Multiple depositions of Ag nanoparticles on chemically modified agarose films for surface-enhanced Raman spectroscopy†
Wen-Lei
Zhai
a,
Da-Wei
Li
a,
Lu-Lu
Qu
a,
John S.
Fossey
ab and
Yi-Tao
Long
*a
aKey Laboratory for Advanced Materials & Department of Chemistry, East China University of Science 4 and Technology, Shanghai, 200237, P.R. China. E-mail: ytlong@ecust.edu.cn; Fax: +86-21-64250032; Tel: +86-21-64250032
bSchool of Chemistry, University of Birmingham, Edgbaston, Birmingham B15 2TT, UK
First published on 7th November 2011
Abstract
A facile and cost-effective approach for the preparation of a surface-enhanced Raman spectroscopy (SERS) substrate through constructing silver nanoparticle/3-aminopropyltriethoxysilane/agarose films (Ag NPs/APTES/Agar film) on various solid supports is described. The SERS performance of the substrate was systematically investigated, revealing a maximum SERS intensity with four layers of the Ag NP deposition. The enhancement factor of the developed substrate was calculated as 1.5 × 107 using rhodamine 6G (R6G) as the probe molecule, and the reproducibility of the SERS signals was established. A high throughput screening platform was designed, manufactured and implemented which utilised the ability to cast agarose to assemble arrays. Quantitative analysis of 4-aminobenzoic acid (4-ABA) and 4-aminothiophenol (4-ATP) was achieved over a ∼0.5 nM–0.1 μM range.
Introduction
Surface-enhanced Raman scattering (SERS) is proving to be a promising tool for non-destructive, ultra-fast and sensitive detection of chemical and biological samples even down to the single molecular level.1–3 It has been used for theoretical investigations of chemical structures and dynamic interface behaviour through providing “fingerprint” information of analyte molecules.4–6 Its sensitivity and negligible interference from water mean it is ideal for use in environmental monitoring and biomedical sensing.7–10 However, the field of applied analysis is still dominated by traditional analytical tools, such as chromatography, infrared spectroscopy, fluorescence and electrochemistry.11 To develop SERS into an attractive and competitive technique for practical applications, more stable, reproducible and economical approaches for SERS substrate preparation are still necessary. Thus far, the majority of reported SERS substrates have been constructed on solid supports, such as silicon wafers, quartz slides and noble metal surfaces. Background interference for these materials is minimal, but they are relatively expensive.12 Therefore, more economical options, such as plastic plates, glass slides and paper, have gradually attracted attention.Numerous methods have been exploited in fabricating SERS substrates, typical methods include wet-chemical reduction,13 electrochemical roughing,14 and metal surface etching.15 Their products present moderate SERS activity, but their stability or uniformity is relatively poor. An ideal SERS substrate should consist of a uniform nanostructure, which should be achieved in order to minimise deviation in the SERS measurement.12 Newly emergent techniques, such as nanosphere lithography (NSL),16 Langmuir–Blodgett (LB) film transfer17,18 or atomic layer deposition (ALD)19 have been used to prepare ordered structures for Raman enhancement. However, these methods depend heavily on specialised equipment and time-consuming procedures, which presents a barrier to future mass production. By contrast, the use of bifunctional molecular modification for the bottom-up assembly/deposition of nanomaterials circumvents these issues and offer the potential for future large-scale/volume applications.20 Using this strategy, one moiety of a bifunctional molecule could anchor to the solid support through a surface polymerization procedure, leaving another moiety “free” to immobilise nanomaterials from a colloid/solution via electrostatic or covalent interactions, and a hierarchical architecture can be achieved through repeating this procedure.21 This approach has been demonstrated as feasible not only for electrochemistry and surface plasmon resonance (SPR) sensors,22,23 but also for SERS substrates.24,25
Recent studies have demonstrated advantages of using agarose gel, (see ESI Fig. S1†) a soft material, to construct SERS substrates.26–28 As a weak Raman scatterer, agarose gel avoids stray light and fluorescence backgrounds in SERS measurements. Moreover, metallic nanoparticles can be stabilised and densely loaded into the matrix structure of agarose gel. Herein, we describe a facile and cost-effective approach to prepare a SERS-active Ag nanoparticles/3-aminopropyltriethoxysilane/agarose film (Ag NPs/APTES/Agar film) on a large area solid support. The preparation method showed strong adhesion of a uniform agarose thin film on a solid support, followed by amine-functionalisation of the agarose film and multiple depositions of Ag NPs (Fig. 1). SERS activity was greatly enhanced after repeating the Ag NP deposition cycle, and after four Ag NP deposition cycles the SERS activity was nearly 10 times higher than after just one deposition; multiple deposition cycles also significantly improved sample-to-sample reproducibility, the relative standard deviation (RSD%) was approximately 8% over 20 successive measurements. We demonstrated that the proposed approach to producing SERS substrates was feasible not only on glass slides but also on polyvinyl chloride (PVC) plates and paper tapes. In addition, spectral differences within the same samples caused by different solid supports were excluded because of the isolation effect of the agarose film. The prepared substrate exhibited high sensitivity, good reproducibility and sufficient stability for at least 6 months.
 | ||
| Fig. 1 The construction of the Ag NPs/APTES/Agar film on a glass slide. | ||
In addition, the array-based SERS substrate was developed by utilizing the plasticity of the agarose film. High-throughput analysis could be accomplished more efficiently by greatly enlarging the sample loading capacity of the substrate and reducing the reagent consumption. At the same time, cross-contamination could effectively be prevented.
Experimental
Silver colloid synthesis
A silver colloid was prepared by sodium citrate reduction in accordance with established procedures:29 all glassware was cleaned with aqua regia (HNO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HCl 1
HCl 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v) overnight and washed with water. Silver nitrate (19 mg) was dissolved in deionised water (100 mL) and heated to boiling. Then sodium citrate (2 mL of a 1% solution) was added dropwise, and heating was maintained for a further 30 min; afterwards, the colloid was cooled to room temperature with a final volume of 60 mL. The mixture was centrifuged at 7000 rpm for 10 min and rinsed with deionised water.
3 v/v) overnight and washed with water. Silver nitrate (19 mg) was dissolved in deionised water (100 mL) and heated to boiling. Then sodium citrate (2 mL of a 1% solution) was added dropwise, and heating was maintained for a further 30 min; afterwards, the colloid was cooled to room temperature with a final volume of 60 mL. The mixture was centrifuged at 7000 rpm for 10 min and rinsed with deionised water.
Agarose thin film preparation and amine-functionalization
A uniform agarose film was adhered to a glass slide, typically, agarose powder (0.4 g) was combined with water (20 mL) and heated to boiling. The hot solution was swiftly applied to a glass slide; upon cooling to room temperature the agarose solution formed a gel. The slide was then dried in an oven to dehydrate the gel.Significantly, agarose may be cast into desired shapes, exploiting this property allowed the construction of multi-welled or array type patterning of the agarose platform through applying a mould in the cooling (gelation) step. Such patterns were preserved after drying the gel into a film.
For the amine-functionalisation of the agarose film, the agarose film/glass slide was immersed in a 10% 3-aminopropyltriethoxysilane (APTES) ethanol solution for 2 h; after gently washing with deionised water a layer of aminosiloxane was generated on the surface of the agarose.
Multiple depositions of Ag NPs
An APTES sol–gel was prepared following a previously reported method,30 where APTES (400 μL) and HCl (1 M, 3 mL) were added to deionised water (27 mL). An APTES sol–gel formed after one hour of vigorous stirring, the material thus formed was stored at 4 °C for later use.To deposit the first layer of the Ag NPs, the amine-functionalised substrate was soaked in Ag NP suspension for 30 min and then rinsed with deionised water. Additional Ag NP layers were formed by repeating the APTES sol–gel formation step followed by further exposure to Ag colloid (2 h); the substrate was washed with deionised water between each step. This procedure could be repeated to furnish the desired number of layers. After Ag NP deposition was complete to the required number of layers, the substrate was heated at 80 °C for 15 min (under a nitrogen atmosphere) to induce cross linking and compact the structure of the Ag NPs/APTES/Agar film.
Spectroscopic measurements
A field-emission scanning electron microscope (Ultra 55, Carl Zeiss Ltd., Germany) was used to characterise the morphology of the prepared substrates. The SERS spectra were recorded using a BWS415 Raman spectrometer (B&W Tek, USA), which utilised up to 300 mW laser power at the excitation wavelength of 785 nm. In this study, all spectra were recorded at a laser power of 5 mW and a 10 s integration time. The Raman spectrometer allowed coupling with a two-axis displacement platform for continuous Raman measurement using a bifurcated optical fibre and Raman probe for the connection.Results and discussion
Silver nanoparticles with an average size of 80 nm (See ESI Fig. S2†) were used in the sensor construction owing to their excellent SERS activity.31 Previous reports have suggested that the SERS performance of substrates modified by metallic nanoparticles is strongly dependent on the amount of nanoparticle depositions.32 In order to optimise the SERS activity of our substrate, the Ag NPs from the colloid were repeatedly deposited. The morphologies of the Ag NPs deposited on indium tin oxide (ITO) glass in different layers were characterized by SEM (see Fig. 2a). A comparison of SEM images indicated an increased density and uniformity of the substrate as the layers of Ag NPs were added. Optical images shown as insets are Ag NPs/APTES/Agar films constructed on glass slides using the same procedure. The colour of the substrate changed from transparent (Fig. 2a, inset i) to deep brown (inset iii), and a metallic luster appeared after the fourth deposition, which further indicated the formation of a dense Ag NP nanostructure. | ||
| Fig. 2 (a) SEM images of Ag NPs deposited on ITO glass with one (i), two (ii), three (iii) and four (iv) layers, respectively. Insets: optical photos of the prepared substrate on the glass slide. (b) SERS spectra of 10−6 M R6G on the prepared substrate with deposition of one (i), two (ii), three (iii) and four (iv) layers of Ag NPs. | ||
Rhodamine 6G (R6G) was employed as the probe molecule for the SERS measurements. The R6G molecules were adsorbed on the as-prepared substrate using a self-assembled monolayer (SAM) method to ensure a homogeneous distribution. Fig. 2b shows the SERS spectra of increasing signal intensity upon additional Ag NP deposition cycles (1 to 4 cycles) acquired upon treatment with a 10−6 M R6G solution. The observed spectral bands matched the Raman spectrum of R6G, and characteristic bands were assigned (see ESI Table S1†). The SERS intensity was enhanced by approximately three orders of magnitude by increasing the number of Ag NP depositions from one to four. A significant SERS effect arises from the close-packed Ag NPs upon four deposition cycles. Particularly, the gaps between the aggregated Ag NPs generate abundant “hot spot” structures for SERS, which are homogeneously distributed on the substrate, making a robust and reproducible enhancement of the Raman signal feasible.
The enhancement factor (EF) for the four-layered substrate was calculated according to the widely accepted formula:
Environmental and biological sensing often requires rapid analysis of a large numbers of samples. Therefore, an array or multi-welled format (akin to 96 well plate format) would be advantageous for SERS applications in these fields.37 Utilising the plasticity of agarose gels, we shaped the substrate into a well-plate pattern. (see ESI Fig. S4†). An optical photo of the resulting substrate is displayed in the inset of Fig. 3a. A typical chip prepared this way contained 40 (2 × 2 mm) wells over a 3 × 2 cm area, each well can then serve as independent test areas for high throughput analysis. Compared to traditional SERS substrates, this well-plate pattern increases the loading capacity of the substrate and reduces the risk of contamination. Furthermore, the patterning of the substrate can be easily regulated by changing the shape of the mould.
 | ||
| Fig. 3 The reproducibility of the SERS signal on the array-based substrate. (a) Successively measured SERS spectra of 10−9 M R6G on 20 wells of the array based substrate. Inset: an optical photo of the array based substrate. (b) The SERS intensity distribution of the 1360 cm−1 band. The red line represents the average intensity of the 20 spectra, ±5% and ±5–10% intensity variation is marked with the yellow and green zones, respectively. | ||
It is noteworthy that the close-packed Ag NP structure improved the SERS performance in terms of enhancement activity and also in terms of signal reproducibility. To investigate the reproducibility of our SERS substrate, a 40 well plate substrate was incubated for 1 h in R6G solution (10−9 M); after rinsing with deionised water and drying, 20 wells on the substrate were randomly selected and their SERS spectra recorded. Fig. 3a shows that the signal intensities from the different wells were relatively consistent. The SERS intensity distribution for the 1360 cm−1 band of R6G is shown in Fig. 3b, which indicated that of the total 20 spectra 15 displayed an intensity variation of less than 5%, while 4 of the remaining 5 spectra were within 10%. These results demonstrate the uniformity of the substrate across the well-plate platform and that it was sufficient to provide reproducible SERS measurements, highlighting the advantages of the present quantitative analysis technique.
To further evaluate the influence of the reproducibility resulting from the deposition process, we also examined the reproducibility of the SERS signal on the substrates with one, two and three layers of Ag NPs (see ESI Fig. S5†). This analysis clearly demonstrated that both intensity and the uniformity of the SERS signal were significantly improved as the number of Ag NP deposition cycles was increased.
The longer term stability (or shelf life) of the prepared substrates was investigated by taking SERS measurements over a 6 month period, over which time the SERS intensities of the R6G solution remained constant (see ESI Fig. S6†). The longer-term stability, as well as the high throughput potential and low cost, making this substrate manifold a promising option for practical sensing applications.
In addition to the glass slide used thus far, the proposed approach could be extended to other solid supports. In proof-of-principle experiments, SERS substrates on polyvinyl chloride (PVC) plates and paper tapes using the same procedure were prepared. Agarose films were coated onto the cleaned supports and modified by APTES followed by multiple depositions of Ag NPs. Fig. 4a shows the SERS spectra of a 1 × 10−7 M R6G solution acquired from both PVC and paper substrates; optical images are shown in the inset. For the same sample, the spectra from the two substrates were similar to each other, except for minor intensity fluctuations. These results imply that spectral differences caused by various supports can be eliminated due to the isolation effect of the agarose film.
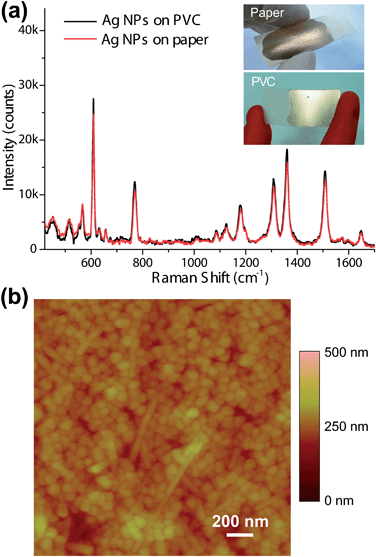 | ||
| Fig. 4 SERS performance and characterization of the substrate constructed on other solid supports. (a) SERS spectra of 10−7 M R6G on prepared substrates using paper tape (red curve) and PVC plate (black curve) as solid supports. Insets: optical photos of the substrates constructed on paper tape (up) and PVC plate (bottom). (b) An AFM image of the prepared substrate constructed on PVC plate. | ||
The surface morphology of the Ag NPs/APTES/Agar film on PVC plate was characterised by atomic force microscopy (AFM) and is presented in Fig. 4b. The close-packed structure of the Ag NPs was confirmed by analysis of AFM images. The density and uniformity of the Ag NP layer was not influenced by changing the solid support as judged by comparison of SEM images in Fig. 2a.
To study the relationship between SERS intensity and the analyte concentration in SERS measurements, 4-aminobenzoic acid (4-ABA) and 4-aminothiophenol (4-ATP) were selected as model analytes and samples of each prepared at concentrations of 0.5, 1, 5, 10, 50 and 100 nM. Samples were transferred to the wells of the plate-based substrate, and the SERS spectra were collected by scanning the laser across the substrate; the measurements were repeated three times for each sample, and the obtained SERS spectra were compared with the Raman spectra of the solid samples (see ESI Figs S7 and S8†). Fig. 5a and b show the SERS spectra of 4-ATP and 4-ABA as a function of concentration, respectively. At all vibrational bands, the SERS intensity mapped onto the variation in sample concentration. Among them, the intensities of two representative bands are plotted against the log of the concentrations. (Fig. 5c and d) A linear relation is apparent over the range 0.5–50 nM for 4-ATP and over the range of 1 nM and 0.1 μM for 4-ABA, which indicated that quantitative analysis of some organic small molecules could be performed on this plate format SERS substrate with ultra-sensitivity and high throughput.
 | ||
| Fig. 5 (a) SERS spectra of 4-aminothiophenol (4-ATP) at concentrations of 5 × 10−10, 1 × 10−9, 5 × 10−9, 1 × 10−8 and 5 × 10−8 M, respectively. (b) SERS spectra of 4-aminobenzoic acid (4-ABA) at concentrations of 1 × 10−9, 5 × 10−9, 1 × 10−8, 5 × 10−8 and 1 × 10−7 M, respectively. The concentration dependence of the SERS intensity at two bands for (c) 4-ATP and for (d) 4-ABA. | ||
Conclusions
In summary, we have demonstrated a simple yet robust method for multiple depositions of Ag NPs on an agarose film as a SERS-active structure. The fabrication route was optimised to obtain a reliable SERS substrate with both sensitivity and reproducibility. A well plate array-based patterning of the substrate improved the loading capacity and accuracy for high-throughput assays. Taking advantage of the chemical and physical properties of the agarose film, the substrate was successfully constructed on glass, PVC and paper supports, and the spectral differences caused by various supports were effectively excluded due to the agarose thin film. The analysis of 4-ATP and 4-ABA in a series of concentrations verified that the small organic molecules with SERS activity could be quantitatively identified with high efficiency using the well-plate shaped substrate. Combined with the development of miniaturised Raman spectrometers,10,38,39 the system reported herein could be produced on the basis of future commercially viable devices for on-site environmental monitoring and other routine SERS applications.Acknowledgements
This research was supported by the National Nature Science Foundation of China (91027035, 21007015), the National Science Fund for Distinguished Young Scholars (21125522), and the Fundamental Research Funds for the Central Universities (WK1013002). YTL is supported by the Program for Professor of Special Appointment (Eastern Scholar) at the Shanghai Institutions of Higher Learning. JSF thanks East China University of Science and Technology for a visiting professorship, the University of Birmingham and ERDF AWM II. The authors thank the Catalysis and Sensing for our Environment (CASE) network.Notes and references
- J. F. Li, Y. F. Huang, Y. Ding, Z. L. Yang, S. B. Li, X. S. Zhou, F. R. Fan, W. Zhang, Z. Y. Zhou, D. Y. Wu, B. Ren, Z. L. Wang and Z. Q. Tian, Nature, 2010, 464, 392–395 CrossRef CAS.
- S. M. Nie and S. R. Emory, Science, 1997, 275, 1102–1106 CrossRef CAS.
- H. Kim, K. M. Kosuda, R. P. Van Duyne and P. C. Stair, Chem. Soc. Rev., 2010, 39, 4802–4844 RSC.
- I. A. Larmour, K. Faulds and D. Graham, Chem. Sci., 2010, 1, 151–160 RSC.
- D. Li, D. W. Li, J. S. Fossey and Y. T. Long, Phys. Chem. Chem. Phys., 2011, 13, 2259–2265 RSC.
- S. P. Song, Y. Qin, Y. He, Q. Huang, C. H. Fan and H. Y. Chen, Chem. Soc. Rev., 2010, 39, 4234–4243 RSC.
- Z. L. Zhang, Y. Q. Wen, Y. Ma, J. Luo, L. Jiang and Y. L. Song, Chem. Commun., 2011, 47, 7407–7409 RSC.
- R. A. Álvarez-Puebla and L. M. Liz-Marzán, Energy Environ. Sci., 2010, 3, 1011–1017 Search PubMed.
- D. W. Li, L. L. Qu, W. L. Zhai, J. Q. Xue, J. S. Fossey and Y. T. Long, Environ. Sci. Technol., 2011, 45, 4046–4052 CAS.
- Y. F. Xie, X. Wang, X. X. Han, X. X. Xue, W. Ji, Z. H. Qi, J. Q. Liu, B. Zhao and Y. Ozaki, Analyst, 2010, 135, 1389–1394 RSC.
- P. L. Stiles, J. A. Dieringer, N. C. Shah and R. P. Van Duyne, Annu. Rev. Anal. Chem., 2008, 1, 601–626 CrossRef CAS.
- X. M. Lin, Y. Cui, Y. H. Xu, B. Ren and Z. Q. Tian, Anal. Bioanal. Chem., 2009, 394, 1729–1745 CrossRef CAS.
- Z. Q. Tian, B. Ren, J. F. Li and Z. L. Yang, Chem. Commun., 2007,(34), 3514–3534 RSC.
- Z. Q. Tian, B. Ren and D. Y. Wu, J. Phys. Chem. B, 2002, 106, 9463–9483 CrossRef CAS.
- M. Pagannone, L. G. Quagliano, L. Mattioli and G. Mattei, J. Raman Spectrosc., 1991, 22, 825–829 CrossRef CAS.
- J. N. Anker, W. P. Hall, O. Lyandres, N. C. Shah, J. Zhao and R. P. Van Duyne, Nat. Mater., 2008, 7, 442–453 CrossRef CAS.
- A. Tao, P. Sinsermsuksakul and P. D. Yang, Nat. Nanotechnol., 2007, 2, 435–440 CrossRef CAS.
- H. Cho, B. Lee, G. L. Liu, A. Agarwal and L. P. Lee, Lab Chip, 2009, 9, 3360–3363 RSC.
- X. Y. Zhang, J. Zhao, A. V. Whitney, J. W. Elam and R. P. Van Duyne, J. Am. Chem. Soc., 2006, 138, 10304–10309 CrossRef.
- O. S. Ivanova and F. P. Zamborini, J. Am. Chem. Soc., 2010, 132, 70–72 CrossRef CAS.
- Y. Shan, J. J. Xu and H. Y. Chen, Nanoscale, 2011, 3, 2916–2923 RSC.
- S. A. Elfeky, F. D'Hooge, L. Poncel, W. Chen, S. P. Perera, J. H. van den Elsen, T. D. James, A. T. Jenkins, P. J. Cameron and J. S. Fossey, New J. Chem., 2009, 33, 1466–1469 RSC.
- V. K. S. Hsiao, J. R. Waldeisen, Y. B. Zhang, P. F. Lloyd, T. J. Bunning and T. J. Huang, J. Mater. Chem., 2007, 17, 4896–4901 RSC.
- K. C. Grabar, P. C. Smith, M. D. Musick, J. A. Davis, D. G. Walter, M. A. Jackson, A. P. Guthrie and M. J. Natan, J. Am. Chem. Soc., 1996, 118, 1148–1153 CrossRef CAS.
- M. K. Fan, G. F. S. Andrade and A. G. Brolo, Anal. Chim. Acta, 2011, 693, 7–25 CrossRef CAS.
- P. Aldeanueva-Potel, E. Faoucher, R. A. Álvarez-Puebla, L. M. Liz-Marzán and M. Brust, Anal. Chem., 2009, 81, 9233–9238 CrossRef CAS.
- S. Abalde-Cela, B. Auguie, M. Fischlechner, W. T. S. Huck, R. A. Álvarez-Puebla, L. M. Liz-Marzán and C. Abell, Soft Matter, 2011, 7, 1321–1325 RSC.
- R. Contreras-Caceres, S. Abalde-Cela, P. Guardia-Giros, A. Fernandez-Barbero, J. Perez-Juste, R. A. Álvarez-Puebla and L. M. Liz-Marzán, Langmuir, 2011, 27, 4520–4525 CrossRef CAS.
- P. C. Lee and D. Meise, J. Phys. Chem., 1982, 86, 3391–3395 CrossRef CAS.
- G. F. S. Andrade, M. K. Fan and A. G. Brolo, Biosens. Bioelectron., 2010, 25, 2270–2275 CrossRef CAS.
- M. W. Meyer and E. A. Smith, Analyst, 2011, 136, 3542–3549 RSC.
- M. K. Fan and A. G. Brolo, Phys. Chem. Chem. Phys., 2009, 11, 7381–7389 RSC.
- W. B. Cai, B. Ren, X. Q. Li, C. X. She, F. M. Liu, X. W. Cai and Z. Q. Tian, Surf. Sci., 1998, 406, 9–22 CrossRef CAS.
- K. W. Kho, Z. X. Shen, H. C. Zeng, K. C. Soo and M. Olivo, Anal. Chem., 2005, 77, 7462–7471 CrossRef CAS.
- D. Li, D. W. Li, Y. Li, J. S. Fossey and Y. T. Long, J. Mater. Chem., 2010, 20, 3688–3693 RSC.
- Y. Su, Q. He, X. H. Yan, J. B. Fei, Y. Cui and J. B. Li, Chem.–Eur. J., 2011, 17, 3370–3375 CrossRef CAS.
- D. Y. Yang, X. Liu, Y. Jin, Y. Zhu, D. D. Zeng, X. Y. Jiang and H. W. Ma, Biomacromolecules, 2009, 10, 3335–3340 CrossRef CAS.
- D. Li, D. W. Li, J. S. Fossey and Y. T. Long, Anal. Chem., 2010, 82, 9299–9305 CrossRef CAS.
- A. M. Mohs, M. C. Mancini, S. Singhal, J. M. Provenzale, B. Leyland-Jones, M. D. Wang and S. M. Nie, Anal. Chem., 2010, 82, 9058–9065 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: the chemical structure of agarose, the SEM characterization of the synthesised Ag NPs, the calculation of the EF, the stability of the SERS substrate. See DOI: 10.1039/c1nr10956a |
| This journal is © The Royal Society of Chemistry 2012 |

