Construction and adsorption properties of microporous tetrazine-based organic frameworks†
Da-Shuai
Zhang
,
Ze
Chang
,
Ying-Bin
Lv
,
Tong-Liang
Hu
and
Xian-He
Bu
*
Department of Chemistry and Tianjin Key Lab on Metal and Molecule-based Material Chemistry, Nankai University, Tianjin, 300071, China. E-mail: buxh@nankai.edu.cn; Fax: +86-22-23502458
First published on 9th November 2011
Abstract
A new class of porous organic materials, tetrazine-based organic frameworks (TzFs), was successfully synthesized and characterized. These materials show narrow micropore size distributions and controllable pore volume affected by different reaction conditions. Also, the tetrazine backbone enhanced the H2 capability of these materials.
Owing to their excellent applications in catalysis,1 gas storage,2 and separation,3 porous materials have received a great deal of attention for many years. Among the most promising porous materials in this field, the past few years have witnessed an increasing number of novel porous materials constructed by pure organic building blocks through various types of reactions, such as covalent organic frameworks (COFs),4 hypercrosslinked polymers (HCPs),5 soluble and crosslinked polymers of intrinsic microporosity (PIMs),6 conjugated microporous polymers (CMPs),7 and porous polymer networks (PPNs).8 More recently, Antonietti and Thomas' group have described the synthesis of crystalline triazine-based organic frameworks (CTFs).9 Also, Zhu's group presented the targeted synthesis of porous aromatic frameworks (PAFs).10 Compared with traditional porous materials, the highly cross-linked nature of these pure organic materials confers their high thermal stability as well as the flexibility for the material design to achieve desirable pore properties.
Though the organic porous materials could achieve high surface area and pore volume with their pure organic backbones, the affinity between gas molecules and framework is relatively low as highly active site (for example, metal node or accessible metal site) is absent in these materials.10c,11 To improve the isosteric heats of gas adsorption, the introduction of heteroatom into framework is proved to be an efficient method.12 In our efforts to find simple and easy way to introduce heteroatoms into organic frameworks, we noticed that s-tetrazine could be an ideal building block. With accessible formation of tetrazine ring under mild condition using nitrile and hydrazine as reactants, N atoms could be introduced into the polymer as well as the construction of the framework backbone. Furthermore, as s-tetrazine has rich and varied physical chemistry that make them applicable in various domains,13 porous organic framework with s-tetrazine backbone may have wide applications as well. Motivated by the above method, we tried to fabricate porous organic frameworks with tetrazine backbone. Herein, we report the synthesis and characterization of microporous tetrazine-based organic frameworks (TzFs) (Scheme 1). The resulted TzFs show narrow pore size distributions and controllable pore volume influenced by reaction conditions. In addition, gas adsorption properties of these materials were studied as well, and the results show increased interaction between the H2 gas molecules and framework.
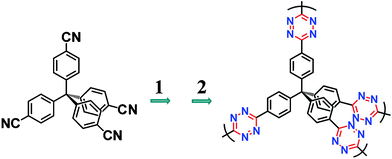 | ||
| Scheme 1 General routes of the synthesis of TzFs. Reaction conditions: 1. hydrazine hydrate and sulphur/solvent. 2. AcOH and NaNO2 solution. | ||
By a two-step formation of the s-tetrazine rings,16 a series of TzFs (Table S1†) were synthesized from tetrahedral tetrakis(4-cyanatophenyl)methane (TCPM) monomer and hydrazine hydrate with sulphur as catalyst under different conditions (Scheme 1 and Scheme S1†). To optimize the reaction conditions, solvents, solvent ratio, reaction time, and reaction temperature were put into our considerations. The pore parameters of these materials (Table S2)† indicate that solvent is the key influence factor for the formation of the frameworks. Take the synthesis of TzF-7 as an example, the addition of ethane-1,2-diol as solvent did not only accelerated the formation of the framework (overnight), but also remarkably increased the surface area and pore volume of the material (Table S2)†.
The structure of TzFs was characterized by FT-IR, solid 13C NMR spectra, elemental analyses (EA), X-ray power diffraction (XRPD) and scanning electron microscope (SEM). As shown in the FT-IR spectrum (Figure S3a)†, the disappearance of the intense nitrile band at 2218 cm−1 suggests a successful polytetrazine reaction, while the appearance of the strong bands at 1604 and 1396 cm−1 suggest the formation of tetrazine rings. Solid 13C NMR experiment can also provide valuable information (Figure S4)†. The major peaks observed at ca. 61, 125, and 145 ppm could be attributed the C atoms in the TCPM monomer, while the minor peaks at ca. 158 and 163 ppm could be ascribed to the carbon within the inside and terminal tetrazine rings, respectively. Also, the absence of signal at 119 ppm, which belongs to the C atom in -CN group, further confirmed the full transformation of -CN groups. Furthermore, XRPD and SEM results confirm that the obtained TzFs are amorphous solid without a well-ordered shape (Figure S2† and Fig. 1). Also, the obvious distinction of the SEM results for TzFs (TzF-6: hydrazine as solvent; TzF-7: ethane-1,2-diol added in reaction) confirm the importance of solvent to the structure of the obtained materials and EA data for TzFs (TzF-6: C 63.58, H 5.44, N 13.95; TzF-7: C 62.59, H 4.99, N 14.43) suggest they should be different compounds with similar structures.
To confirm the stability of TzFs, thermogravimetric analysis (TGA) was performed (Figure S3b)†. The result indicates that the framework of TzF could be stable up to 217 °C before the collapse of the framework. The 5.4% loss of weight between 217 and 346 °C should be attributed to the decomposition of s-tetrazine rings into nitriles and N2 molecules, which agrees well with the experimental data reported before.14 This decomposable characteristic makes the TzFs eco-friendly and easy to recycle.
In order to comprehend the porosity of TzFs shown here, gas sorption properties of TzF-6 and TzF-7 were investigated at low pressure. As Fig. 2a shows, the nitrogen adsorption isotherms obtained at 77 K for both TzF-6 and TzF-7 show type I behavior, which indicates the microporous nature of these materials. The Brunauer–Emmett–Teller (BET) and Langmuir methods were used to analyze these curves. The analysis reveals that TzF-7 exhibits higher BET and Langmuir surface areas (571 cm2 g−1 and 764 cm2 g−1, respectively) and larger pore volume (1.09 cm3 g−1). On the other hand, fitting the nitrogen isotherm of TzF-6 gives the BET and Langmuir surface areas as 444 and 593 cm2 g−1 and a total pore volume of 0.46 cm3 g−1. The higher surface area and pore volume of TzF-7 might be ascribed to the more loose surface (Fig. 1b) than that of TzF-6 (Fig. 1a). In TzF-6, the desorption curve exhibits a hysteresis, which might be caused by the mesopore structure in the framework. Pore size distribution curves for both TzF-6 and TzF-7 were obtained by analysing the N2 adsorption isotherms using non-local density functional theory (NL-DFT), and the results prove the narrow pore size distributions and different pore sizes in TzF-6 and TzF-7 (Fig. 2a, inset). It should be noticed that there are significant differences between their pore size distributions, which well-supported by the fact mentioned above that pore volume is affected by different reaction conditions.
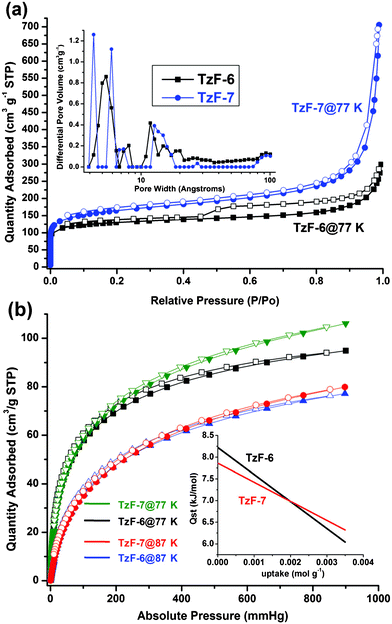 | ||
| Fig. 2 Nitrogen adsorption/desorption isotherms of TzF-6 and TzF-7 at 77 K (a): black, TzF-6 and blue, TzF-7), and inset: micropore size distribution of them (black, TzF-6 and blue, TzF-7). Hydrogen adsorption and desorption isotherms of TzF-6 and TzF-7 at 77 K and 87 K (b): black, TzF-6 at 77 K and blue, TzF-6 at 87 K; olive, TzF-7 at 77 K and red, TzF-7 at 87 K, and inset: the high value and slow decrease of the heat of adsorption (black, TzF-6 and red, TzF-7). (Adsorption branch is labelled with filled symbols). | ||
Hydrogen adsorption and desorption isotherms were also measured to explore the potential gas storage application of TzFs. Fig. 2b shows the similar sorption curves of TzF-6 and TzF-7 at 77 K and 87 K with typical type-I behaviour. The hydrogen adsorption isotherms at 77 K reveal an uptake of 94 cm3 g−1 for TzF-6 and 106 cm3 g−1 for TzF-7 at 900 mmHg, respectively. To calculate the H2 isosteric heat of adsorption of TzF-6 and TzF-7, the hydrogen adsorption data were analyzed using a Virial Method15 (see ESI for details†) from the respective adsorption isotherms at 77 K and 87 K. The isosteric heat of adsorption is 8.2–6.0 kJ mol−1 for TzF-6 and 7.8–6.3 kJ mol−1 for TzF-7, respectively, depending on the degree of H2 loading (Fig. 2b, inset). These values are comparable to many other porous organic polymers,10c,11–12 and approached the usual range of MOFs.16 The relatively high initial value and slowly decreasing of the value indicate a strong interaction between the adsorbed hydrogen molecules and framework. For further interpretation, the Virial parameters obtained by fitting were analyzed (see Figure S8 and Figure S9)†. The results reveal that the A1 values are very negative (−840.9 and −729.0 g mol−1 for TzF-6 at 77 K and 87 K, respectively; −765.3 and −687.2 g mol−1 for TzF-7 at 77 K and 87 K, respectively), which suggests a strong H2–H2 interaction inside the framework. On the other hand, the relatively low A0 values (−13.3 and −14.8 ln (mol g−1Pa−1) for TzF-6 at 77 K and 87 K, respectively; −13.5 and −14.9 ln (mol g−1Pa−1) for TzF-7 at 77 K and 87 K, respectively) suggest a strong H2-framwork interaction which might be attributed to the tetrazine rings decorated pores.17 Additionally, it should be noted that TzF-6 has a higher Qst value than that of TzF-7, the reason of which may be given by the more negative A1 values for TzF-6 than that of TzF-7. Also, it can be seen that TzF-6 shows single pore size distribution (Fig. 2a, inset, black) at 4.6–6.4 Å, just equal to about two H2 molecules (2 × 2.8 Å), while TzF-7 shows double peaks (Fig. 2a, inset, blue) at 4.0–4.3 Å and 5.8–6.0 Å, just the latter suitable for two H2 molecules. These results coincide with the less negative A0 values of TzF-6 than that of TzF-7. As a result, the more negative A1 values and less negative A0 values demonstrate TzF-6 has stronger H2–H2 and H2–framework interactions than that of TzF-7. These distinctions are in accord with the fact discussed earlier that pore size distributions of them are significantly different, in other words, pore volume of TzFs can be well controlled within a certain range by different reaction conditions. Furthermore, we explore the storage capability of TzF-7 for other relative gases, including O2, CO2 and CH4. For O2, the adsorption experiment shows similar behaviour to that of N2 adsorption at 77 K (Figure S7†). For CO2, a moderate amount of adsorption could be achieved at 273 K and 303 K, however, only a small quantity of CH4 could be adsorbed in the same conditions. These adsorption properties may be applicable for CO2 capture or gas purification.18
In summary, a class of unprecedented porous organic frameworks based on tetrazine backbone was successfully synthesized in a facile approach. Different reaction mixtures were considered to be the main origin of different pore volume and pore size distributions among the TzFs. Also, these materials show enhanced hydrogen adsorptions due to the tetrazine rings decorated pores. Given the promising perspective of these novel materials, we are currently engaged in optimizing the reaction conditions and modifying the frameworks by Diels–Alder cycloaddition reactions. Further studies of this work are underway in our lab.
Acknowledgements
The financial support from the 973 Program (2012CB821703), NSFC (21031002 and 51073079) and the NSF of Tianjin, China (10JCZDJC22100).References
- (a) L. Ma, J. M. Falkowski, C. Abney and W. Lin, Nat. Chem., 2010, 2, 838–846 CrossRef CAS; (b) J. S. Seo, D. Whang, H. Lee, S. I. Jun, J. Oh, Y. J. Jeon and K. Kim, Nature, 2000, 404, 982–986 CrossRef CAS; (c) X. Du, Y. Sun, B. Tan, Q. Teng, X. Yao, C. Su and W. Wang, Chem. Commun., 2010, 46, 970–972 RSC.
- (a) M. Eddaoudi, J. Kim, N. Rosi, D. Vodak, J. Wachter, M. O'Keeffe and O. M. Yaghi, Science, 2002, 295, 469–472 CrossRef CAS; (b) J.-R. Li, R. J. Kuppler and H.-C. Zhou, Chem. Soc. Rev., 2009, 38, 1477–1504 RSC.
- (a) B. Chen, C. Liang, J. Yang, D. S. Contreras, Y. L. Clancy, E. B. Lobkovsky, O. M. Yaghi and S. Dai, Angew. Chem., Int. Ed., 2006, 45, 1390–1393 CrossRef CAS; (b) O. Kanie, I. Ohtsuka, T. Ako, S. Daikoku, Y. Kanie and R. Kato, Angew. Chem., Int. Ed., 2006, 45, 3851–3854 CrossRef CAS.
- (a) H. M. El-Kaderi, J. R. Hunt, J. L. Mendoza-Cortes, A. P. Cote, R. E. Taylor, M. O'Keeffe and O. M. Yaghi, Science, 2007, 316, 268–272 CrossRef CAS; (b) X. Feng, L. Chen, Y. Dong and D. Jiang, Chem. Commun., 2011, 47, 1979–1981 RSC; (c) E. L. Spitler, M. R. Giovino, S. L. White and W. R. Dichtel, Chem. Sci., 2011, 2, 1588–1593 RSC.
- (a) J. Germain, J. Hradil, J. M. J. Fréchet and F. Svec, Chem. Mater., 2006, 18, 4430–4435 CrossRef CAS; (b) J.-Y. Lee, C. D. Wood, D. Bradshaw, M. J. Rosseinsky and A. I. Cooper, Chem. Commun., 2006, 2670–2672 RSC; (c) M. P. Tsyurupa and V. A. Davankov, React. Funct. Polym., 2006, 66, 768–779 CrossRef CAS; (d) C. D. Wood, B. Tan, A. Trewin, H. Niu, D. Bradshaw, M. J. Rosseinsky, Y. Z. Khimyak, N. L. Campbell, R. Kirk, E. Stöckel and A. I. Cooper, Chem. Mater., 2007, 19, 2034–2048 CrossRef CAS.
- (a) B. S. Ghanem, K. J. Msayib, N. B. McKeown, K. D. M. Harris, Z. Pan, P. M. Budd, A. Butler, J. Selbie, D. Book and A. Walton, Chem. Commun., 2007, 67 RSC; (b) N. B. McKeown, P. M. Budd, K. J. Msayib, B. S. Ghanem, H. J. Kingston, C. E. Tattershall, S. Makhseed, K. J. Reynolds and D. Fritsch, Chem.–Eur. J., 2005, 11, 2610–2620 CrossRef CAS; (c) N. B. McKeown, B. Gahnem, K. J. Msayib, P. M. Budd, C. E. Tattershall, K. Mahmood, S. Tan, D. Book, H. W. Langmi and A. Walton, Angew. Chem., Int. Ed., 2006, 45, 1804–1807 CrossRef CAS.
- (a) A. I. Cooper, Adv. Mater., 2009, 21, 1291–1295 CrossRef CAS; (b) J.-X. Jiang, F. Su, A. Trewin, C. D. Wood, N. L. Campbell, H. Niu, C. Dickinson, A. Y. Ganin, M. J. Rosseinsky, Y. Z. Khimyak and A. I. Cooper, Angew. Chem., Int. Ed., 2007, 46, 8574–8578 CrossRef CAS; (c) J.-X. Jiang, F. Su, A. Trewin, C. D. Wood, H. Niu, J. T. A. Jones, Y. Z. Khimyak and A. I. Cooper, J. Am. Chem. Soc., 2008, 130, 7710–7720 CrossRef CAS; (d) E. Stöckel, X. Wu, A. Trewin, C. D. Wood, R. Clowes, N. L. Campbell, J. T. A. Jones, Y. Z. Khimyak, D. J. Adams and A. I. Cooper, Chem. Commun., 2009, 212 RSC; (e) R. Dawson, D. J. Adams and A. I. Cooper, Chem. Sci., 2011, 2, 1173 RSC.
- W. Lu, D. Yuan, D. Zhao, C. I. Schilling, O. Plietzsch, T. Muller, S. Bräse, J. Guenther, J. Blümel, R. Krishna, Z. Li and H.-C. Zhou, Chem. Mater., 2010, 22, 5964–5972 CrossRef CAS.
- (a) M. J. Bojdys, J. Jeromenok, A. Thomas and M. Antonietti, Adv. Mater., 2010, 22, 2202–2205 CrossRef CAS; (b) P. Kuhn, M. Antonietti and A. Thomas, Angew. Chem., Int. Ed., 2008, 47, 3450–3453 CrossRef CAS; (c) P. Kuhn, A. l. Forget, D. Su, A. Thomas and M. Antonietti, J. Am. Chem. Soc., 2008, 130, 13333–13337 CrossRef CAS.
- (a) H. Ren, T. Ben, E. Wang, X. Jing, M. Xue, B. Liu, Y. Cui, S. Qiu and G. Zhu, Chem. Commun., 2010, 46, 291–293 RSC; (b) J. H. Lan, D. P. Cao, W. C. Wang, T. Ben and G. S. Zhu, J. Phys. Chem. Lett., 2010, 1, 978–981 CrossRef CAS; (c) T. Ben, H. Ren, S. Ma, D. Cao, J. Lan, X. Jing, W. Wang, J. Xu, F. Deng, J. M. Simmons, S. Qiu and G. Zhu, Angew. Chem., Int. Ed., 2009, 48, 9457–9460 CrossRef CAS.
- H. Furukawa and O. M. Yaghi, J. Am. Chem. Soc., 2009, 131, 8875–8883 CrossRef CAS.
- (a) P. Pandey, A. P. Katsoulidis, I. Eryazici, Y. Wu, M. G. Kanatzidis and S. T. Nguyen, Chem. Mater., 2010, 22, 4974–4979 CrossRef CAS; (b) D. C. Zhong, J. B. Lin, W. G. Lu, L. Jiang and T. B. Lu, Inorg. Chem., 2009, 48, 8656–8658 CrossRef CAS.
- G. Clavier and P. Audebert, Chem. Rev., 2010, 110, 3299–3314 CrossRef CAS.
- R. A. Carboni and R. V. Lindsey, J. Am. Chem. Soc., 1959, 81, 4342–4346 CrossRef CAS.
- (a) J. H. Cole, D. H. Everett, C. T. Marshall, A. R. Paniego, J. C. Powl and F. Rodriguez-Reinoso, Chem. Soc., Faraday Trans. 1: Phys. Chem. Condens. Phases, 1974, 70, 2154–2169 CAS; (b) I. P. O'Koye, M. Benham and K. M. Thomas, Langmuir, 1997, 13, 4054–4059 CrossRef CAS; (c) C. R. Reid, I. P. O'Koy and K. M. Thomas, Langmuir, 1998, 14, 2415–2425 CrossRef CAS; (d) C. R. Reid and K. M. Thomas, Langmuir, 1999, 15, 3206–3218 CrossRef CAS.
- (a) Z. Guo, G. Li, L. Zhou, S. Su, Y. Lei, S. Dang and H. Zhang, Inorg. Chem., 2009, 48, 8069–8071 CrossRef CAS; (b) M. Dincă, A. Dailly, C. Tsay and J. R. Long, Inorg. Chem., 2008, 47, 11–13 CrossRef; (c) B. Schmitz, U. Muller, N. Trukhan, M. Schubert, G. Férey and M. Hirscher, ChemPhysChem, 2008, 9, 2181–2184 CrossRef CAS; (d) Z. Y. Guo, G. H. Li, L. Zhou, S. Q. Su, Y. Q. Lei, S. Dang and H. J. Zhang, Inorg. Chem., 2009, 48, 8069–8071 CrossRef CAS; (e) A. J. Lan, K. H. Li, H. H. Wu, L. Z. Kong, N. Nijem, D. H. Olson, T. J. Emge, Y. J. Chabal, D. C. Langreth, M. C. Hong and J. Li, Inorg. Chem., 2009, 48, 7165–7173 CrossRef CAS; (f) Z. Chang, D.-S. Zhang, T.-L. Hu and X.-H. Bu, Cryst. Growth Des., 2011, 11, 2050–2053 CrossRef CAS.
- Z. Chang, D.-S. Zhang, Q. Chen, R.-F. Li, T.-L. Hu and X.-H. Bu, Inorg. Chem., 2011, 50, 7555–7562 CrossRef CAS.
- (a) Z. Wang, B. Zhang, H. Yu, L. Sun, C. Jiao and W. Liu, Chem. Commun., 2010, 46, 7730–7732 RSC; (b) X. Si, C. Jiao, F. Li, J. Zhang, S. Wang, S. Liu, Z. Li, L. Sun, F. Xu, Z. Gabelica and C. Schick, Energy Environ. Sci., 2011, 4, 4522–4527 RSC.
Footnote |
| † Electronic Supplementary Information (ESI) available: experimental details, characterization of the materials and gas sorption isotherms. See DOI: 10.1039/c1ra00571e/ |
| This journal is © The Royal Society of Chemistry 2012 |

