Design of a dual-output fluorescent DNA logic gate and detection of silver ions and cysteine based on graphene oxide†
Wan Yi
Xie
,
Wei Tao
Huang
,
Nian Bing
Li
* and
Hong Qun
Luo
*
Key Laboratory on Luminescence and Real-Time Analysis, Ministry of Education, School of Chemistry and Chemical Engineering, Southwest University, Chongqing 400715, P. R. China. E-mail: linb@swu.edu.cn; luohq@swu.edu.cn; Fax: +86-23-68253237; Tel: +86-23-68253237
First published on 8th November 2011
Abstract
In the presence of graphene oxide, upon formation of cytosine–Ag–cytosine the fluorescence wavelength of FAM-labeled DNA exhibited a red shift, and its intensity significantly increased. A novel fluorescent DNA sensor for Ag+ and cysteine detection, and a dual-output fluorescent DNA INHIBIT logic gate are designed.
Silver ions have been shown to be highly toxic to aquatic life,1 thus the detection in aqueous solution is important to environmental monitoring and biomedical science. Over the past few decades, it was found that some metal ions including Ag+ ions can selectively bind to a few native or artificial bases in DNA duplexes, in this way “metal-base pairs” are created. Due to the special selectivity, a number of Ag+ sensors have been studied, such as G-quadruplex-hemin DNAzyme based colorimetric sensors2 and oligonucleotide-based fluorescent probes.3 In order to increase the signal-to-noise ratio of DNA probes, highly efficient nanoquenchers, such as Au nanoparticles (AuNPs) and single-walled carbon nanotubes (SWNTs), replaced the traditional organic fluorophores and quenchers.4Graphene is recently predicted through theoretical calculations to be a super quencher with a long-range resonance energy transfer (LrRET) property.5 Here, we present a rapid assay for highly sensitive and selective detection of Ag+, based on graphene oxide and silver-specific oligonucleotides. Graphene oxide (GO) was synthesized by a modified Hummers method,6 and tapping-mode atomic force microscope (AFM) studies revealed that the thickness of GO was of ∼1 nm, characteristic of a fully exfoliated GO sheet, and the chemical structure of GO was characterized by X-ray photoelectron spectroscopy (XPS). (Fig. S1, ESI†). Scheme 1 shows a schematic representation of this new nanoswitch design. We employed two separate C-rich ssDNA (DNA1: 5′-CTC TCT CTC TCT CTC TCT CTC-FAM-3′, DNA2: 5′-CAC ACA CAC ACA CAC ACA CAC-3′) as silver–specific oligonucleotides. In the absence of Ag+, the ssDNA is in a flexible single strand, which could readily form stable GO/DNA complexes in aqueous solution by means of stacking interactions between nucleotide bases and GO.7 Therefore, GO adsorbs the unbound ssDNA and quenches the fluorescence of FAM (fluorescein derivative)-labeled DNA1. In the presence of Ag+, Ag+ may bind between the N3 nitrogen atoms of cytosine, resulting in the C–C mismatched base pairs forming C–Ag+–C base pairs, and we found that not only the FAM fluorescence intensity (FI) was significantly increased, but also the fluorescence emission wavelength (λem) of FAM exhibited a red shift. Cysteine (Cys), a sulfur-containing amino acid, plays a crucial role in the human body.8 It is a potential neurotoxin,9 a biomarker for various medical conditions,10 and a disease-associated physiological regulator.11 The determination of Cys is important and of general interest. Cys is a strong Ag+-binder and can be used to unfold the C–Ag+–C structures, resulting in the change of the double-stranded DNA to two ssDNA, which are adsorbed on GO and the fluorescence is quenched and the fluorescence emission wavelength is gradually restored. As expected, a new detection of Ag+ ions and Cys has been successfully constructed. In addition, a new GO-based Ag+/Cys-driven dual-output fluorescent DNA INHIBIT logic gate has also been designed.
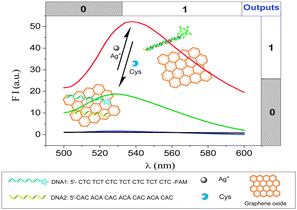 | ||
| Scheme 1 Schematic representation of Ag+ and Cys detection based on graphene oxide and silver-specific oligonucleotides. | ||
The sensing conditions for this part of the experiment, including GO concentration, the addition order of DNA1, DNA2, Ag+, and GO of this system, and reaction time for fluorescence quenching and restoration were all investigated and optimized (Fig. S2, ESI†). GO can significantly reduce the background fluorescence of FAM-labeled DNA. As shown in Fig. S3 (ESI†), the influence of GO on the detection was evaluated by analyzing the change of fluorescence intensity, ΔFI (a.u.), defined as ΔFI = |FI − FI0|. The change in the fluorescence intensity of DNA1/DNA2 solution caused by the addition of Ag+ is about 26 (a.u.) in the absence of GO; however, in the presence of GO was about 51; fluorescence enhancement is thus observed. Furthermore, when Cys was added to the two solutions above, the change in the FI is about 21 in the presence of GO, whereas it is only about 4 in the absence of GO. This comparison clearly demonstrates that the GO greatly improved the sensitivity for the detection of both Ag+ and Cys.
In addition, as shown in Fig. S3A (ESI†), in the presence of GO, λem exhibits a red shift with the addition of Ag+. However, the addition of Cys makes the fluorescence emission peak shift back to shorter wavelength. Similarly, in the absence of GO (Fig. S3B, ESI†), with the addition of Ag+ the λem exhibits a red shift, whereas the addition of Cys can restore it. These data suggest that the red shift is not due to GO but rather due to the interaction between Ag+ and FAM-labeled DNA. In order to understand whether the interaction between Ag+ and 6-FAM causes the red shift, the fluorescence spectrum of fluorescein was investigated on addition of AgNO3 (Fig. S4, ESI†). However, there were no obvious change either in fluorescence intensity or emission wavelength. Thus the interaction between Ag+ and 6-FAM can be ruled out. These results suggest that Ag+ ion binds directly to N3 of cytosine residues to form the C–Ag+–C base pairs, and the binding of Ag+ may change the electronic properties of the base pairs, and the selective electronic interactions among the base pairs, leading to the observed red-shift.12
To study the feasibility of the Ag+ sensing system, FIapex and λem of the sensor as a function of Ag+ concentration was measured. The effect of different concentrations of Ag+ on FIapex and λem of DNA1/DNA2 in the presence of GO is shown in Fig. 1A. As the concentration of Ag+ was increased, the resulting FIapex of FAM was intensified. A linear relationship (R2 = 0.9900) was observed with Ag+ concentrations from 20 to 1500 nM (Fig. 1B) and the detection limit for Ag+ was 14 nM (based on 3σ/slope). In addition, from the results shown in Fig. 1C, A linear relationship (R2 = 0.9834) of the Ag+ concentration with λem was observed in the concentration range of 60–1000 nM. We also examined the selectivity of the Ag+ sensing system, and the fluorescence response to other common metal ions were investigated at a concentration of 10 μM (Fig. S5A and B, ESI†). It is clear that only Ag+ gives obvious fluorescence intensity and wavelength change, while other tested metal ions do not.
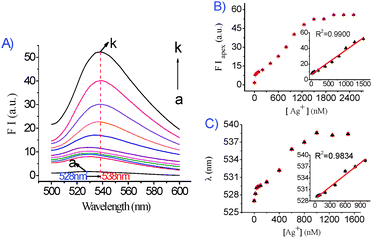 | ||
| Fig. 1 (A) Effect of different concentrations of Ag+ on the fluorescence spectra of the DNA1/DNA2/Ag+/GO system. DNA1/DNA2: 50 nM/50 nM; GO: 0.037 mg mL−1. Ag+ (from a–k, nM): 0, 20, 40, 60, 100, 200, 400, 600, 800, 1000, 1500. (B) Fluorescence intensity at the fluorescence emission peak plotted against the concentration of Ag+. The inset is the linear relationship between the fluorescence intensity recorded at the apex with an excitation wavelength of 480 nm and Ag+ concentration in the range 0–1500 nM. (C) The fluorescence emission wavelength plotted against the concentration of Ag+. The inset is the linear relationship of the Ag+ concentration to the fluorescence emission wavelength. Fluorescence intensity was recorded at the apex with an excitation wavelength of 480 nm. | ||
Another sensing application of the DNA1/DNA2/Ag+/GO platform was to detect Cys, based on the competition between C–Ag+–C base pairs and Cys for binding to Ag+. As indicated in Fig. 2A, the FIapex and the λem of the DNA1/DNA2/Ag+/GO system are sensitive to Cys and decrease as the concentration of Cys increases. A linear relationship (R2 = 0.9836) (Fig. 2B) was observed in the Cys concentration range of 60–500 nM. This sensor has a detection limit of 44 nM for Cys. In this experiment, the fluorescence emission wavelength was gradually restored with the addition of Cys, and a linear relationship (R2 = 0.9787) was obtained when the Cys concentration ranged from 100 to 500 nM (Fig. 2C). To determine the selectivity of the Cys-sensing system, the fluorescence response to the other 19 essential amino acids was investigated at a concentration of 5 μM. It is clear that only Cys can significantly decrease the fluorescence intensity (Fig. S6A, ESI†) and restore the fluorescence emission wavelength (Fig. S6B, ESI†). This result suggests that the C–C mismatch site binds Ag+ strongly enough to effectively compete against all tested amino acids other than Cys, which contributes to excellent selectivity over other amino acids for this DNA1/DNA2/Ag+/GO based sensing platform. We further investigated the effect of glutathione (GSH) and homocysteine (Hcy), on the DNA1/DNA2/Ag+/GO system. As shown in Fig. S7 (ESI†), it is clear that the responses of GSH and Hcy are similar to that of Cys.
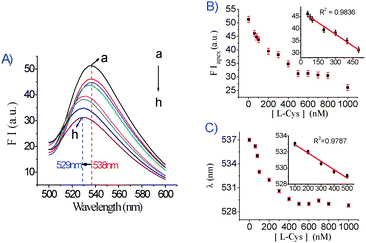 | ||
| Fig. 2 (A) Fluorescence emission spectra of DNA1/DNA2/Ag+/GO (DNA1/DNA2: 50 nM/50 nM Ag+: 1.5 μM/GO: 0.037 mg mL−1 , λex = 480 nm) in the absence and presence of different concentrations of Cys (nM): (a) 0, (b) 60, (c) 80, (d) 100, (e) 200, (f) 300, (g) 400, (h) 500. (B) The fluorescence intensity at the fluorescence emission peak plotted against the concentration of Cys. The inset is the linear relationship between the fluorescence intensity and the Cys concentration. Fluorescence intensity was recorded at the apex with an excitation wavelength of 480 nm. (C) The fluorescence emission wavelength was plotted against the concentration of Cys. The inset is the linear relationship between the fluorescence emission wavelength and the Cys concentration. Fluorescence intensity was recorded at the apex with an excitation wavelength of 480 nm. | ||
The Ag+ and Cys-sensing method has several potential advantages: (1) GO acted as an excellent “nanoquencher” for the fluorophore. (2) The simultaneous changes in the fluorescence intensity and the emission wavelength for detection of Ag+ and Cys provide advantages of high sensitivity and selectivity with convenience. In view of these advantages, our study provides a new strategy for potential application for monitoring Ag+ in environmental samples. The determination results of Ag+ in tap water and river water samples are given in Table S1 (see ESI†).
For a new generation of molecular logic gates, researchers have focused on DNA, due to the well-regulated structures and the ability to store genetic information13 and a range of diverse DNA logic gates have been designed in recent years.14 The Ag+/Cys–mediated fluorescence changes in the sensing system enabled the design of a DNA INHIBIT logic gate (Fig. 3A). Ag+ and Cys were defined as the two inputs for the logic gate; FIapex and the wavelength of the λem were defined as two outputs. The four possible input combinations were (0, 0), (1, 0), (0, 1) and (1, 1) shown in the truth table (Fig. 3B). For input, the presence of Ag+ or Cys was defined as 1 and the absence as 0. FIapex and λem here serve as the outputs (1 or 0) with thresholds. As regards the output, FIapex, we assigned logic 1 to the situation when the fluorescence intensity was corresponding to the input (1, 0), and logic 0 to the fluorescence intensity 0.5-fold lower than that of (1, 0) (Fig. 3C). Simultaneously, we designed another logic gate in parallel using the wavelength shift (Fig. 3D). If the red shift of the emission wavelength was larger than 5 nm relative to 528 nm, the output was defined as logic 1, and the intervening shifts (from 0 to 5 nm) were invalid, which were logic 0. Some common cations and other amino acids have no obvious influence on the outputs. This is a new concept for performance of DNA logic gates and it is the first example of a GO-based Ag+/Cys-driven dual-output fluorescent DNA INHIBIT logic gate.
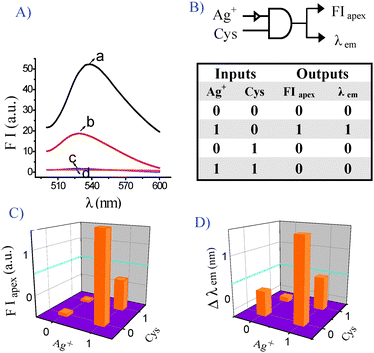 | ||
| Fig. 3 Operation of Ag+/Cys-driven DNA as a two-input and two-output INHIBIT logic gate. (A) Fluorescence spectra of the treatment with Ag+ and Cys after the addition of GO (0.037 mg mL−1) and DNA1/DNA2 (50 nM/50 nM): (a) 1.5 μM Ag+, FIapex = 52.27, λem = 538.2 nm; (b) 1.5 μM Ag+ + 1.5 μM Cys, FIapex = 18.75, λem = 528 nm; (c) no input, FIapex = 1.67, λem = 526.2 nm; (d) 1.5 μM Cys, FIapex = 1.14, λem = 525 nm. (B) Truth table for the two-input and two-output INHIBIT logic gate; (C) Fluorescence intensity at FIapex in the form of a bar representation; (D) Fluorescence wavelength in the form of a bar representation. | ||
We gratefully acknowledge the National Natural Science Foundation of China (No. 20975083) and the Municipal Science Foundation of Chongqing City (No. CSTC-2008BB 4013) and the 211 Project of Southwest University (the Third Term).
Notes and references
- (a) T. P. Morgan and C. M. Wood, Environ. Toxicol. Chem., 2004, 23, 1261–1267 CrossRef CAS; (b) C. Hogstrand and C. M. Wood, Environ. Toxicol. Chem., 1998, 17, 547–561 CrossRef CAS.
- (a) T. Li, L. L. Shi, E. K. Wang and S. J. Dong, Chem.–Eur. J., 2009, 15, 3347–3350 CrossRef CAS; (b) X. H. Zhou, D. M. Kong and H. X. Shen, Anal. Chem., 2010, 82, 789–793 CrossRef CAS; (c) X. H. Zhou, D. M. Kong and H. X. Shen, Anal. Chim. Acta, 2010, 678, 124–127 CrossRef CAS.
- (a) A. Ono, S. Q. Cao, H. Togashi, M. Tashiro, T. Fujimoto, T. Machinami, S. Oda, Y. Miyake, I. Okamoto and Y. Tanaka, Chem. Commun., 2008, 4825–4827 RSC; (b) Y. H. Lina and W. L. Tseng, Chem. Commun., 2009, 6619–6621 RSC; (c) Y. Q. Wen, F. F. Xing, S. J. He, S. P. Song, L. H. Wang, Y. T. Long, D. Li and C. H. Fan, Chem. Commun., 2010, 46, 2596–2598 RSC; (d) C. Zhao, K. G. Qu, Y. J. Song, C. Xu, J. S. Ren and X. G. Qu, Chem.–Eur. J., 2010, 16, 8147–8154 CAS.
- (a) H. Wang, Y. X. Wang, J. Y. Jin and R. H. Yang, Anal. Chem., 2008, 80, 9021–9028 CrossRef CAS; (b) J. Ling and C. Z. Huang, Anal. Methods, 2010, 2, 1439–1447 RSC; (c) L. B. Zhang, T. Li, B. L. Li, J. Li and E. K. Wang, Chem. Commun., 2010, 46, 1476–1478 RSC.
- (a) R. S. Swathi and K. L. Sebastian, J. Chem. Phys., 2008, 129, 054703 CrossRef CAS; (b) R. S. Swathi and K. L. Sebastian, J. Chem. Phys., 2009, 130, 086101 CrossRef CAS; (c) R. S. Swathi and K. L. Sebastian, J. Chem. Sci., 2009, 121, 777–787 CrossRef CAS.
- W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- (a) C. H. Lu, H. H. Yang, C. L. Zhu, X. Chen and G. N. Chen, Angew. Chem., Int. Ed., 2009, 48, 4785–4787 CrossRef CAS; (b) S. J. He, B. Song; D. Li, C. F. Zhu, W. P. Qi, Y. Q. Wen, L. H. Wang, S. P. Song, H. P. Fang and C. H. Fan, Adv. Funct. Mater., 2010, 20, 453–459 CrossRef CAS.
- L. C. Gruen, Biochim. Biophys. Acta, 1975, 386, 270–274 CAS.
- X. F. Wang and M. S. Cynader, J. Neurosci., 2001, 21, 3322–3331 CAS.
- M. T. Goodman, K. McDuffie, B. Hernandez, L. R. Wilkens and J. Selhub, Cancer, 2000, 89, 376–382 CrossRef CAS.
- W. Droge and E. Holm, FASEB J., 1997, 11, 1077–1089 CAS.
- (a) S. S. Tan, Y. N. Teo and E. T. Kool, Org. Lett., 2010, 12, 4820–4823 CrossRef CAS; (b) S. S. Tan, S. J. Kim and E. T. Kool, J. Am. Chem. Soc., 2011, 133, 2664–2671 CrossRef CAS.
- L. M. Adleman, Science, 1994, 266, 1021–1024 CAS.
- (a) J. H. Guo, D. M. Kong and H. X. Shen, Biosens. Bioelectron., 2010, 26, 327–332 CrossRef CAS; (b) A. Saghatelian, N. H. Volcker, K. M. Guckian, V. S. Lin and M. Z. Ghadiri, J. Am. Chem. Soc., 2003, 125, 346–347 CrossRef CAS; (c) T. Li, E. K. Wang and S. J. Dong, J. Am. Chem. Soc., 2009, 131, 15082–15083 CrossRef CAS; (d) X. M. Li, L. Sun and T. R. Ding, Biosens. Bioelectron., 2011, 26, 3570–3576 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and other data. See DOI: 10.1039/c1cc15449d |
| This journal is © The Royal Society of Chemistry 2012 |
