from the NP solution over time using centrifugation. We also characterized the potential degradation of Ag NPs in egg water and developing embryos over time for 120 h by measuring LSPR spectra of single Ag NPs in situ in real time using DFOMS-MSIS.
Breeding of zebrafish embryos
We housed wild-type adult zebrafish (Aquatic Ecosystems) in a stand-alone system (Aquatic Habitats), and maintained and bred them, as we described previously.16–18 Briefly, we placed two pairs of mature zebrafish in a clean 10 gallon breeding tank, and used a light (14 h)–dark (10 h) cycle to trigger breeding and fertilization of their embryos. We collected cleavage-stage embryos (0.75–2.25 hours-post-fertilization, hpf), transferred each of them into a Petri-dish and thoroughly rinsed them with egg water, prior to study of the transport of single Ag NPs into single embryos and characterization of their effects on embryonic development. All experimental procedures involving embryos and zebrafish were performed in compliance with the Old Dominion University’s IACUC guidelines.Real-time imaging of diffusion of single Ag NPs into/in embryos
We placed the cleavage-stage embryos in self-made microchambers containing egg water and the Ag NPs (1 pM) and tracked the diffusion of single Ag NPs into the embryos and inside embryos using DFOMS-MSIS (Fig. 3–5).31 Single Ag NPs were distinguished from embryonic debris and tissues using their distinctive plasmonic optical properties (colors). Note that embryonic debris and tissues appear white under dark-field illumination. LSPR spectra of single Ag NPs acquired by DFOMS-MSIS were used to determine their sizes using optical nano rulers, as we described previously.16–18Quantitative study of dose-dependent toxicity of the Ag NPs (95.4 ± 16.0 nm)
The cleavage-stage embryos were incubated with various concentrations of the Ag NPs (0, 2 × 10−4, 2 × 10−3, 1.0, and 2.0 pM) or (0, 5.7 × 10−4, 5.7 × 10−3, 2.8, 5.7 μg mL−1), and the supernatants, in 24-well plates at 28.6 °C for 120 h (Fig. 6 and 7). The supernatants were collected from the third-washed NPs that had been incubated with DI water, egg water or the embryos for 120 h. Each well in the plates contains egg water (2 mL) with four embryos and a given concentration of the NPs or the supernatants. The developing embryos in each well were imaged at representative embryonic developmental stages (2, 10, 24, 48, 72, 96, and 120 hpf) using an inverted microscope (Zeiss Axiovert 100) equipped with a CCD camera (CoolSnap EZ, Roper Scientific).Study of the embryos in egg water alone and in the supernatants (absence of the NPs) serve as control experiments to determine potential effects of residual chemicals (sodium citrate, silver nitrate, related by-products) from NP synthesis or potential release of Ag+ or degradation of Ag NPs, on embryonic development.
Quantitative imaging of single Ag NPs embedded in individual zebrafish
The cleavage-stage embryos were incubated with given concentrations of the Ag NPs continuously (chronically) for 120 h, and developed to normal or deformed zebrafish. We rinsed them with DI water to remove any external NPs, fixed them using a tissue processor (Microm STP-120 Spin, Thermo Fisher Scientific), and sectioned them to prepare ultrathin-layer tissue samples (1–2 μm thickness) using a microtome (HM 360 rotary microtome, Thermo Fisher Scientific), as we described previously.17 Finally, the number and sizes of individual NPs embedded in the tissues of interest were quantitatively determined using their size-dependent LSPR spectra acquired by DFOMS-MSIS (Fig. 8 and 9). A minimum of three slices of the tissues of each normal and deformed given organ (e.g., eye, brain) of each normal and deformed zebrafish were analyzed for each measurement. A minimum of 15 representative normal and deformed zebrafish were characterized.Data analysis and statistics
For characterization of sizes, shapes, LSPR spectra, and stability (non-aggregation) of single Ag NPs, 300 individual NPs were studied for each sample with a minimum of 100 NPs for each measurement. For real-time imaging of transport and diffusion mechanisms of single NPs into and in embryos over time, a minimum of 15 embryos were studied for each given concentration with 5 embryos per measurement. For study of dose-dependent effects of the NPs on embryonic development, a total number of 36 embryos with 9 replicates of 4 embryos per measurement were studied for each NP concentration and each control experiment.Using conventional statistical analysis methods (t-test and ANOVA), we found significant dose-dependent nanotoxicity (differences of the embryos that developed to normal or deformed zebrafish, or became dead), as they were incubated with various concentrations of Ag NPs (0–2 pM), with the confidence level of 90% (or P = 0.10) for t-test and 95% (or P = 0.05) for ANOVA.
In this study, we characterized the effects of Ag NPs on embryonic development at single-embryo and single-NP resolution. These approaches enable us to observe the rare events of interest, which otherwise would be buried under ensemble measurements. Thus, conventional statistical analysis is irrelevant in this study. Representative and distinctive observations, especially rare deformed zebrafish, were illustrated in Fig. 7 and summarized in Table S1 (ESI‡).
Results and discussion
Synthesis and characterization of the Ag NPs (95.4 ± 16.0 nm)
We synthesized and purified the Ag NPs as described in the Experimental section. The TEM image of the purified Ag NPs and their size distribution (Fig. 1A and B) show polygon-shaped NPs with average sizes of 95.4 ± 16.0 nm. The dark-field optical image of single Ag NPs (Fig. 1C) shows that the majority of single NPs exhibit plasmonic green color with some being yellow and red. LSPR spectra of single NPs (Fig. 1D) show peak wavelengths (λmax) with full-width-of-half-maximum (FWHM), λmax (FWHM), at 558 (78), 610 (89), and 704 (91) nm.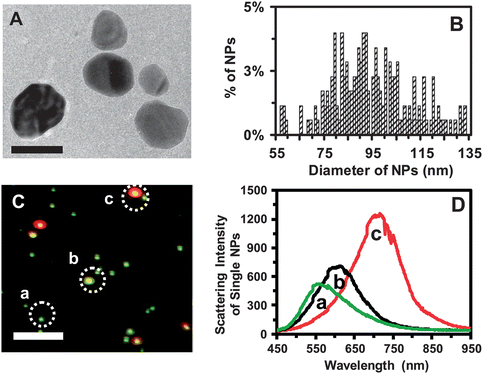 | ||
| Fig. 1 Characterization of sizes, shapes, and plasmonic optical properties of single Ag NPs. (A) HRTEM image shows the sizes, shapes and morphologies of single Ag NPs. (B) Histogram of size distribution of single Ag NPs determined by HRTEM shows their average sizes of 95.4 ± 16.0 nm. (C) Representative dark-field optical image of single Ag NPs shows individual plasmonic green, yellow and red NPs. (D) LSPR spectra of single Ag NPs (plasmonic green, yellow, and red) show λmax (FWHM) at 558 (78), 610 (89) and 704 (91) nm, respectively. Scale bars are 100 nm in (A) and 2 μm in (C). The scale bar in (C) shows the distances among single NPs, but not their sizes, owing to the optical diffraction limit. | ||
We determined the sizes of single Ag NPs using their LSPR spectra, by correlating the distribution (%) of their LSPR spectra measured by DFOMS-MSIS with their sizes determined by HRTEM, using the calibration approaches as we reported previously.31,32 For this NP solution, the NPs with sizes of 74–95 nm show plasmonic green color with λmax of LSPR spectra of 544–560 nm, and the NPs with 96–120 nm display plasmonic yellow and red colors with λmax of LSPR spectra of 562–728 nm. The size-dependent LSPR spectra of single NPs enable their sizes to be determined in situ in real time at the nanometer scale using DFOMS-MSIS.
Notably, plasmonic properties (LSPR spectra) of single Ag NPs depend highly upon their sizes, shapes and surrounding environments (e.g., medium).27–29,31,33 None of the single NPs has the exactly identical sizes, shapes, morphologies, and surrounding environments (e.g., surface adsorbates). Thus, individual Ag NPs with slightly different sizes, morphologies, or surface adsorbates can exhibit distinctive LSPR spectra. That is why we observed different plasmonic colors from the similar sizes of single Ag NPs in different solutions, and the same plasmonic colors of distinctive sizes of single Ag NPs in different environments.7,8,11,16–18,31,32,35 Thus, it is essential to calibrate the sizes and shapes of single Ag NPs with their LSPR spectra for each NP solution.
We characterized the stability (non-aggregation) of the purified Ag NPs suspended in egg water over 120 h at single-NP resolution using DFOMS-MSIS. The number of single NPs in 60 images acquired by DFOMS at each time point over 120 h remains essentially unchanged with around 133 NPs per image (Fig. 2A), suggesting that the NPs in egg water are very stable. If NPs were aggregated, the number of NPs in solution would have decreased and their sizes would have increased over time, which would have made the study of dose- and size-dependent nanotoxicity unreliable and irrelevant. Thus, it is crucial to study the stability of the NPs and their nanotoxicity in situ in real time at single-NP resolution. In this study, DFOMS-MSIS serves as a high-throughput tool to characterize the number and sizes of single NPs in situ and in real time.
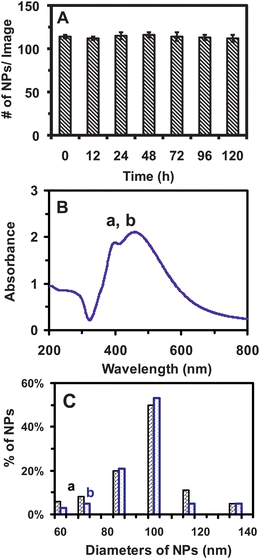 | ||
| Fig. 2 Characterization of stability (non-aggregation) of Ag NPs in egg water (1.0 mM NaCl). (A) The average numbers of single Ag NPs per image acquired by DFOMS, for Ag NPs (2 pM) incubated with egg water at 28 °C for 0, 12, 24, 48, 72, 96, and 120 h, are 133 ± 2, 130 ± 2, 135 ± 3, 136 ± 4, 135 ± 3, 139 ± 4, and 130 ± 3, respectively. In total, 60 images are acquired with 20 images per measurement at each time point, enabling sufficient statistics that represent bulk analysis of the NPs at single-NP resolution. Each image represents an effective detection volume (area) to measure the number of single NPs in egg water over time for 120 h. (B) UV-Vis absorption spectra of Ag NPs (2 pM) incubated with egg water at 28 °C for (a) 0 and (b) 120 h show that the peak absorbance of 2.1 at 459 nm (FWHM = 100 nm) and the absorbance of 1.9 at a shoulder peak of 392 nm (FWHM = 76 nm) remain essentially unchanged, and the NPs are stable in egg water for 120 h. (C) Histograms of size distributions of Ag NPs incubated with egg water at 28 °C for (a) 0 and (b) 120 h measured using DLS show that their average diameters are (95.4 ± 16.4) and (97.5 ± 15.5) nm, respectively. | ||
We further characterized the stability of NPs using ensemble measurements, UV-vis spectroscopy and DLS. UV-vis spectra of the NPs in egg water over 120 h show that their peak absorbance of 2.1 at 459 nm (FWHM = 100 nm) and absorbance of 1.9 at a shoulder peak of 392 nm (FWHM = 76 nm) remain unchanged (Fig. 2B). Size distributions of NPs incubated in egg water over 120 h, measured by DLS (Fig. 2C), show that their average diameters (95.4 ± 16.4 and 97.5 ± 15.5 nm) remain essentially unchanged. The sizes of hydrated NPs became slightly larger after being suspended in egg water for 120 h, similar to those we observed previously.16–18 Unlike SND, DLS is unable to determine the sizes of single NPs, but the ensemble average of size distribution of a population of the NPs. Small portions of any given sized NPs in the population are often buried under the ensemble averages and smaller NPs may not be detected due to the weaker scattering intensity than the larger NPs.
Taken together, the NPs are very stable (non-aggregation) in egg water over 120 h (embryonic developmental duration), enabling us to investigate their size- and dose-dependent transport into embryos and their effects on embryonic development over time.
Analysis and characterization of molar concentrations of purified Ag NPs and their supernatants
We use molar concentrations of the Ag NPs to study dose-dependent transport and nanotoxicity. The molar concentrations of single Ag NPs (but not atoms or ions) are calculated and characterized, as we described previously.16,17,36 Briefly, we calculate the weight of Ag (WAg) generated by the complete reduction of AgNO3 by multiplying the moles of added AgNO3 with the atomic weight of Ag (107.87 g mol−1), because the Ag+ cations are completely reduced to Ag due to the presence of an excess amount of reducing agent (sodium citrate) during the synthesis. We calculate the volume of generated Ag (VAg) by dividing WAg by the density of Ag (d = 10.5 g cm−3), as VAg = WAg/d, and compute the number of the Ag NPs, diameter (Dia) = 95.4 ± 16.0 nm, by dividing the VAg with the volume of one Ag NP (πDia3/6). We then determine the moles of Ag NPs by dividing the number of NPs with Avogadro constant (6.02 × 1023), and the molar concentrations of NPs by dividing their moles by the solution volume. The results show that the NP concentration in 520 mL is 0.15 nM.We acquire UV-vis absorption spectra of a series of unwashed Ag NP solutions, and plot their baseline-subtracted absorbance (0, 0.049, 0.102, 0.223, 0.454, and 0.972) versus molar concentrations of the NPs (0, 0.6, 1.2, 2.4, 4.9, and 10.2 pM). The plot shows a linear calibration curve with a linear regression of 1.0 and slope of (9.4 ± 0.6) × 1010 M−1. Using the Beer–Lambert law (A = εbC), we determine that the molar absorptivity (extinction coefficient) of unwashed Ag NPs at λmax of 459 nm is (9.4 ± 0.6) × 1010 M−1 cm−1.
We wash the NPs to remove any residual chemicals from solution using centrifugation. The NPs are well resuspended in DI water to produce the first-time washed NP solution. UV-vis absorption spectra of the first-time washed Ag NP solution show that their λmax remain unchanged, indicating that the sizes of Ag NPs remain unchanged during the centrifugation, which is confirmed by HRTEM and DLS. Using the same approaches, we determine the molar absorptivity of the first-washed Ag NP solution, and prepare and characterize the second- and third-washed Ag NPs using UV-vis spectroscopy, HRTEM and DLS. The results show that λmax of the spectra and sizes of the NPs remain unchanged with the molar absorptivity of the third-washed colloid Ag NPs of (3.3 ± 0.4) × 1011 M−1 cm−1.
We use DFOMS to image and detect trace amounts of individual Ag NPs in the supernatants collected from the third-washed NPs. If we observed any Ag NPs in the supernatant, we would further remove them from the supernatant using ultra-centrifugation (L90, Beckman). We collect the last-washed supernatants with any potential residual byproducts and chemicals except Ag NPs and use them to treat the embryos, which serves as a blank control experiment. Note that any potential residual chemicals in the supernatant should also be present in the NPs. Thus, if we observe the effects of NPs on embryonic development, but not the supernatant, we can conclude that the effects are attributed to the NPs, but not to residual chemicals resulting from NP synthesis.
Typically, the supernatants from thrice-washed NPs can provide successful control experiments (no effects on embryonic development as those embryos observed in egg water). Thus, the thrice-washed NPs are sufficiently pure and can be used to study their effects on embryonic development. This approach ensures that we study the effects of the Ag NPs on embryonic development, but not ions or other chemicals potentially presented in the NPs.
The number of NPs (molar concentration) is directly proportional to the weight and surface area of the NPs. Therefore, the molar concentration of NPs accurately represents the size, number, and surface properties of the NPs. Thereby, the dose-dependent effects of NPs on embryonic development in molar concentrations represent the dependence of nanotoxicity on the sizes, number and surface properties of NPs, and accurately reflect dose-dependent nanotoxicity. Note that individual NPs (like different molecules) are independent entities and have their own NP (molecular) weights and physicochemical properties. Weight of different sizes of the Ag NPs cannot be appropriately described by the same atomic weight of Ag. Thus, the concentration of the NPs cannot be accurately expressed by the weight/volume (w/v) of the Ag atom, as widely used in nanotoxicity studies. Furthermore, the NP concentration in w/v cannot accurately express the surface properties and doses of the NPs. For instance, the different sizes of NPs with the same w/v have different numbers of the NPs, and thereby different surface area and charges. Thus, if one uses w/v to express the doses of NPs, it cannot accurately reflect the dependence of nanotoxicity on the number and surface properties of the NPs.
Real-time imaging of transport mechanisms of single Ag NPs into/in embryos
To determine whether the Ag NPs as large as 95.4 ± 16.0 nm (57–133 nm) can enter embryos and their related transport mechanisms, we incubate the NPs with cleavage-stage embryos and directly track diffusion of single NPs into and in embryos in real time using DFOMS. The image of the single live embryo in Fig. 3A illustrates the chorionic layer (CL), chorionic space (CS) and inner mass of embryo (IME). The snap-shots of sequential optical images (Fig. 3B–D) show that single NPs diffuse from egg water into the CS through chorionic pores (chorionic pore canals, CPCs) on the CLs; single NPs diffuse in the CS, and from the CS into the IME, respectively. Arrays of chorionic pores on the CLs are observed and their diameters (0.5–0.7 μm) are determined in vivo in real time using DFOMS (Fig. 3B), which agree well with those measured previously by DFOMS,16–18 and SEM (scanning electron microscopy).40 We clearly visualize single embryonic cells and directly observe single NPs diffusing into embryonic cells on the surface of IME in vivo in real time using DFOMS (Fig. 3D).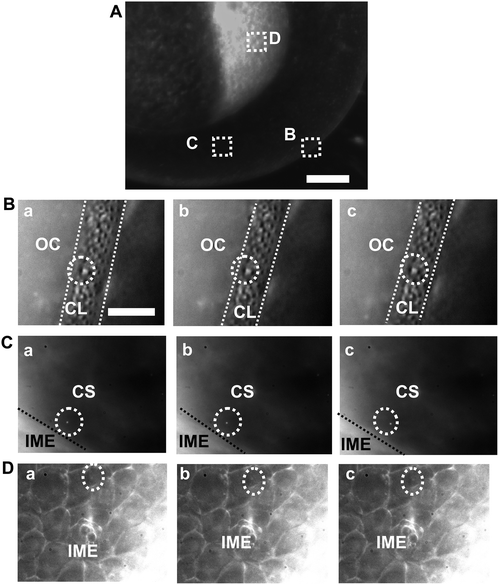 | ||
| Fig. 3 Real-time probing of transport and diffusion of single Ag NPs into/in embryos. (A) Optical image of cleavage-stage embryo shows: (B) the chorionic layer (CL), (C) chorionic space (CS), and (D) inner mass of embryos (IME) as squared, where the transports of single Ag NPs are studied in real time. (B–D) Sequential dark-field optical images of: (B) the CL (as highlighted by dashed lines), (C) the CS (the interface of CS with IME are marked by dashed lines), and (D) the IME, showing the diffusion of single Ag NPs (as circled) from outside the chorion (OC) in egg water into the CL; in the CS; and from the CS into the IME, respectively. The time interval between each sequential image (temporal resolution) is 1.1 s. Scale bars are 125 μm in (A) and 20 μm in (B–D). | ||
Single Ag NPs exhibit distinctive plasmonic colors and they can be effectively distinguished from embryonic cells, membranes and debris which appear white under dark-field illumination. Sizes of single NPs are determined in vivo in real time by their size-dependent LSPR spectra using optical nano rulers as we described previously.8,31
The shortest exposure time (the highest speed) and monochrome (black/white) mode of the CCD camera are used to rapidly track diffusion of the single NPs in real time (Fig. 3). LSPR spectra of the single NPs are acquired using color-mode of the CCD camera (CCD-MSIS) (Fig. 4) at time points of interest between each set of sequential optical images acquired by its monochrome mode (Fig. 3), to determine the sizes of NPs. Note that monochrome mode of the CCD camera can directly collect the photons scattered by single NPs without passing through the optical filters (or grating). In contrast, photons have to pass through an LCTF (or be dispersed by a grating) to generate the spectra (colors) of individual NPs (sorting out the photons at any given wavelength). Thus, the monochrome-mode CCD is more sensitive and can acquire sequential optical images with sufficient signal-to-noise-ratio more rapidly than its color mode. Thus, we use the monochrome mode to acquire real-time sequential images (videos) of diffusion of single Ag NPs (Fig. 3), and utilize the color modes to acquire LSPR spectra of single Ag NPs (Fig. 4). The spectra of single Ag NPs enable them to be distinguished from embryonic cells, tissues and debris (Fig. 4), and allow us to determine their sizes using optical nano rulers as we described previously.8,31
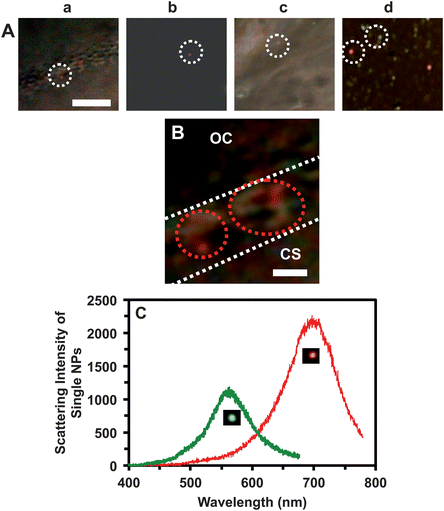 | ||
| Fig. 4 Real-time characterization of sizes and mechanisms of single Ag NPs diffusing into/in cleavage-stage embryos using their plasmonic optical properties determined by DFOMS-MSIS. (A) Snap-shot color images of those sequential optical images in Fig. 3B–D show that individual plasmonic Ag NPs diffuse: (a) through the CL, (b) in the CS, (c) in the CS into the IME, and (d) in the IME. (B) Zoom-in color image of (a) shows that the Ag NPs are trapped in chorionic pores, and some of them are aggregated in the pores. (C) LSPR spectra of the single Ag NPs as those circled in (A) show λmax (FWHM) at 557 (65) and 698 (88) nm. Scale bars in (A–B) are 20 and 2 μm, respectively. | ||
The results in Fig. 3 show that single Ag NPs as large as 95.4 ± 16.0 nm can diffuse through the chorionic pores into the CS, and into the IME. The majority of the Ag NPs pass through the chorionic pores without clogging the pores. However, a few of the single Ag NPs, that are trapped inside the chorionic pores for a longer period of time (minutes), aggregate with the Ag NPs that diffuse through the same pores to generate larger Ag NPs (Fig. 4B). The aggregation causes the red-shift and broader LSPR spectra of single Ag NPs with higher scattering intensity, and clogs the chorionic pores and their transportation, which might have led to adverse effects of the Ag NPs on embryonic development.
We further determine the diffusion mechanisms and modes (e.g., random Brownian motion, directed or restricted diffusion) of single Ag NPs as the NPs diffuse into/in the embryos using diffusion theories. We track the diffusion trajectories of single Ag NPs in real time (Fig. 5a). Note that viscosities inside live embryos vary widely upon local embryonic environments, which alter over time as embryos develop. Therefore, we use real-time squared displacement (RTSD, diffusion distance at each time interval) instead of mean-squared displacement (MSD, average distance over time), to determine the diffusion modes and diffusion coefficient of single NPs, as they diffuse into and through embryos.
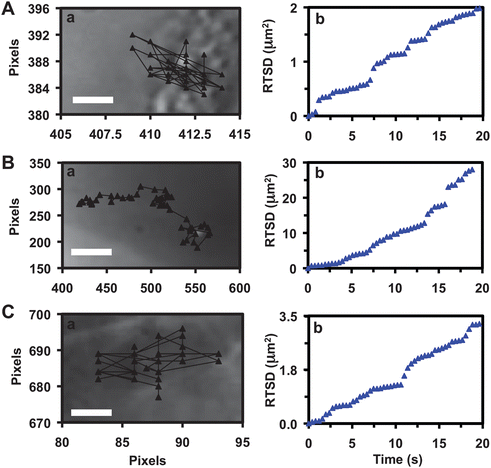 | ||
| Fig. 5 Characterization of transport mechanisms and diffusion modes of single Ag NPs into/in the embryos. Plots of (a) diffusion trajectories and (b) real-time squared displacement (RTSD) as a function of time of single plasmonic green NPs: (A) as they enter the extra-surface of CL, shows restricted diffusion with diffusion coefficients of (3.9 ± 7.5) × 10−11 cm2 s−1; (B) in CS near the surface of IME and (C) in IME, both shows simple Brownian motion with diffusion coefficients of (7.5 ± 6.0) × 10−9 and (7.8 ± 8.2) × 10−10 cm2 s−1, respectively. Scale bars in (A–C) are 0.1, 3 and 0.3 μm, respectively. | ||
Plots of RTSD of single NPs versus time (Fig. 5b) show stepwise linearity, as single NPs diffuse through chorionic pores, in the CS, and into the IME. As described in diffusion theories,41–43 the linear plots of MSD versus time indicate simple random Brownian motion and the steps show restricted diffusion. Thus, the results in Fig. 5b indicate that passive diffusion of single NPs (but not directed diffusion) enable them to enter embryos. The plot in Fig. 5Ab shows several steps, indicating that individual NPs are frequently trapped in chorionic pores as they diffuse from egg water into the embryos. Similar phenomena are observed as the NPs diffuse from the CS into the IME, showing their dense interfaces.
We determine diffusion coefficients (D) of single NPs by dividing the slopes of the linear portion of the plots (Fig. 5b) by four using two-dimensional (2D) random walk theory (RTSD = 4DΔt).44 They are (3.9 ± 7.5) × 10−11, (7.5 ± 6.0) × 10−9 and (7.8 ± 8.2) × 10−10 cm2 s−1 for single NPs diffusing through the CL into the CS, in the CS, and into the IME, respectively. Diffusion coefficients of the single NPs in egg water measured using the same approaches are (2.8 ± 0.5) × 10−8 cm2 s−1. The results show highly heterogeneous embryonic environments and higher viscosity of the IME and the CL than the CS. Diffusion coefficients of single NPs are inversely proportional to viscosities of the medium, as described by the Stokes–Einstein equation, D = kT/(6πηa), where k is the Boltzmann constant; T is the temperature; a is the radius of single NPs; and η is the viscosity of the medium where NPs diffuse in.44 Thus, the results show that embryonic environments are orders of magnitude more viscous than egg water.
In our previous studies, we observed high viscosity gradients of embryonic environments in CS.16,18 In this study, the large standard deviations of diffusion coefficients of single Ag NPs in embryos also show high heterogeneities (viscosity gradients) of embryonic environments. The high heterogeneities and rapid changes of embryonic environments in fast developing live embryos make the comparison of diffusion coefficients of various sized NPs impossible. Thus, we measure the diffusion of the similar sizes (colors) of single Ag NPs in the same embryos simultaneously, in order to effectively probe viscosity gradients of the embryos in vivo in real time. The results further demonstrate that single Ag NPs can serve as effective optical probes to study embryonic nanoenvironments.
Embryos as ultrasensitive and high-throughput in vivo assays
Optical images of zebrafish embryos (Fig. 6A) show well-defined developmental stages at cleavage (0.75–2.25 hpf), late segmentation (24 hpf), early hatching (48 hpf), and late hatching stages (72 hpf), and a fully developed zebrafish larvae (120 hpf). The images show that the embryos are transparent. A massive amount (thousands) of embryos can be generated rapidly at very low cost; they can be characterized simultaneously; and each embryo completes its full development within 120 h. In contrast, embryos of other in vivo model organisms (e.g., mouse, rat) develop in the wombs, and take months to fully develop. Thus, it would have taken months and years to characterize thousands of such embryos. Therefore, unlike other in vivo model organisms, zebrafish embryos can serve as high-throughput in vivo assays to screen biocompatibility and toxicity of nanomaterials.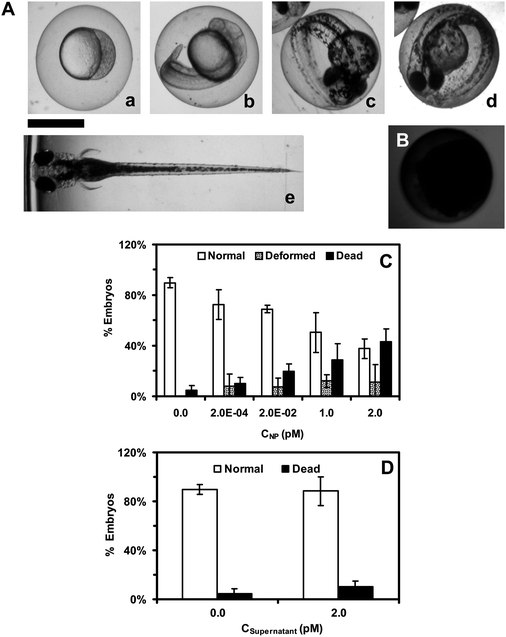 | ||
| Fig. 6 Study of toxic effects of Ag NPs (95.4 ± 16.0 nm) on embryonic development. (A) Optical images of normally developing embryos at (a) cleavage-stage (0.75–2.25 hpf); (b) late segmentation stage (24 hpf); (c) early hatching stage (48 hpf); (d) late hatching stage (72 hpf); (e) fully developed larvae (120 hpf). (B) Dead embryo. (C) Histograms of distributions of embryos that developed to normal or deformed zebrafish or became dead versus NP concentration. (D) Control experiments: histograms of the distributions of embryos that develop to normal zebrafish or become dead either in egg water alone or versus supernatant concentration. A total of 36 embryos are studied for each NP concentration and control in (C) and (D). The means and standard deviations (error bars) at each given concentration from each three replicates are presented. Scale bar is 500 μm for all images in (A) and (B). | ||
The earlier developing embryos (cleavage-stage embryos) can be highly susceptible to toxic effects of external substances (e.g., drugs, NPs) because they are the foundations for the later developmental stages. Therefore, they can serve as more sensitive assays to screen the biocompatibility and toxicity of nanomaterials than later-stage embryos or fully developed fish. Notably, cleavage-stage embryos undergo drastic changes to formulate the development of different organs, and their related developmental mechanisms remain unknown.19–21 Thus, we choose cleavage-stage embryos as ultrasensitive in vivo assays to study transport and toxicity of the NPs.
Study of dose-dependent biocompatibility and toxicity of the Ag NPs
To determine the dose-dependent toxicity of the NPs, we incubate cleavage-stage embryos with various concentrations (0–2.0 pM or 0–5.7 μg mL−1) of purified and stable Ag NPs (95.4 ± 16.0 nm) in egg water, and image the embryos in vivo in real time for 120 h as they develop to the full larvae. This experimental design enables the NPs to passively diffuse into embryos without external intervention and the study of their effects on embryonic development in situ in real time. These approaches mimic the potential transport and effects of the NPs on aquatic and eco-living organisms should the NPs be released into the environments. To determine effects of any potential residual chemicals from NPs synthesis and potential release of Ag+ from the NPs over time, we conduct two types of control experiments simultaneously by incubating the embryos with egg water alone and with the highest concentration of the supernatants collected from the third-washed NPs that have been incubated with egg water and the embryos for 0–120 h.The embryos at representative development stages (Fig. 6A) are imaged and monitored over time for 120 h until they are fully developed. We characterize normal zebrafish (Fig. 6A), dead embryos (Fig. 6B), and deformed zebrafish (Fig. 7A), based upon their morphologies and phenotypes. The number of embryos that develop to normal and deformed zebrafish and become dead is determined and plotted against molar concentrations of NPs and supernatants. The results in Fig. 6C show that embryonic development depends highly upon the NP concentration (dose). As the NP concentration increases from 0 to 2.0 pM, the number of embryos that develop to normal zebrafish decreases, while the number of embryos that develop to deformed zebrafish and become dead increases.
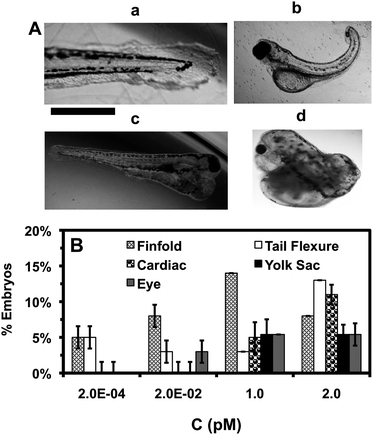 | ||
| Fig. 7 Study of dependence of types of zebrafish deformities upon NP concentration. (A) Representative optical images of deformed zebrafish show: (a–c) finfold abnormalities; (b) tail/spinal cord flexure; (c and d) cardiac malformation and yolk sac edema; and (d) eye abnormality. Scale bar = 500 μm. (B) Histograms of distributions of the embryos that develop to multiple types of deformed zebrafish show their high dependence upon NP concentration. As NP concentration increases, the number of embryos with severe deformities and various types of deformities increase. A total of 36 embryos are studied for each NP concentration. The means and standard deviations (error bars) at each given concentration from each three replicates are presented. The percentages of embryos are calculated by dividing the number of embryos developed into deformed zebrafish at each given concentration with the total number of embryos developed to deformed zebrafish at all concentrations. | ||
We examine individual morphological defects (phenotypes) of deformed zebrafish and find various types of deformation, which include abnormal finfold and tail/spinal cord flexture, cardiac malformation, yolk sac edema, and eye deformities (Fig. 7A). The number of embryos that develop to deformed zebrafish with high severity and multiple deformities increases as the NP concentration increases (Fig. 7B; Table S1 in ESI‡), showing dose-dependent phenotypes.
Finfold deformities (e.g., disorganized and improperly arranged finfolds) and abnormal tail and spinal cord flexture (Fig. 7A:ab) are observed in all studied concentrations. Note that normal zebrafish shows well organized finfold rays and straight tails (spinal cord) (Fig. 6A). Interestingly, severe deformations, such as cardiac malformations (e.g., edema of the pericardial sac region and cardiac arrhythmia) and yolk sac edema with swollen and enlarged yolk sac (Fig. 7Ac) are observed only at higher NP concentrations (≥1 pM). Deformed zebrafish with cardiac abnormalities also frequently show swollen and enlarged yolk sac (yolk sac edema). In normal zebrafish (Fig. 6A), the yolk sac (a bulbous area containing yolk that provides nutrients to embryonic development) is much smaller than the deformed zebrafish, because they should shrink during the later stages of normal embryonic development.45–47 Notably, severe eye abnormalities and head edema are observed, starting at very low NP concentrations (≥0.02 pM) and the number of deformed zebrafish with eye abnormalities increases as the NP concentration increases. Such eye abnormalities are rarely observed for the embryos that are exposed to toxic heavy ions (e.g., Cd2+) or smaller Ag NPs (11.6 ± 3.5 nm) even at its high concentrations.16,48
Strikingly, individual zebrafish with one type of severe deformation (e.g., cardiac, eye or head) is typically accompanied with several other types of deformations (e.g., finfold, tail and yolk sac edema) (Fig. 7Ad). Such interesting phenomena suggest that embryonic developmental pathways may be highly regulated and one pathway may depend highly upon others. Thus, one type of deformity may lead to multiple deformations in the same individual zebrafish.
We perform two control experiments simultaneously by incubating the embryos with egg water alone (blank control) and with the highest concentration of supernatants collected from the last-washed Ag NPs, which have been thoroughly washed and incubated with egg water and embryos for 0–120 h. We use the supernatants to determine potential toxic effects of trace chemicals (e.g., Ag+) resulting from the synthesis or degradation of the NPs over time, which serve as control experiments to validate that the observed toxicity (Fig. 6 and 7) is attributed to the NPs, but not other chemicals. The results (Fig. 6D) show that over 95% of embryos develop to normal zebrafish, and none of the embryos develop to deformed zebrafish, which indicates that Ag NPs do not release a sufficient amount of silver cation (Ag+) to affect the embryonic development.
If the Ag NPs released any sufficient amount of Ag+ that caused significant effects on embryonic development, we would have observed them in the control experiment (as embryos were treated by the supernatants over 120 h). Furthermore, we would have observed the blue-shift of LSPR spectra of single Ag NPs, owing to their shrinking sizes, should they release Ag+. We have detected the change of single molecules on the surface of single Ag NPs using their LSPR spectra acquired by our DFOMS-MSIS with 1 nm spectral resolution.6,10,38 We also use AAS to analyze the Ag+ in the supernatants from the NPs that have been incubated with egg water for 120 h. The result shows that the trace amount of Ag+ of less than 0.024 ppt (parts per trillion) in the supernatant remains unchanged over the 120 h incubation and indicates no release of Ag+ from the NPs. Taken together, the deformed zebrafish and high percent of dead embryos observed in Fig. 6 and 7 are attributed to the Ag NPs, but not any potential contaminated chemicals or release of Ag+.
High-throughput quantitative imaging and analysis of single Ag NPs embedded in tissues of zebrafish
To address why some embryos develop normally while others become deformed or dead, we further determine distributions of the NPs in the tissues of developed zebrafish. The cleavage-stage embryos are incubated with the Ag NPs (≤2 pM) continuously (chronically) for 120 h to develop to normal or deformed zebrafish. We then rinse and fix the zebrafish and prepare the ultrathin sections (1–2 μm thickness) of their tissue samples as described in the Experimental section. Individual NPs embedded in various tissues of normal and deformed zebrafish are characterized using DFOMS-MSIS. The results (Fig. 8) show that the NPs are embedded in both normal and deformed zebrafish and the NPs are well distributed in the organs (e.g., eyes, brains and hearts) of zebrafish, which indicates that the NPs diffuse into embryos and stay inside the embryos throughout their developmental stages (120 h).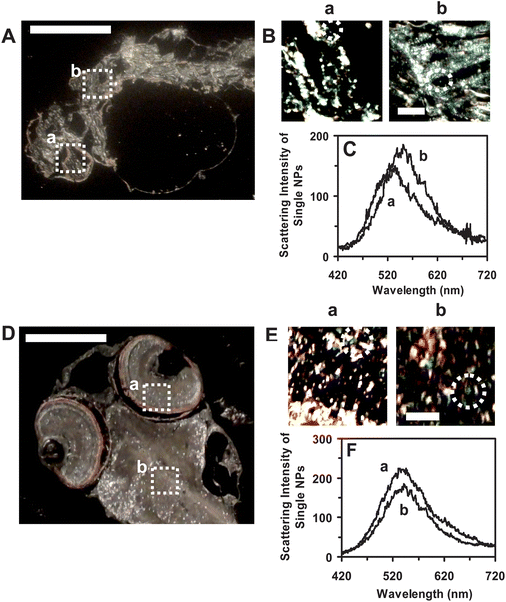 | ||
| Fig. 8 Quantitative imaging and characterization of individual Ag NPs embedded in the tissues of (A–C) deformed zebrafish and (D–F) normal zebrafish using DFOMS-MSIS. Optical image of ultrathin longitudinal section of fixed (A) deformed zebrafish with five types of deformities; and (D) normal zebrafish. (a) Eye and (b) brain tissues are squared in (A) and (D). (B) and (E): zoom-in optical images of the tissue sections of (a) and (b) as highlighted in (A) and (D) show single Ag NPs embedded in the tissues, respectively. (C) and (F): LSPR spectra of the individual Ag NPs circled in (B) and (E) show distinctive λmax (FWHM) of (C): (a) 556 (97); (b) 549 (104) nm; and (F): (a) 552 (89); (b) 554 (109), respectively. The scale bars are 500 μm in (A) and (D) and 50 μm in (B) and (E). | ||
We quantitatively analyze the number and sizes of individual Ag NPs embedded in the tissues using their distinctive plasmonic optical properties (colors), and size-dependent LSPR spectra (Fig. 8C and F). Unlike a conventional spectrograph that acquires LSPR spectra of single NPs one at a time, DFOMS-MSIS permits us to simultaneously acquire LSPR spectra of a massive number of single NPs, which enables us to achieve high-throughput quantitative analysis of individual Ag NPs embedded in the tissues of interest with both spatial and temporal resolutions.
Plots of the number of given plasmonic colors (sizes) of single NPs in normal and deformed zebrafish show that more NPs are embedded in eye and brain tissues of the deformed zebrafish than those of the normal zebrafish (Fig. 9). The result shows dose-dependent toxic effects of the NPs on embryonic development at single-NP resolution. The larger number of NPs accumulated inside the embryos throughout their development may lead to their deformation and death. Nonetheless, individual embryos might have various degrees of tolerance to toxic effects of the Ag NPs. Embryos with the high degree of tolerance could survive and develop to normal zebrafish, while those with the low degree of tolerance could develop to deformed zebrafish or become dead. Such individuality phenomena could not be detected should ensemble measurements were utilized. Therefore, this study further underscores the importance of study of effects of the NPs on embryonic development at single-NP and single-embryo resolution.
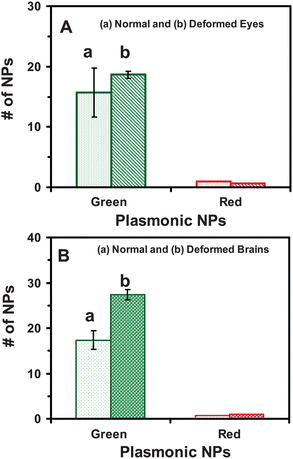 | ||
| Fig. 9 Quantitative analysis of number and sizes of the individual Ag NPs embedded in tissues of the deformed and normal zebrafish. Histograms of the number and sizes (colors) of single Ag NPs embedded in: (A) eye and (B) brain tissues of (a) normal and (b) deformed zebrafish. Tissues of 15 deformed and normal zebrafish each are analyzed. The minimum of 3 slices of each type of the tissues are characterized for each analysis. Each image serves as an effective detection volume (area) for quantitative analysis of the number of NPs embedded in the tissues. The means and standard deviations (error bars) of each given type (plasmonic green and red) of the NPs from each three replicated measurements are presented. | ||
New findings distinguished from our previous studies
All findings from this study are distinctive and cannot be extrapolated from any previous studies. Although several other studies have reported the size-dependent nanotoxicity of the Ag NPs,24,25 these reported studies did not use purified and stable Ag NPs and thus cannot conclude whether those observations are attributed to the sizes of the NPs or residual chemicals in the NPs. Doses and sizes of the NPs were neither characterized at single-NP resolution nor in situ in real time during nanotoxicity studies. Thus, the in situ doses and sizes of the Ag NPs used in these reported studies are entirely unknown, which makes their findings unreliable.24,25 Note that, in order to accurately determine the sizes and doses of Ag NPs, one must characterize them at single-NP resolution and in situ in real time, due to the high heterogeneity, activity and instability (aggregation) of the Ag NPs.In our previous studies,16,17 we demonstrated that the smaller purified Ag NPs (11.6 ± 3.5 nm) and Au NPs (11.6 ± 0.9 nm) were stable (non-aggregated) in egg water over the entire embryonic development (120 h). We found that these smaller NPs passively diffused into developing embryos via chorionic pores. The small Ag NPs show dose-dependent toxic effects on embryonic development, while the Au NPs are much more biocompatible with embryonic development. These previous studies effectively show the chemical-dependent nanotoxicity, because the identical experimental conditions (except different chemical compositions of NPs) are used.
These previous results neither predict nor conclude the effects of larger Ag NPs (95.4 ± 16.0 nm) on the embryonic development. It is entirely unknown whether such larger NPs can be stable in egg water, whether they can enter the embryos and whether they are more biocompatible or toxic than the smaller NPs. In fact, it remains unknown whether the biocompatibility and toxicity of the nanomaterials (e.g., Ag NPs) are size-dependent, and whether the larger Ag NPs are more toxic than smaller Ag NPs.
In this study, we have synthesized and purified larger Ag NPs (95.4 ± 16.0 nm) with nearly the same shape as those smaller Ag NPs used in our previous studies.16 We found that these larger Ag NPs are stable (non-aggregated) in egg water over the entire duration of embryonic development (120 h). We have further developed our single-NP imaging microscopy and spectroscopy by coupling DFOMS with MSIS, which enables high-throughput quantitative analysis of the number and sizes of single Ag NPs in vivo in situ in real time and in embedded tissues of interest. With these distinct capabilities, we found that such large Ag NPs still can passively diffuse into embryos (IME) via their chorionic pores, and they stay inside the embryos throughout the embryonic development (120 h). This study shows the dose-dependent toxic effects of these larger Ag NPs on the embryonic development at single-NP resolution.
In comparison with our previous study of the smaller Ag NPs (11.6 ± 3.5 nm) (0–0.71 nM),16 we observe more various types of abnormalities with much more severe deformities in this study (Fig. 6 and 7; Table S1‡). The large Ag NPs (95.4 ± 16.0 nm) exhibit much higher toxicity toward embryonic development than the small Ag NPs (11.6 ± 3.5 nm). For example, more than half of the embryos become dead when they are exposed to the large Ag NPs (95.4 ± 16.0 nm) with the concentration (2 pM) that is four orders of magnitude lower than small Ag NPs for 120 h. More than 20% of the embryos become dead and another 20% of embryos develop into severely deformed zebrafish as they are exposed to the lower concentration of the NPs (0.2 pM, 95.4 ± 16.0 nm) for 120 h. In contrast, all embryos (>85%, the same amount as the control experiment) survive and develop into normal zebrafish, when they are exposed to the same concentration of the small Ag NPs (11.6 ± 3.5 nm) over 120 h. None of the embryos develop into deformed zebrafish as they are exposed to the small Ag NPs with the concentration as high as 30 pM (≤30 pM).
Taken together, these important findings offer reliable new evidences and insights into the size-dependent nanotoxicity at single-NP resolution. We have demonstrated the high-throughput quantitative analysis of individual Ag NPs embedded in the tissues using DFOMS-MSIS, and the importance of study of transport and toxicity of nanomaterials at single-NP resolution in situ in real time. This study shows that LSPR spectra of single NPs can be used to study size-dependent nanotoxicity. One can now use similar approaches, such as single-NP fluorescence or Raman spectroscopy, to study the transport and toxicity of nanomaterials with given optical and spectral properties.
Summary
In summary, we have synthesized, purified and characterized the Ag NPs (95.4 ± 16.0 nm) that are stable (non-aggregated) in egg water (medium of embryos; 1.0 mM NaCl) over the entire embryonic development (120 h). We have developed single-NP plasmonic microscopy and spectroscopy (DFOMS-MSIS) for quantitative analysis of doses and sizes of individual Ag NPs in situ in real time throughout their incubation with the embryos for 120 h. The results show that individual NPs passively diffuse into the cleavage-stage embryos through chorionic pores, and stay inside the embryos throughout the entire embryonic development (120 h), demonstrating that they can potentially concentrate trace contaminants (e.g., NPs) from aquatic systems. The majority of the NPs pass through the chorionic pores, while a few Ag NPs are trapped inside the pores and are aggregated with other Ag NPs, leading to clogging of the pores and hindering their transportation. We observe dose-dependent toxic effects of the NPs on the embryonic development, and that higher doses of the NPs cause higher levels of toxic effects on embryonic development. As the NP concentrations increase, the number of embryos that developed into normal zebrafish decrease, while the number of embryos that become dead and develop to deformed zebrafish increase. The NPs are found in the normal and deformed zebrafish with a larger number of the NPs in the tissues of the deformed zebrafish than those of the normal zebrafish. In comparison with our previous studies of the effects of smaller Ag NPs (11.6 ± 3.5 nm) on embryonic development, we find size-dependent toxic effects of Ag NPs on embryonic development and larger Ag NPs are more toxic than smaller Ag NPs. Taken together, our studies show that single-NP microscopy and spectroscopy can effectively characterize the transport and toxicity of the NPs in vivo in situ in real time, and zebrafish embryos can serve as ultrasensitive in vivo assays to rapidly screen biocompatibility and toxicity of the NPs. These new findings and tools are invaluable for the future study of nanotoxicity and rational design of biocompatible nanomaterials.Acknowledgements
This work is supported in part by NSF (NIRT: CBET 0507036) and NIH (R01 GM0764401). K.J.L., P.D.N. and L.M.B. are grateful for the support of NSF-GRAS (CBET 1042533), Dominion Scholar Fellowship and NIH-GRAS (R01 3GM0764401-S), respectively.References
- R. Misra, S. Acharya and S. K. Sahoo, Drug Discovery Today, 2010, 15, 842–850 CrossRef CAS.
- M. K. Teli, S. Mutalik and G. K. Rajanikant, Curr. Pharm. Des., 2010, 16, 1882–1892 CrossRef CAS.
- X.-H. N. Xu and R. P. Patel, in Handbook of Nanostructured Biomaterials and their Applications in Nanobiotechnology, ed. H. S. Nalwa, American Scientific Publishers, Los Angeles, CA, 2005, vol. 1, pp. 435–456 Search PubMed.
- M. Ahamed, M. S. Alsalhi and M. K. Siddiqui, Clin. Chim. Acta, 2010, 411, 1841–1848 CrossRef CAS.
- T. Huang, P. D. Nallathamby, D. Gillet and X.-H. N. Xu, Anal. Chem., 2007, 79, 7708–7718 CrossRef CAS.
- T. Huang and X.-H. N. Xu, Nanoscale, 2011, 3, 3567–3572 RSC.
- X.-H. N. Xu, J. Chen, R. B. Jeffers and S. V. Kyriacou, Nano Lett., 2002, 2, 175–182 CrossRef CAS.
- P. D. Nallathamby, K. J. Lee, T. Desai and X.-H. N. Xu, Biochemistry, 2010, 49, 5942–5953 CrossRef CAS.
- K. Chaloupka, Y. Malam and A. M. Seifalian, Trends Biotechnol., 2010, 28, 580–588 CrossRef CAS.
- T. Huang, P. D. Nallathamby and X.-H. N. Xu, J. Am. Chem. Soc., 2008, 130, 17095–17105 CrossRef CAS.
- P. D. Nallathamby and X.-H. N. Xu, Nanoscale, 2010, 2, 942–952 RSC.
- A. J. Haes and R. P. Van Duyne, J. Am. Chem. Soc., 2002, 124, 10596–10604 CrossRef CAS.
- J. Fabrega, S. R. Fawcett, J. C. Renshaw and J. R. Lead, Environ. Sci. Technol., 2009, 43, 7285–7290 CrossRef CAS.
- J. M. Hillegass, A. Shukla, S. A. Lathrop, M. B. MacPherson, N. K. Fukagawa and B. T. Mossman, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2010, 2, 219–231 CrossRef CAS.
- C. Buzea, I. Pacheco, II and K. Robbie, Biointerphases, 2007, 2, MR17–MR71 CrossRef.
- K. J. Lee, P. D. Nallathamby, L. M. Browning, C. J. Osgood and X.-H. N. Xu, ACS Nano, 2007, 1, 133–143 CrossRef CAS.
- L. M. Browning, K. J. Lee, T. Huang, P. D. Nallathamby, J. Lowman and X.-H. N. Xu, Nanoscale, 2009, 1, 138–152 RSC.
- P. D. Nallathamby, K. J. Lee and X.-H. N. Xu, ACS Nano, 2008, 2, 1371–1380 CrossRef CAS.
- J. den Hertog, Biosci. Rep., 2005, 25, 289–297 CrossRef.
- A. J. Hill, H. Teraoka, W. Heideman and R. E. Peterson, Toxicol. Sci., 2005, 86, 6–19 CrossRef CAS.
- P. Kahn, Science, 1994, 264, 904–905 CAS.
- H. Teraoka, W. Dong and T. Hiraga, Congenital Anomalies, 2003, 43, 123–132 CrossRef CAS.
- L. I. Zon and R. T. Peterson, Nat. Rev. Drug Discovery, 2005, 4, 35–44 CrossRef CAS.
- P. V. AshaRani, G. L. Mun, M. P. Hande and S. Valiyaveettil, ACS Nano, 2009, 3, 279–290 CrossRef CAS.
- O. Bar-Ilan, R. M. Albrecht, V. E. Fako and D. Y. Furgeson, Small, 2009, 5, 1897–1910 CrossRef CAS.
- B. J. Shaw and R. D. Handy, Environ. Int., 2011, 37, 1083–1097 CrossRef CAS.
- C. F. Bohren and D. R. Huffman, in Absorption and Scattering of Light by Small Particles, Wiley, New York, 1983, pp. 287–380 Search PubMed.
- K. L. Kelly, E. Coronado, L. L. Zhao and G. C. Schatz, J. Phys. Chem. B, 2003, 107, 668–677 CrossRef CAS.
- U. Kreibig and M. Vollmer, in Optical Properties of Metal Clusters, Springer, Berlin, 1995, pp. 14–123 Search PubMed.
- G. Mie, Ann. Phys., 1908, 25, 377–445 CrossRef CAS.
- P. D. Nallathamby, T. Huang and X.-H. N. Xu, Nanoscale, 2010, 2, 1715–1722 RSC.
- T. Huang and X.-H. N. Xu, J. Mater. Chem., 2010, 20, 9867–9876 RSC.
- P. Mulvaney, Langmuir, 1996, 12, 788–800 CrossRef CAS.
- K. J. Lee, L. M. Browning, T. Huang, F. Ding, P. D. Nallathamby and X.-H. N. Xu, Anal. Bioanal. Chem., 2010, 397, 3317–3328 CrossRef CAS.
- X.-H. N. Xu, W. J. Brownlow, S. V. Kyriacou, Q. Wan and J. J. Viola, Biochemistry, 2004, 43, 10400–10413 CrossRef CAS.
- X.-H. N. Xu, S. Huang, W. Brownlow, K. Salatia and R. Jeffers, J. Phys. Chem. B, 2004, 108, 15543–15551 CrossRef CAS.
- S. C. Gebhart, R. C. Thompson and A. Mahadevan-Jansen, Appl. Opt., 2007, 46, 1896–1910 CrossRef.
- T. Huang, L. M. Browning and X.-H. N. Xu, Nanoscale, 2012, 4, 2797–2812 RSC.
- T. Huang, W. Cai, H. E. Elsayed-Ali and X. H. N. Xu, Nanoscale, 2012, 4, 380–385 RSC.
- D. M. Rawson, T. Zhang, D. Kalicharan and W. L. Jongebloed, Aquacult. Res., 2000, 31, 325–336 CrossRef.
- A. Kusumi and Y. Sako, J. Cell Biol., 1994, 125, 1251–1264 CrossRef.
- A. Kusumi, Y. Sako and M. Yamamoto, Biophys. J., 1993, 65, 2021–2040 CrossRef CAS.
- H. Qian, M. P. Sheetz and E. L. Elson, Biophys. J., 1991, 60, 910–921 CrossRef CAS.
- I. Tinoco, K. Sauer, J. Wang and J. D. Puglisi, in Physical Chemistry-Principles and Applications in Biological Sciences, Prentice Hall, New Jersey, 2002, pp. 274–290 Search PubMed.
- Z. Gong and V. Korzh, in Fish Development and Genetics: The Zebrafish and Medaka Models, World Scientific Publishing Co., Singapore, 2004, pp. 87–424 Search PubMed.
- Y. W. Kunz, Developmental Biology of Teleost Fishes, Springer, Netherlands, 2004, pp. 267–428 Search PubMed.
- W. S. Hoar and D. J. Randall, Fish Physiology: The Physiology of Developing Fish, Part A, Eggs and Larvae, Academic Press, New York, 1988, pp. 1–48 Search PubMed.
- A. V. Hallare, M. Schirlinga, T. Luckenbacha, H.-R. Köhler and R. Triebskorn, J. Therm. Biol., 2005, 30, 7–17 CrossRef CAS.
Footnotes |
| † This article is part of a themed issue highlighting the targeted study of single units, such as molecules, cells, organelles and pores – The “Single” Issue, guest edited by Henry White. |
| ‡ Electronic supplementary information (ESI) available: Summary of dose-dependent nanotoxicity of the Ag NPs (95.4 ± 16.0 nm). See DOI: 10.1039/c2an35293a |
| This journal is © The Royal Society of Chemistry 2012 |
