Assembling carbon-coated α-Fe2O3 hollow nanohorns on the CNT backbone for superior lithium storage capability†
Zhiyu
Wang
a,
Deyan
Luan
b,
Srinivasan
Madhavi
bc,
Yong
Hu
d and
Xiong Wen (David)
Lou
*ac
aSchool of Chemical and Biomedical Engineering, Nanyang Technological University, 70 Nanyang Drive, Singapore, 637457, Singapore. E-mail: xwlou@ntu.edu.sg; Tel: +65 6316 8879
bSchool of Materials Science and Engineering, Nanyang Technological University, 50 Nanyang Avenue, 639798, Singapore
cEnergy Research Institute @ NTU, Nanyang Technological University, 50 Nanyang Drive, Singapore, 637553, Singapore
dInstitute of Physical Chemistry, Zhejiang Normal University, Jinhua, 321004, P.R.China
First published on 25th November 2011
Abstract
Novel hierarchical nanostructures composed of carbon coated α-Fe2O3 hollow nanohorns on carbon nanotube (CNT) backbones have been constructed by direct growth and thermal transformation of β-FeOOH nanospindles on CNTs, followed by carbon nanocoating. When evaluated as a potential anode material for lithium-ion batteries, such hierarchical structures exhibit superior lithium storage capabilities by virtue of their advantageous structural features.
Broader contextLithium-ion batteries (LIBs) power millions of laptop computers and other portable electronics. Despite great commercial success, the performance of LIBs based on current electrode materials is approaching the limit. High-energy density and high-power LIBs with greater safety are also vitally important for widespread use of electric cars, which have regained interest recently in view of fast depletion of fossil fuels and associated environmental concerns and climate change. Therefore, there is an urgent need to develop new high-performance electrode materials with significantly higher capacities and/or power densities for next-generation LIBs. In this contribution, we describe a green and simple method to prepare a novel electrode material with hierarchically nanostructured frameworks by assembling carbon-coated α-Fe2O3 hollow nanohorns onto carbon nanotube (CNT) backbones. The combination of highly conductive CNTs, hollow nanostructures with large surface area and shortened diffusion pathway, and high-capacity iron oxide makes the resultant hybrid material a truly durable electrode material for lithium-ion batteries. |
With the advantages of high energy density, long lifespan and environmental benignity, lithium-ion batteries (LIBs) have become the predominant power source for portable electronics for many years.1 The continuously surging demand in emerging large-scale energy applications such as low-emission electric vehicles, renewable power plants and electric grids boosts a great deal of interest in seeking for high-performance electrode materials with the capability to store and deliver more energy efficiently. Among the available alternative anode materials, iron oxides (mainly α-Fe2O3 and Fe3O4) have always been regarded as very appealing candidates because of their much higher capacity than that of conventional graphite (372 mA h g−1), as well as nontoxicity, high corrosion resistance and low processing cost.2 In principle, the lithium storage capacity of iron oxides is mainly delivered through the reversible conversion reaction between lithium ions and metal oxide forming metal nanocrystals dispersed in a Li2O matrix. This process usually causes fast capacity fading due to drastic volume variation and severe destruction of the electrode upon electrochemical cycling. The sluggish kinetics for charge transfer and ionic diffusion, as well as high intrinsic resistance also induces additional performance degradation of metal oxide based electrodes, especially at high current densities. So far, an enormous amount of effort has been made to circumvent the above issues by designing iron oxide based nanostructures with optimized particle sizes, shapes and component compositions.2–17 However, the fabrication of truly durable iron oxide electrodes with satisfactory high-rate capability and high specific capacity is still a great challenge.
The use of hybrid nanostructures, where the nanostructures of active materials are assembled onto or embedded into the conductive matrix via chemical bonding or noncovalent forces, has been regarded as one of the most effective approaches towards high-performance electrode materials for LIBs.12,18–29 In such hybrid structures, in addition to all the desired functions of each constituent, some strong synergetic effect can be achieved by integrating the individual components, hence realizing the full potential of the nanocomposite materials. In practice, the feasibility of this concept has been well demonstrated by recent successes in synthesis of various composite nanostructures such as FeF3/carbon nanotubes (CNTs),23Fe3O4/SWNT,27 and carbon coated Fe3O4 nanospindles.29 Here, we report a novel hierarchical nanostructure composed of carbon coated α-Fe2O3 hollow nanohorns grafted on CNT backbones (denoted as CNT@Fe2O3) by bottom-up assembly of β-FeOOH nanospindles on CNTs and subsequent in situ phase and structure transformation and further modification with carbon nanocoating. In theory, such a unique hybrid structure is expected to manifest greatly improved electrochemical performance because of the integration of several advantageous structural features. Specifically, the highly conductive and flexible CNT backbone can provide a three-dimensional (3D) electronic network to facilitate the charge transfer.23,27 Second, the hollow α-Fe2O3 nanohorns grafted on the CNT backbone are beneficial to the enhanced electrochemical activity and mechanical integrity of the electrode due to their large surface area, nanoscale diffusion length of typically a few nanometres and sufficient internal void space.30 Furthermore, the outmost continuous carbon nanocoating around the overall architecture may serve as a structural buffering layer to cushion the internal strain associated with lithium uptake while preventing the Fe2O3 nanostructures from being electrically isolated upon cycling.29,31 Benefited from the enhanced structural stability and kinetics for lithium storage, our hybrid materials exhibit an exceptionally stable capacity retention of 420–800 mA h g−1 at high current densities of 500–3000 mA g−1.
The synthesis strategy for the fabrication of CNT@Fe2O3 hierarchical structures is illustrated in Scheme 1. In the first step, β-FeOOH nanospindles are spontaneously grown and assembled on CNT backbones (denoted as CNT@FeOOH) by forced hydrolysis of FeCl3 without the need for any structure-directing agent. Prior to this growth, the CNTs are first treated by acidic reflux to attach carboxylic or hydroxyl groups that facilitate the surface nucleation and anchoring of β-FeOOH.21 Afterwards, β-FeOOH nanospindles on CNTs are easily transformed to α-Fe2O3 hollow nanohorns as a result of thermal dehydroxylation and simultaneous lattice shrinkage at 400 °C.32 Lastly, an outmost layer of amorphous carbon is deposited around the scaffold of CNT@Fe2O3 architectures by hydrothermal carbonization of glucose, leading to the formation of carbon coated CNT@Fe2O3 hierarchical hybrid structures.
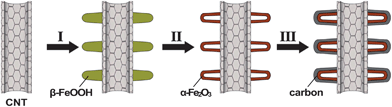 | ||
| Scheme 1 Schematic illustration of the formation of carbon coated α-Fe2O3 hollow nanohorns on the CNT backbone: (I) heterogeneous growth of β-FeOOH nanospindles on CNTs by force hydrolysis of Fe3+ ions; (II) thermal transformation of β-FeOOH nanospindles to α-Fe2O3 hollow nanohorns on CNTs by annealing CNT@FeOOH structures in air and (III) carbon nanocoating of CNT@Fe2O3 hierarchical structures by hydrothermal carbonization of glucose. | ||
The crystallographic structure and phase purity of the CNT@FeOOH sample are determined by X-ray powder diffraction (XRD), as shown in Fig. S1†. All the diffraction peaks can be assigned to tetragonal β-FeOOH (JCPDS no. 75-1594) except the one from (002) planes of CNTs at ca. 26°. A panoramic view by a scanning electron microscope (SEM) reveals that this sample consists of sinuous and highly entangled CNTs uniformly decorated with nanospindles on the entire surface (Fig. 1a–c). The formation of CNT@FeOOH hierarchical structures is further evidenced by transmission electron microscopy (TEM) observation, as characterized by radial assembly of high density β-FeOOH nanospindles on the curvature of long CNT backbones (Fig. 1d–f). The β-FeOOH nanospindles possess a length of around 20–50 nm and a diameter of several nanometres. The selected area electron diffraction (SAED) analysis (inset of Fig. 1e) is superimposed by the diffraction rings from polycrystalline FeOOH nanospindles and a pair of short arc from (002) diffraction of the CNTs, indicating the assembly of FeOOH nanostructures onto CNT backbones. High-resolution TEM (HRTEM) characterization further evidences the close integration of FeOOH structures on CNTs, as shown in Fig. S2a†. Although the exact mechanism is unclear yet, we suggest the preferential electrostatic adsorption of Fe3+ ions to polar oxygen-containing groups on CNTs, the subsequent hydrolysis of Fe3+ and further olation/oxolation of FeO6 units may be responsible for the heterogeneous nucleation and growth of β-FeOOH nanostructures on CNT backbones. In this process, a low initial concentration of FeCl3 (e.g., 0.03 M) results in growth of sparse nanospindles on CNTs, while an excessive amount of FeCl3 (e.g., 0.36 M) induces severe homogeneous growth of monodisperse β-FeOOH particles (Fig. S3†). In other words, the growing density of β-FeOOH nanospindles on CNT backbones can be readily tuned to achieve a desirable loading amount of iron content without reduction in product quality by simply varying the initial concentration of FeCl3 in solution.
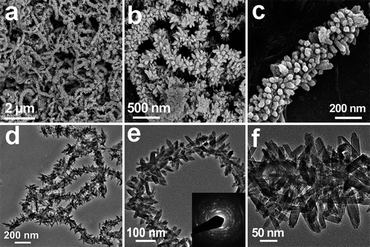 | ||
| Fig. 1 (a–c) FESEM images and (d) TEM images of CNT@FeOOH hierarchical structures; (e and f) TEM images showing the high density of β-FeOOH nanospindles on the CNT backbone. | ||
The hierarchical characteristics of CNT@FeOOH nanoarchitectures enable their composition of individual components to be varied without damaging the overall structure. By careful annealing in air, CNT@Fe2O3 hierarchical structures can be derived through phase transformation of β-FeOOH to polycrystalline hexagonal α-Fe2O3 (hematite, JCPDS no. 33-0664) on CNT backbones, as confirmed by XRD analysis (Fig. S1†) and SEM examination (Fig. 2a–c). TEM examination shows that the thermal conversion of β-FeOOH nanospindles to α-Fe2O3 nanohorns undergoes a topotactic transformation process due to their structural similarity, as shown in Fig. 2c and d. During the annealing process, a different amount of porosity is introduced in the resulting structures as a result of the volume contraction associated with the transformation from low density β-FeOOH (3 g cm−3) to denser hematite with a density of 5.3 g cm−3.32 Further investigation reveals that the β-FeOOH nanospindles become porous but can still retain their original shape (Fig. S4a†) at 250 °C. While increasing the temperature to 300 °C, numerous small pores with irregular shapes emerge within the nanospindles (Fig. S4b†), which eventually coalesce to form well-developed hollow interiors if annealed at 400 °C (Fig. 2e and f). Accordingly, the specific surface area (calculated by the Brunauer–Emmett–Teller method) of the samples increases from about 66 m2 g−1 (for CNT@FeOOH) to 110 m2 g−1 after annealing (Fig. S5†) while the overall hierarchical nanostructure remains the same, as characterized by SAED and HRTEM examinations (inset of Fig. 2d and S2b†). Furthermore, the robust structure of CNT@Fe2O3 nanoarchitectures provides the feasibility of exploiting nanocoating of carbon around the scaffold by controlled hydrothermal carbonization of glucose. After carbon coating, there is no apparent change in the morphology of the products (Fig. 2g) and the overall carbon content in the sample is around 25–30 wt% (by thermogravimetric and CHN elemental analyses, Fig. S6†). TEM characterization indicates that the entire surface of CNT@Fe2O3 hierarchical structures has been covered with a uniform and continuous amorphous carbon overlayer with a thickness of a few nanometres (Fig. 2h and i and S7†). With a large surface area of about 96 m2 g−1, such a hybrid nanostructure might hold great promise in offering a sufficient interface to facilitate the electrochemical reactions.
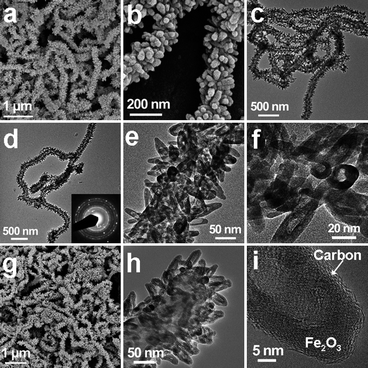 | ||
| Fig. 2 (a and b) FESEM images and (c and d) TEM images of CNT@Fe2O3 hierarchical structures; (e and f) TEM images revealing the formation of hollow nanohorns on CNT backbones; (g) FESEM image and (h) TEM image of carbon-coated CNT@Fe2O3 hierarchical structures; (i) HRTEM image of uniform carbon nanocoating on α-Fe2O3 hollow nanohorns. | ||
It is well known that the electrochemical performance is not only highly dependent on the intrinsic crystalline texture and surface properties, but also greatly related to the morphology and assembled structure of active materials.1,19 In carbon coated CNT@Fe2O3 hierarchical structures, the α-Fe2O3 nanohorns are largely separated from each other because they are grown directly on the CNT backbones, thus making them fully accessible to lithium ions in the electrolyte. This is in distinct contrast to the electrode composed of nanoparticles, where severe agglomeration is likely to occur thus eliminating most of the active interfaces for lithium storage. Meanwhile, the extremely reduced dimensions of free-standing α-Fe2O3 nanohorns, adequate interspaces between them, and sufficient internal porosity ensure maximum electrode stability by effectively mitigating the internal mechanical stress induced by the large volume variation of the electrode upon cycling.1,30 On the other hand, the conductive CNT network also plays a positive role to improve the electrochemical performance of CNT@Fe2O3electrodes by endowing a three-dimensional electronic path for fast and stable charge transfer and lowering the internal electrode resistance.23,27 Furthermore, the continuous and elastic amorphous carbon overlayer further strengthens the structural integrity of CNT@Fe2O3 hierarchical structures, which will improve the cycle life.29,31
Motivated by these structural features, we have evaluated the electrochemical lithium storage properties of carbon coated CNT@Fe2O3 hierarchical structures for their potential use as an anode material for LIBs. Fig. 3a shows representative discharge/charge voltage profiles of this material at a current density of 500 mA g−1 within a cut-off voltage window of 0.01–3.0 V. The initial discharge and charge capacities are found to be 1060 and 580 mA h g−1, respectively. The irreversible capacity loss of 45% may be mainly attributed to irreversible processes such as inevitable formation of inorganic solid electrolyte interface (SEI) film (mainly Li2CO3 and alkyl carbonates) and electrolyte decomposition, which are common to most anode materials.2–15 From the second cycle onwards, the capacity of carbon-coated CNT@Fe2O3 hierarchical structures increases gradually from 660 mA h g−1 to 820 mA h g−1 within 100 cycles with a high Coulombic efficiency of around 97–98% (Fig. S8†). Such a capacity rise with cycling is not uncommon for various nanostructured metal oxide electrodes.24,27,33–38 The reversible formation of organic polymeric/gel-like layer by electrolyte decomposition at low potential has been suggested as one of the possible reasons for this phenomenon because of its capability of coating around the active materials to ensure the mechanical cohesion and delivering excess capacity at low potential through a so-called “pseudo-capacitance-type behavior”.36 In the CoO/Li system, such an effect has resulted in the stable and gradual capacity increase during the electrochemical cycling.36,37 Moreover, the activation of the active materials may also contribute to the capacity rise in metal oxide electrodes. To investigate this, the differential capacity versus voltage curves of 1st, 15th, 30th, 50th, 75th and 100th cycles are plotted in Fig. 3c for carbon-coated CNT@Fe2O3 structures. The gradually increased intensity of the peaks at around 0.9 V corresponds to the enhanced reversibility of the conversion reaction between Li2Fe2O3 and Fe(0) for lithium storage. That is, the activation effect may also apply on our material although the detailed mechanism involved is not yet fully understood. With the aim of demonstrating the advantages of carbon coated CNT@Fe2O3 hierarchical structures on lithium storage, the cycling performance of the CNT@Fe2O3 without carbon coating and α-Fe2O3 nanoparticles is also investigated under the same conditions. The CNT@Fe2O3 architectures deliver an initial discharge capacity of 1200 mA h g−1, which then stabilizes at around 500 mA h g−1 after 100 cycles. This performance, although not as good as that of carbon coated CNT@Fe2O3, is much better than that of iron oxide nanocapsules,5 clearly indicating the beneficial effect of the CNT backbone. Whereas for α-Fe2O3 nanoparticles, the capacity decays much faster to less than 300 mA h g−1 within 30–50 cycles. Apparently, both the hierarchical structure and carbon nanocoating contribute to the significantly improved electrochemical properties of our carbon coated CNT@Fe2O3 materials.
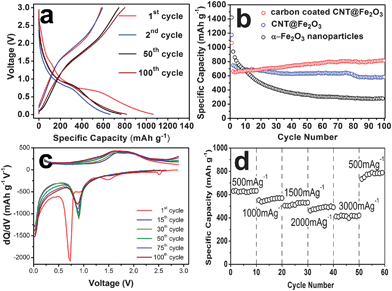 | ||
| Fig. 3 (a) Discharge/charge voltage profiles of carbon coated CNT@Fe2O3 hierarchical structures. (b) Comparative cycling performance of CNT@Fe2O3 structures with and without carbon coating and α-Fe2O3 particles. For these tests, the discharge/charge and cycling curves are taken between 0.01 and 3.0 V at a current density of 500 mA g−1. (c) The differential capacity versus voltage plots of carbon coated CNT@Fe2O3 structures. (d) The rate capability of carbon coated CNT@Fe2O3 hierarchical structures at different current densities. | ||
In addition to the cycling performance, the high-rate capability is also of great importance especially for high-power applications. Benefitted from the unique hierarchical structure and the presence of carbon nanocoating, our materials exhibit excellent cycling response to a continuously varying current rate although α-Fe2O3 electrodes are generally observed to suffer from sluggish kinetics. Even cycled at very high rates of 1000–3000 mA g−1, comparable capacities of 420–500 mA h g−1 can still be maintained, as shown in Fig. 3d. Such a remarkable high-rate performance is superior to that of most α-Fe2O3 based electrodes, which lose almost all of the capacity upon cycling under similar high current densities.2–15 Moreover, the phenomenon of slight capacity rise is also observed upon cycling at high current densities. After deep cycling at 3000 mA g−1, a constant capacity of around 800 mA h g−1 can be restored when cycling at a lower current density of 500 mA g−1.
It has been proposed that the lithium storage in the α-Fe2O3 is primarily based on a multiple step reaction, in which the phase transition between hexagonal LixFe2O3, cubic Li2Fe2O3 and Fe(0) is involved as follows:11,39
| α-Fe2O3 + xLi+ + xe− → α-LixFe2O3 | (1) |
| α-LixFe2O3 + (2 − x)Li+ + (2 − x)e− → α-Li2Fe2O3 | (2) |
| α-Li2Fe2O3 + 4Li+ + 4e− → 2Fe0 + 3Li2O | (3) |
At the initial stage of lithium intercalation (reaction (1)), a small amount of lithium is inserted into the α-Fe2O3 to form hexagonal α-LixFe2O3 without changing the crystal structure. Then the α-LixFe2O3 is converted to cubic Li2Fe2O3 due to the excess lithium intercalation (reaction (2)). Finally, fine Fe(0) nanoclusters are generated and dispersed in the Li2O matrix as a result of the complete reduction of Li2Fe2O3 (reaction (3)). For the overall process, the first two reactions are largely irreversible, whereas the last one is highly reversible for lithium storage. To further investigate the mechanism of lithium storage in carbon coated CNT@Fe2O3 structures, the differential capacity versus voltage curves of various cycles (Fig. 3c) are carefully studied. At the 1st cycle, three peaks at 1.5 V, 1.0 V and 0.7 V are observed, corresponding to the formation of hexagonal α-LixFe2O3, the phase transition from LixFe2O3 to cubic Li2Fe2O3 and complete reduction to Fe(0) plus SEI film formation, respectively. The broad peak at 1.8–1.9 V corresponds to the restoration of Fe(0) to Fe(II). The subsequent curves show good reproducibility with a cathodic and anodic peak pair at around 0.9 V and 1.8–1.9 V for reversible conversion between Fe(II) and Fe(0). The initial peaks at 1.5 V and 0.7 V disappear as a result of irreversible phase transition from α-LixFe2O3 to cubic Li2Fe2O3 and SEI film formation. Apparently, the lithium storage mechanism of CNT@Fe2O3 structures is similar to other iron oxide electrodes.9,10,14,40,41 However, in favor of the presence of conductive CNT backbones, our composite material exhibits much lower resistance than the α-Fe2O3 nanoparticles, as evidenced by the drastically reduced diameter of the semicircle at high-frequency region in the electrochemical impedance spectroscopy (EIS) patterns (Fig. S9†). As a result of lower contact and charge-transfer impedances, lithium ion diffusion and electron transfer are facilitated to give the greatly enhanced electrochemical performance of the CNT@Fe2O3 composites.
In summary, an advanced carbon coated CNT@Fe2O3 hierarchical nanostructure has been constructed by bottom-up assembly of β-FeOOH nanospindles on CNT backbones and thermal transformation to hollow α-Fe2O3 nanohorns followed by carbon nanocoating. In virtue of greatly enhanced electrode stability and kinetics for lithium storage, this unique hybrid structure exhibits a very stable capacity retention of 800 mA h g−1 over 100 cycles at a current density of 500 mA g−1 and exceptional high-rate capability at high current densities of 1000–3000 mA g−1. It is believed that the structural design of electrodes demonstrated in this work will have important implications on the fabrication of high-performance electrode materials for lithium-ion batteries.
Acknowledgements
The authors are grateful to the National Research Foundation (Singapore) for financial support through the Clean Energy Research Programme (CERP; NRF2009EWTCERP001-036). Y. Hu acknowledges the financial support from Natural Science Foundation of China (21171146).Notes and references
- P. G. Bruce, B. Scrosati and J. M. Tarascon, Angew. Chem., Int. Ed., 2008, 47, 2930–2946 CrossRef CAS.
- J. Chen, L. N. Xu, W. Y. Li and X. L. Gou, Adv. Mater., 2005, 17, 582–586 CrossRef CAS.
- J. S. Chen, T. Zhu, X. H. Yang, H. G. Yang and X. W. Lou, J. Am. Chem. Soc., 2010, 132, 13162–13164 CrossRef CAS.
- J. P. Liu, Y. Y. Li, H. J. Fan, Z. H. Zhu, J. Jiang, R. M. Ding, Y. Y. Hu and X. T. Huang, Chem. Mater., 2010, 22, 212–217 CrossRef CAS.
- H. S. Kim, Y. Piao, S. H. Kang, T. Hyeon and Y. E. Sung, Electrochem. Commun., 2010, 12, 382–385 CrossRef CAS.
- J. M. Ma, J. B. Lian, X. C. Duan, X. D. Liu and W. J. Zheng, J. Phys. Chem. C, 2010, 114, 10671–10676 CAS.
- C. Z. Wu, P. Yin, X. Zhu, C. Z. OuYang and Y. Xie, J. Phys. Chem. B, 2006, 110, 17806–17812 CrossRef CAS.
- N. Yanna, P. Zhang, Z. Guo, P. Munroe and H. Liu, Electrochim. Acta, 2008, 53, 4213–4218 CrossRef.
- H. Liu, G. X. Wang, J. Park, J. Wang and C. Zhang, Electrochim. Acta, 2009, 54, 1733–1736 CrossRef CAS.
- M. V. Reddy, T. Yu, C. H. Sow, Z. X. Shen, C. T. Lim, G. V. S. Rao and B. V. R. Chowdari, Adv. Funct. Mater., 2007, 17, 2792–2799 CrossRef CAS.
- D. Larcher, C. Masquelier, D. Bonnin, Y. Chabre, V. Masson, J. B. Leriche and J. M. Tarascon, J. Electrochem. Soc., 2003, 150, A133–A139 CrossRef CAS.
- Y. Zhao, J. X. Li, Y. H. Ding and L. H. Guan, Chem. Commun., 2011, 47, 7416–7418 RSC.
- P. C. Wang, H. P. Ding, T. Bark and C. H. Chen, Electrochim. Acta, 2007, 52, 6650–6655 CrossRef CAS.
- W. Zhou, L. J. Lin, W. J. Wang, L. L. Zhang, Q. O. Wu, J. H. Li and L. Guo, J. Phys. Chem. C, 2011, 115, 7126–7133 CAS.
- H. J. Kim, K. I. Choi, A. Q. Pan, I. D. Kim, H. R. Kim, K. M. Kim, C. W. Na, G. Z. Cao and J. H. Lee, J. Mater. Chem., 2011, 21, 6549–6555 RSC.
- C. J. Jia, L. D. Sun, Z. G. Yan, L. P. You, F. Luo, X. D. Han, Y. C. Pang, Z. Zhang and C. H. Yan, Angew. Chem., Int. Ed., 2005, 44, 4328–4333 CrossRef CAS.
- D. Jagadeesan, U. Mansoori, P. Mandal, A. Sundaresan and M. Eswaramoorthy, Angew. Chem., Int. Ed., 2008, 47, 7685–7688 CrossRef CAS.
- L. Taberna, S. Mitra, P. Poizot, P. Simon and J. M. Tarascon, Nat. Mater., 2006, 5, 567–573 CrossRef.
- R. Liu, J. Duay and S. B. Lee, Chem. Commun., 2011, 47, 1384–1404 RSC.
- A. Magasinski, P. Dixon, B. Hertzberg, A. Kvit, J. Ayala and G. Yushin, Nat. Mater., 2010, 9, 353–358 CrossRef CAS.
- Y. Hou, Y. W. Cheng, T. Hobson and J. Liu, Nano Lett., 2010, 10, 2727–2733 CrossRef CAS.
- L. F. Cui, Y. Yang, C. M. Hsu and Y. Cui, Nano Lett., 2009, 9, 3370–3374 CrossRef CAS.
- S. W. Kim, D. H. Seo, H. Gwon, J. Kim and K. Kang, Adv. Mater., 2010, 22, 5260–5264 CrossRef CAS.
- H. X. Zhang, C. Feng, Y. C. Zhai, K. L. Jiang, Q. Q. Li and S. S. Fan, Adv. Mater., 2009, 21, 2299–2304 CrossRef CAS.
- A. L. M. Reddy, M. M. Shaijumon, S. R. Gowda and P. M. Ajayan, Nano Lett., 2009, 9, 1002–1006 CrossRef CAS.
- S. W. Lee, N. Yabuuchi, B. M. Gallant, S. Chen, B. S. Kim, P. T. Hammond and Y. Shao-Horn, Nat. Nanotechnol., 2010, 5, 531–537 CrossRef CAS.
- C. M. Ban, Z. C. Wu, D. T. Gillaspie, L. Chen, Y. F. Yan, J. L. Blackburn and A. C. Dillon, Adv. Mater., 2010, 22, E145–E149 CrossRef CAS.
- F. Y. Cheng, J. Liang, Z. L. Tao and J. Chen, Adv. Mater., 2011, 23, 1695–1715 CrossRef CAS.
- W. M. Zhang, X. L. Wu, J. S. Hu, Y. G. Guo and L. J. Wan, Adv. Funct. Mater., 2008, 18, 3941–3946 CrossRef CAS.
- X. W. Lou, L. A. Archer and Z. C. Yang, Adv. Mater., 2008, 20, 3987–4019 CrossRef CAS.
- X. W. Lou, C. M. Li and L. A. Archer, Adv. Mater., 2009, 21, 2536–2539 CrossRef CAS.
- Y. Piao, J. Kim, H. Bin Na, D. Kim, J. S. Baek, M. K. Ko, J. H. Lee, M. Shokouhimehr and T. Hyeon, Nat. Mater., 2008, 7, 242–247 CrossRef CAS.
- Y. F. Shi, B. K. Guo, S. A. Corr, Q. H. Shi, Y. S. Hu, K. R. Heier, L. Q. Chen, R. Seshadri and G. D. Stucky, Nano Lett., 2009, 9, 4215–4220 CrossRef CAS.
- S. H. Lee, Y. H. Kim, R. Deshpande, P. A. Parilla, E. Whitney, D. T. Gillaspie, K. M. Jones, A. H. Mahan, S. B. Zhang and A. C. Dillon, Adv. Mater., 2008, 20, 3627–3632 CrossRef CAS.
- Y. Yu, C. H. Chen and Y. Shi, Adv. Mater., 2007, 19, 993–997 CrossRef CAS.
- S. Laruelle, S. Grugeon, P. Poizot, M. Dollé, L. Dupont and J. M. Tarascon, J. Electrochem. Soc., 2002, 149, A627–A634 CrossRef CAS.
- P. Poizot, S. Laruelle, S. Grugeon, L. Dupont and J. M. Tarascon, Nature, 2000, 407, 496–499 CrossRef CAS.
- Z. Y. Wang, J. S. Chen, T. Zhu, S. Madhavi and X. W. Lou, Chem. Commun., 2010, 46, 6906–6908 RSC.
- D. Larcher, D. Bonnin, R. Cortes, I. Rivals, L. Personnaz and J. M. Tarascona, J. Electrochem. Soc., 2003, 150, A1643–A1650 CrossRef CAS.
- X. L. Wu, Y. G. Guo, L. J. Wan and C. W. Hu, J. Phys. Chem. C, 2008, 112, 16824–16829 CAS.
- B. Sun, J. Horvat, H. S. Kim, W. S. Kim, J. Ahn and G. X. Wang, J. Phys. Chem. C, 2010, 114, 18753–18761 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures, XRD, BET and more TEM images, as well as electrochemical data. See DOI: 10.1039/c1ee02831f |
| This journal is © The Royal Society of Chemistry 2012 |
