 Open Access Article
Open Access ArticleSynthesis of chiral ionic liquids by ion cross-metathesis: en route to enantioselective water–ionic liquid extraction (EWILE), an eco-friendly variant of the ELLE process†‡
Viacheslav
Zgonnik
,
Chantal
Zedde
,
Yves
Génisson
,
Marie-Rose
Mazières
and
Jean-Christophe
Plaquevent
*
Laboratoire de Synthèse et Physicochimie de Molécules d'Intérêt Biologique UMR-CNRS 5068, Université Paul Sabatier, 118 route de Narbonne, 31062, Toulouse, France. E-mail: plaquevent@chimie.ups-tlse.fr; Fax: 00 33(0)561556011; Tel: 00 33(0)561556511
First published on 2nd February 2012
Abstract
From two initial ILs two other ILs are obtained by simultaneous ion exchange. The hydrophobic ions unite in the hydrophobic layer, whereas the hydrophilic ions gather in water. This protocol is explored as a variant of the ELLE process, in which an enantiomer of a racemic mixture is preferentially extracted with water.
Ionic liquids (ILs) are salts that are liquid under normal physical conditions. They possess several unique properties, such as a near-zero vapour pressure, which make them non-toxic by inhalation and not flammable, and a good ability to dissolve various substances. These characteristics, along with many others, render their use favourable for green chemistry.1 The field of ILs is growing rapidly: during the last decade the amount of published articles was multiplied by ten.
Generally, the synthesis of ionic liquids consists of two main steps: the formation of the cation and the anion exchange through ion metathesis.1 The basic concept of ion metathesis is the formation of a new pair of salts, which can be easily separated based on their distinct physical properties. Commonly, ion metathesis involves the anion exchange of halide salts with metal salts. This method has limitations in the preparation of pure ILs due to the contamination by metal halide salts and is not easily applied at the industrial scale because of its high cost. Also, ILs are positioned as “green solvents”, but their production often uses large quantities of organic solvents, mainly for the purification of ionic liquids from halogen impurities. Thus there is a continuous need for new methods of preparation of ILs that should overcome present-day obstacles of their widespread usage.
Herein we report a simple and fully atom economic way to obtain new families of ionic liquids. Starting from two initial ILs, two new ILs are obtained in quantitative yield by mutual ion exchange based on their physico-chemical properties.2 The driving force of this process is hydrophobic/hydrophilic interaction. This means that when two ILs are exposed to each other as a biphasic aqueous system, their constituent ions redistribute depending on their hydrophobicity/hydrophilicity. Otherwise saying, the hydrophobic ions unite together in the hydrophobic layer, whereas the hydrophilic ions gather in the hydrophilic layer. We call this new process “ionic liquids ion cross-metathesis” (ILICM).
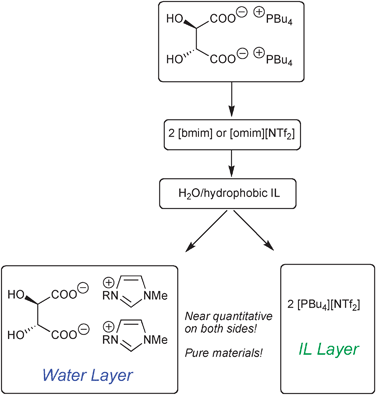
In regards with our continuous interest in chiral ionic liquids (CILs),3 we illustrated the principle of ILICM using tartaric acid-based ILs and imidazolium-based ILs. Tetrabutylphosphonium tartrates are highly hydrophilic substances despite the presence of the hydrophobic tetrabutylphosphonium. However, when dissolved in a hydrophobic IL such as [bmim][NTf2], then extracted by water, the following phenomenon was observed: the highly hydrophilic ions (tartaric carboxylate and imidazolium) unite together in water, while the hydrophobic ions (tetrabutylphosphonium and NTf2−) gather in the IL (Scheme 1).
 | ||
| Scheme 1 | ||
To respect electro-neutrality of both phases, the tartate dicarboxylate anion must move to water layer along with two cations. Worthy of note is that those countercations are not the former tetrabutylphosphoniums, but the more hydrophilic cations present in the mixture, i.e. the imidazoliums, which are much less hydrophobic than tetrabutylphosphonium cations. The exchange is complete, i.e. equivalent quantity of cations is extracted to ensure the electro-neutrality of the solution. To ensure quantitative exchange the presence of two immiscible phases is required. In our example it was water–hydrophobic IL ([bmim]–, [omim]– or [PBu4]–[NTf2]), and ILs were used in excess with respect to tartaric carboxylate. Fig. 1 depicts the behaviour of [PBu4][NTf2], which exhibits a paraffin-like structure and stands as a “ball” in the aqueous layer during stirring.
![Separation of [PBu4][NTf2] (paraffin ball) from water layer in ILICM process.](/image/article/2012/CC/c2cc17799d/c2cc17799d-f1.gif) | ||
| Fig. 1 Separation of [PBu4][NTf2] (paraffin ball) from water layer in ILICM process. | ||
Two CILs were prepared using this method: [bmim]2-[(R,R)-Trtr] and [omim]2-[(R,R)-Trtr] (Scheme 1). In the literature, [bmim]2-[(R,R)-Trtr] was previously prepared using [bmim][OH] (obtained via an ion-exchange resin), which was neutralised with tartaric acid.4 Its [omim]2 congener is new. Both compounds were obtained from water solution in 100% yield and excellent purity.5
The following issue was: what would happen if the chiral ion of the CIL (tartrate moiety in our examples) was exposed to a racemic counter-ion? In other words, is this kind of process able to induce chiral recognition? The system that combines the principles of enantiomeric recognition and solvent extraction in one single technique is often called enantioselective liquid–liquid extraction (ELLE).8 This technology is very promising for obtaining enantiopure compounds and becomes the object of much attention since the development of appropriate equipment that reduces the time and cost of the separation of enantiomers.8 Prerequisite for ELLE processes is the simultaneous presence of a chiral host/racemic substrate pair and of two immiscible phases. In our opinion, chiral ionic liquids could be excellent candidates to be applied in ELLE, because they could play both the roles of the chiral selector and of the solvent;9 in addition, the use of a hydrophobic CIL layer combined with water extraction would perfectly fit the main principles of green chemistry.
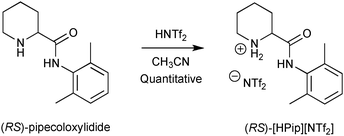
The model substrate that we selected for further studies was suggested by Dr Martin Hedberg (AstraZeneca) during the Intenant European network.10 It is a molecule of pharmaceutical interest called pipecoloxylidide, the (S) enantiomer of which has properties of local anaesthetic. The first step consisted of preparing its NTf2 salt according to Scheme 2 (quantitative yield).
 | ||
| Scheme 2 | ||
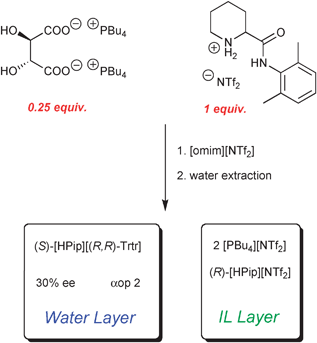
The biphasic chiral extraction system was established by mixing the chiral ionic liquid [PBu4]2[(R,R)-Trtr] and (R,S)-[HPip][NTf2] in [omim][NTf2]. The commercial hydrophobic IL [omim][NTf2] was used to increase the fluidity of the system. The IL phase was mixed for 2 hours in order to allow the molecules of the chiral host and of the racemate to interact. After that, water was added and the biphasic system was stirred for additional 12 hours. A relatively small quantity of water (ca. 20 equiv.) was used with the goal to extract preferentially one of the enantiomers.
As before, tartaric salts partition into water because of hydrophilicity. To respect electro-neutrality of both phases, the tartate dicarboxylate anion must move to water layer along with some cations, choosing the most hydrophilic from the mixture. But as there is excess of pipecoloxylidide moieties, tartaric acid preferentially chooses one enantiomer as its counter-cation. In our example, the natural (R,R)-enantiomer of tartaric acid preferentially extracts into the water layer of the (S)-enantiomer of pipecoloxylidide, which is precisely the eutomeric stereoisomer in this series (Scheme 3).
 | ||
| Scheme 3 | ||
We checked many different parameters, such as the nature of the IL co-solvent, the temperature and the incubation time to determine their influence both on enantiomeric excess (ee) and yield (see ESI†). Without IL co-solvent the mixture is too viscous. Heating increases dramatically the ee, reaching the maximum of 30% at 50 °C, when at 0 °C and at 180 °C no ee was observed (see Fig. 2).
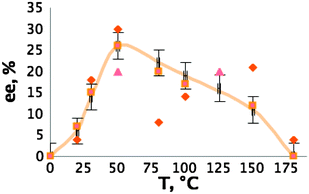 | ||
| Fig. 2 Role of the temperature (different shapes of points represent different runs). | ||
Also, incubation time is important, because no ee was recorded without it. The best example shows ee of 30% and operational selectivity of 2. This represents the first example of the use of chiral ionic liquids in ELLE in the absence of any metallic ions.11
In conclusion, the described IL ion cross-metathesis is a new and efficient method for the handy synthesis of ILs with 100% atom economy. ELLE results in CILs are very promising as a new and eco-friendly method for liquid–liquid resolution. Further studies regarding the mechanism of interactions, scaling effect, recycling of ILs and breadth of selectivity are planned in our research team.
We wish to thank Dr M. Hedberg (AstraZeneca) for the generous gift of racemic and enantiopure pipecoloxylidide, and to express our gratitude for supporting this study within INTENANT and the grant FP7-NMP2-SL2008-214129 by the European Union (http://www.intenant.eu/).
Notes and references
- (a) Ionic Liquids in Synthesis, ed. P. Wasserscheid and T. Welton, Wiley-VCH, Weinheim, 2nd edn, 2008 Search PubMed; (b) J. P. Hallet and T. Welton, Chem. Rev., 2011, 111, 3508–3576 CrossRef.
- For relevant previous examples of mutually immiscible ILs, see for example: A. Arce, M. J. Earle, S. P. Katdare, H. Rodriguez and K. R. Seddon, Chem. Commun., 2006, 2548–2550 RSC.
- (a) C. Baudequin, D. Brégeon, J. Levillain, F. Guillen, J.-C. Plaquevent and A.-C. Gaumont, Tetrahedron: Asymmetry, 2005, 16, 3921–3945 CrossRef CAS; (b) J.-C. Plaquevent, J. Levillain, F. Guillen, C. Malhiac and A.-C. Gaumont, Chem. Rev., 2008, 108, 5035–5060 CrossRef CAS.
- (a) C. Wang, Y. Zheng, S. Cai, J. Ma and R. Li, React. Kinet. Catal. Lett., 2008, 95, 129–134 CrossRef CAS; (b) S. Bose, A. B. Wijeratne, A. Thite, G. A. Kraus, D. W. Armstrong and J. W. Petrich, J. Phys. Chem. B, 2009, 113, 10825–10829 CrossRef CAS.
- Note: special remark should be made on the fact that little attention has been paid by chemists working on the synthesis of new ILs to the characterization and the purity of ionic entities. In our work, the synthesis of chiral ionic liquids consisted only of mixing two water solutions of commercially available compounds: tartaric acid and [PBu4][OH], which we showed to be contaminated by commercially starting [PBu4][Cl] (up to 15%). This impurity is silent both in NMR and positive mass spectrometry analyses and this is a pitfall when determining ILs purity. This points out the importance to pay particular attention to the characterization methods when taking the data from previous researches. The problem of non-reproducibility of the literature data for ILs was discussed recently.6,7.
- A. Stark, P. Behrend, O. Braun, A. Müller, J. Ranke, B. Ondruschka and B. Jastorff, Green Chem., 2008, 10, 1152–1161 RSC.
- J. C. Pastre, C. R. D. Correia and Y. Génisson, Green Chem., 2008, 10, 885–889 RSC.
- For a recent and comprehensive review in the field, see: B. Schuur, B. J. V. Verkuijl, A. J. Minnaard, J. G. de Vries, H. J. Heeres and B. L. Feringa, Org. Biomol. Chem., 2011, 9, 36–51 CAS.
- V. Zgonnik, S. Gonella, M.-R. Mazières, F. Guillen, G. Coquerel and J.-C. Plaquevent, Org. Process Res. Dev., 2012 DOI:10.1021/op200082a.
- L. K. Thalén, M. H. Hedberg and J.-E. Bäckvall, Tetrahedron Lett., 2010, 51, 6802–6805 CrossRef.
- F. Tang, Q. Zhang, D. Ren, Z. Nie, Q. Liu and S. Yao, J. Chromatogr., A, 2010, 1217, 4669–4674 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures, compound characterisation data. See DOI: 10.1039/c2cc17799d |
| ‡ This article is part of the ChemComm 'Chirality' web themed issue. |
| This journal is © The Royal Society of Chemistry 2012 |
