Effect of electrodeposition temperature on the electrochemical performance of a Ni(OH)2 electrode
Yin-Mei
Wang
,
Dan-Dan
Zhao
,
Yong-Qing
Zhao
*,
Cai-Ling
Xu
* and
Hu-Lin
Li
Key Laboratory of Nonferrous Metal Chemistry and Resources Utilization of Gansu Province, and College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou, 730000, P. R. China. E-mail: yqzhao@lzu.edu.cn; xucl@lzu.edu.cn; Fax: +86-931-891-2582; Tel: +86-931-891-2589
First published on 5th December 2011
Abstract
The effect of the electrodeposition temperature on the electrochemical performance of Ni(OH)2 electrode was investigated in this report. Ni(OH)2 was electrodeposited directly on nickel foam at different temperatures. The crystalline structure, morphology and specific surface area of the prepared Ni(OH)2 were characterized by X-ray powder diffraction (XRD), field emission scanning electronic microscopy (FESEM) and Brunauer–Emmett–Teller (BET). Electrochemical techniques such as cyclic voltammetry (CV), chronopotentiometry, and electrochemical impedance spectra (EIS) were carried out to systematically study the electrochemical performance of various Ni(OH)2 electrodes in 1 M KOH electrolyte. The results demonstrated that the electrodeposition temperature had obviously affected the properties of the Ni(OH)2. A pure α-Ni(OH)2 phase could be observed at low temperature. When the temperature increased to 65 °C, the β-Ni(OH)2 phase together with α-Ni(OH)2 phase were present. Moreover, the sample synthesized at 65 °C possessed a porous honeycomb-like microstructure and the corresponding specific capacitance was up to 3357 F g−1 at a charge–discharge current density of 4 A g−1, which suggested its potential application as an electrode material for supercapacitors.
1. Introduction
Recently, Ni(OH)2 has attracted an increasing scientific and technological interest because it is widely used as the electrode material in nickel-based alkaline secondary batteries including nickel–metal hydride (Ni–MH), nickel–cadmium (Ni–Cd), nickel–zinc (Ni–Zn) and nickel–iron (Ni–Fe).1–4 In particular, Ni(OH)2 is also an attractive candidate for supercapacitor applications5–13 due to its high theoretical specific capacitance, well-defined redox behavior, and relatively low cost.5 For example, Cheng et al.14 reported a specific capacitance of 696 F g−1 for sol–gel-derived Ni(OH)2 xerogels synthesized by the sol–gel method. Lang et al.15 and Hu et al.16 obtained the loose-packed α-Ni(OH)2 for supercapacitors through a chemical precipitation method and a nanoporous Ni(OH)2 film using a chemical bath deposition method, with the specific capacitance of 2055 F g−1 and 2200 F g−1 at a discharging density of 0.625 A g−1 and 1 A g−1, respectively.In the process of fabricating the electrode, the method of depositing activated material on the substrate is one of the most important elements to affect the performance of the supercapacitor. Unlike the above conventional approaches for preparing Ni(OH)2 materials, the electrodeposition technique is an ideal method for synthesizing nanomaterials because of its unique principle and flexibility in the control of the structure and morphology of the materials.17–21 We have previously reported that a 3D Ni(OH)2 electrode can be prepared via an electrodeposition method.22 Ni(OH)2 coatings are directly electrodeposited on nickel foam and the nanocrystalline “α” phase of Ni(OH)2 is confirmed by the X-ray powder diffraction analysis. A maximum specific capacitance of 3152 F g−1 at 4 A g−1 is obtained.
According to previous literature,17,23–25 the effect of deposition conditions on Ni(OH)2 such as the deposition potential, the concentration of the electrolyte, the loading mass, and so forth, have been investigated. However, to the best of our knowledge, there is no report on the effect of the electrodeposition temperature on the electrochemical performance of Ni(OH)2 up to now. In this work, the electrodeposition temperature is believed to play a crucial role in the preparation of Ni(OH)2 films. Generally, Ni(OH)2 exists in two basic polymorphic forms: α-Ni(OH)2 and β-Ni(OH)2, which are transformed into γ-NiOOH and β-NiOOH, respectively, during charging.26 The main differences between the α- and β-type Ni(OH)2 phase reside in the different stacking of the layers and the interlamellar distances. It is reported that all α-Ni(OH)2 synthesized have a lower tap-density than β-Ni(OH)2.27–29 In this study, a pure α-Ni(OH)2 phase can be observed at low temperatures. When the temperature increased to 65 °C, the β-Ni(OH)2 phase together with the α-Ni(OH)2 phase are present. This suggests that a mixed structure of α-Ni(OH)2 and β-Ni(OH)2 with higher density is synthesized at 65 °C. At 65 °C, a specific capacitance as high as 3357 F g−1 is attained at a charge–discharge current density of 4 A g−1 in 1 M KOH electrolyte , which is rather high compared with those of the corresponding Ni(OH)2 materials at relatively low discharge current densities in the literature.5,16,23 The superior capacitive behaviour of the Ni(OH)2 electrode can be attributed to the high density biphase structure, the porous honeycomb-like surface morphology, the short ion diffusion path, and especially the highly enhanced BET surface area.
2. Experimental section
2.1 Preparation of Ni(OH)2 electrodes
All the reagents in this experiment were of analytical purity and used as received without further purification. Ni(OH)2 was directly electrodeposited on a piece of nickel foam (1 cm2 in area) from 0.1 M Ni(NO3)2 aqueous solution at a constant potential of −0.7 V vs. the saturated calomel electrode (SCE). The Ni(OH)2 samples prepared at different electrodeposition temperatures were labeled as NH20, NH30, NH40, NH50 and NH65, which corresponded to 20 °C, 30 °C, 40 °C, 50 °C and 65 °C, respectively. The mass of the Ni(OH)2 loading was about 0.7 mg estimated by Faraday's law, and the corresponding deposition charge quantity was approximately 1.1656 C. Before the electrodeposition, the nickel foam substrate was first rinsed with acetone and hydrochloric acid to clean and etch the metal surface. After deposition, the as-made Ni(OH)2 samples were washed several times with deionized water and dried naturally in the atmosphere to study.2.2 Materials characterization
The X-ray powder diffraction pattern (XRD; a Rigaku D/MAX 2400 diffractometer, Japan; monochromated Cu Kα radiation, k = 1.5418 Å; 40.0 kV, 60.0 mA) was used to characterize the crystalline structure of the Ni(OH)2 samples deposited at different temperatures. The surface morphologies of the as-prepared Ni(OH)2 materials were observed by means of field emission scanning electron microscopy (FESEM, JEOL JSM-4800). A nitrogen adsorption and desorption apparatus (Micromeritics ASAP 2010, America) was employed to investigate the surface area and the pore size distribution of the synthesized materials. All samples were outgassed at the temperature of 80 °C for 10 h under vacuum before measurements were recorded.2.3 Electrochemical measurements
The electrochemical properties of Ni(OH)2 electrodes were investigated in 1 M KOH electrolyte using a three-electrode system: the prepared Ni(OH)2 as the working electrode, a 1.5 cm × 1.5 cm platinum plate as auxiliary electrode and a standard Hg/HgO electrode as a reference electrode. Cyclic voltammetry (CV) was carried out between −0.05 to 0.8 V at scan rates varying from 2 to 50 mV s−1. The charge and discharge of the electrode was evaluated between 0.05 and 0.55 V at the charge/discharge current densities of 4, 8, 16, and 32 A g−1, respectively. Electrochemical impedance spectroscopy (EIS) of the prepared electrodes was performed at 0.25 V (near the circuit potential). Data were collected in the frequency range 10−2 to 105 Hz. All electrochemical experiments were conducted with a CHI660D workstation at room temperature (25 °C).3. Results and discussion
3.1 Powder X-ray diffraction (XRD) analysis
Fig. 1 shows the XRD spectra of Ni(OH)2 samples prepared at different temperatures. As can be seen, the XRD patterns of all Ni(OH)2 samples exhibit the characteristics of the α-Ni(OH)2 at 11.35°, 22.73°, 33.46°, 39.35° and 59.84° according to JCPDS card No.38–0715. However, when the temperature increased to 65 °C, the additional diffraction signals at 19.65°, 38.96°, 52.32°, 70.28° and 72.60° are also observed, which is coincident with the β-Ni(OH)2 (JCPDS No.14-0117). That is to say, the Ni(OH)2 sample obtained at 65 °C consists of α-Ni(OH)2 and β-Ni(OH)2 phases, but the samples obtained at lower temperatures were the pure phase of α-Ni(OH)2. The formation of the biphase Ni(OH)2 structure with high density makes it possible to exhibit excellent electrochemical performance for the NH65 sample.27–29 The difference in diffraction peaks between the Ni(OH)2 samples indicates that the deposition temperature has obviously affected the crystalline structure of the Ni(OH)2 samples.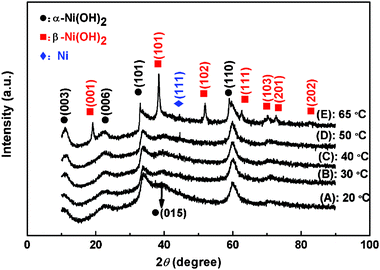 | ||
| Fig. 1 XRD patterns of Ni(OH)2 electrodes obtained at different electrodeposition temperatures: (A) 20 °C; (B) 30 °C; (C) 40 °C; (D) 50 °C; (E) 65 °C. | ||
3.2 Surface morphology
In order to investigate the Ni(OH)2 surface morphology evolution with the increase of electrodeposition temperature, the FESEM images of the Ni(OH)2 samples obtained at different temperatures are shown in Fig. 2. It was found that the deposition temperatures had a remarkable influence on the surface morphologies of the samples. The surface microstructures dramatically changed with increasing temperatures. The NH20 sample displayed a clear island-like structure composed of the very tiny fibers (Fig. 2a). Elevating the temperature to 30 °C, the Ni(OH)2 fibers disappeared. Meanwhile, the sample surface was prone to aggregate into large particles (Fig. 2b). When the electrodeposition temperature further increased to 40 °C (Fig. 2c) and 50 °C (Fig. 2d), the surface of samples could not find the apparent island-like structure, but existed as available cracks, and the fibers became smaller than that of the NH20 sample. Comparing the sample of NH40 with NH50, the fibers became more tenuous and the cracks became more regular with the increase of deposition temperature from 40 to 50 °C. Eventually, when the electrodeposition temperature rose to 65 °C (Fig. 2e), the cracks almost died away and the surface of the sample created a porous honeycomb-like microstructure, which probably led to a high specific surface area and provided the structural foundation for the excellent electrochemical performance.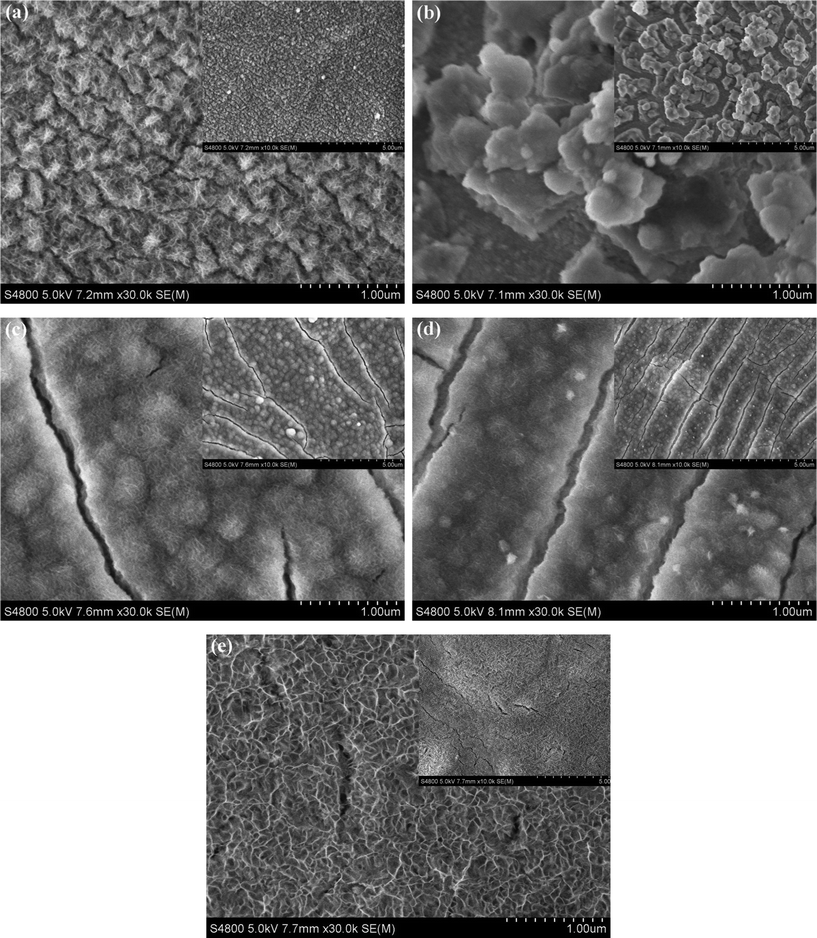 | ||
| Fig. 2 FESEM photographs of Ni(OH)2 electrodes obtained at different electrodeposited temperatures: (a) 20 °C; (b) 30 °C; (c) 40 °C; (d) 50 °C; (e) 65 °C. The insets are the corresponding figures at low magnification. | ||
3.3 Specific surface area analysis
Fig. 3 shows the nitrogen adsorption and desorption isotherms and the pore size distributions of Ni(OH)2 samples. The specific surface areas were calculated by employing the Brunauer–Emmett–Teller (BET) method and the pore size distributions (PSD) were obtained by means of the Barrett–Joyner–Halenda (BJH) equation using the adsorption isotherm branch. The BET specific surface areas of NH20, NH30, NH40, NH50 and NH65 samples were found to be 2.82, 2.66, 2.16, 6.06, and 80.53 m2 g−1, respectively. Analyses of the PSD revealed the pore size distributions of NH20, NH30, NH40, NH50 and NH65 samples were mainly in the diameter range 4.9–40.0, 6.4–48.1, 7.7–46.5, 6.3–48.6 and 2.0–15.0 nm, respectively. The sudden jump of the surface area of NH65 sample than that of others can be firstly assumed to be related to the smallest pore diameter.30 Moreover, based on the previous study,15,30 we presumed that the large difference in the surface areas of the Ni(OH)2 samples fabricated at different temperatures were resulting from the great difference among their surface morphologies. Meanwhile, the characteristic mesoporous structures of all the samples may be particularly suitable for use as the electrode material for supercapacitors because electrolytes can readily access and fill the inner-surface of the material to give a large electrode area.31 As a result, the surface areas and the pore properties of the prepared materials have always shown a corresponding impact on the electrochemical properties as discussed later.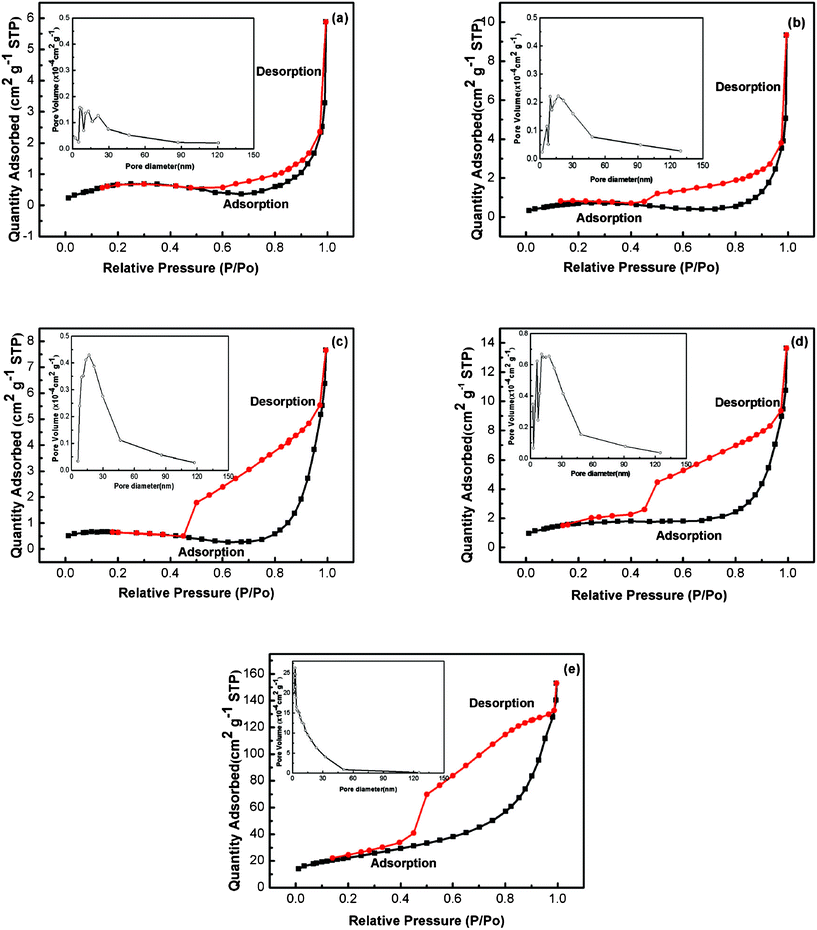 | ||
| Fig. 3 Nitrogen adsorption and desorption isotherms of Ni(OH)2 electrodes obtained at different electrodeposition temperatures: (a) 20 °C; (b) 30 °C; (c) 40 °C; (d) 50 °C; (e) 65 °C. The insets are the corresponding BJH pore size distributions derived from the adsorption branch. | ||
3.4 Electrochemical properties
Fig. 4 presents the CV curves of Ni(OH)2 samples within −0.05 to 0.8 V potential range at a scan rate of 20 mV s−1 in 1 M KOH electrolyte. For all the electrodes, one anodic oxidation peak and one cathodic reduction peak are observable on the CV curves, which is related to the reversible electron transfer process of Ni(OH)2 described by the following well-accepted reaction:32,33| α-Ni(OH)2 + OH− ↔ γ-NiOOH + H2O + e− | (1) |
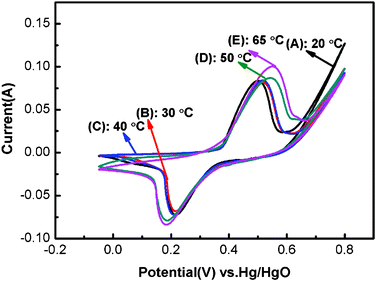 | ||
| Fig. 4 Cyclic voltammetry curves of Ni(OH)2 electrodes obtained at different electrodeposition temperatures within −0.05 to 0.8 V potential range at a scan rate of 20 mV s−1 in 1 M KOH electrolyte: (A) 20 °C; (B) 30 °C; (C) 40 °C; (D) 50 °C; (E) 65 °C. | ||
Additionally, a shoulder anodic peak is found on the CV curves of NH50 and NH65, which might be attributed to the change from β-Ni(OH)2 to β-NiOOH.10,34 According to the XRD analysis, the NH65 sample consists of α-Ni(OH)2 and β-Ni(OH)2 phase, therefore it is reasonable for the presence of a shoulder anodic peak. However, the β-Ni(OH)2 phase couldn't obviously be observed from the XRD pattern of NH50 sample, possibly due to the very low content. This is also consistent with the α-Ni(OH)2 being converted to γ-NiOOH at a lower potential than β-Ni(OH)2 to β-NiOOH.35 Moreover, the redox peak position of NH50 and NH65 samples is shifted to the positive and negative potentials, respectively. And the peak height is also higher than that of NH20, NH30 and NH40 samples, which gives rise to the large capacitance.
Generally, the potential difference (ΔEa,c) between the anodic and cathodic peak can be employed to characterize the reversibility of the redox reaction.36 Electrode materials with small ΔEa,c are desired to have excellent reversibility.37 Moreover, the charge process of the Ni(OH)2 electrode usually occurs in competition with a parasitic oxygen evolution reaction,38 which will obscure the basic line of oxidation peak current and limit the electrochemical performance of the Ni(OH)2 electrodes.29,38 Thus, the difference between the oxygen evolution potential and the oxidation peak potential (ΔEo,a) of the samples is also an important parameter for judging the performance of electrode materials. The large ΔEo,a value suggests the high charge efficiency of the electrode and the high utilization of active material before oxygen evolution during the charge–discharge.39 The details of CV curves are listed in Table 1.
| Sample | E pa/V | E pc/V | ΔEa,c/V | Eo2 | ΔEo,a |
|---|---|---|---|---|---|
| NH20 | 0.499 | 0.216 | 0.283 | 0.587 | 0.088 |
| NH30 | 0.515 | 0.215 | 0.300 | 0.613 | 0.098 |
| NH40 | 0.517 | 0.209 | 0.308 | 0.622 | 0.105 |
| NH50 | 0.543 | 0.187 | 0.356 | 0.653 | 0.110 |
| NH65 | 0.550 | 0.188 | 0.362 | 0.667 | 0.117 |
As can be seen in Table 1, the potential difference (ΔEa,c) is becoming larger from the NH20 to NH65 sample, which suggests that the charge–discharge process occurs less reversibly with the deposited temperature increasing from 20 to 65 °C. Moreover, we note that the oxygen evolution overpotential (ΔEo,a) is also shifted to a positive value from the NH20 to NH65 sample, thus more active material can be utilized during charge–discharge and the charge efficiency of the sample also can be markedly improved with increasing the deposited temperature from 20 to 65 °C.
Typical CV curves of all samples at various scan rates are displayed in Fig. 5. As the scan rate increases from 2 to 50 mV s−1, the peak current becomes larger and larger and all the oxidation peaks and reduction peaks on CV curves can be observed clearly and the peak shapes are similar to each other. But, the peak potential is shifted to the anodic and cathodic direction, respectively, because of an increasing involvement of polarization at high scan rate.40
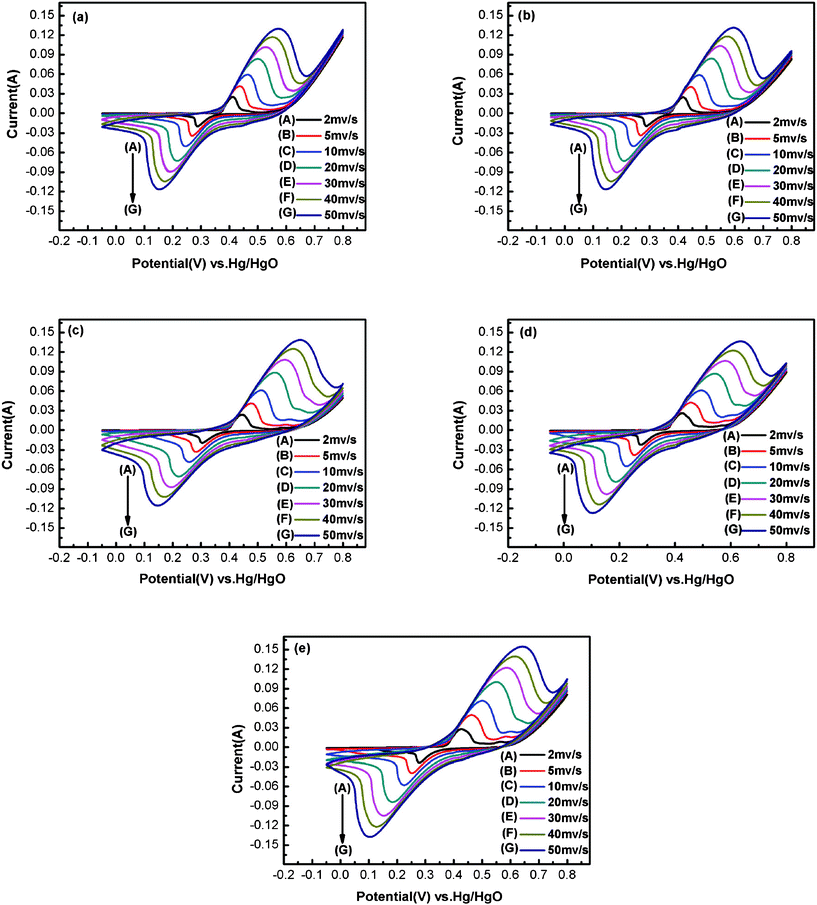 | ||
| Fig. 5 Cyclic voltammetry curves of Ni(OH)2 electrodes obtained at different electrodeposition temperatures and different scan rates: (a) 20 °C; (b) 30 °C; (c) 40 °C; (d) 50 °C; (e) 65 °C. | ||
Fig. 6 shows the plots of the anodic peak current (ip) vs. the square root of the scan rate (v1/2) obtained from Fig. 5. The linear relationships between ip and v1/2 can be found in Fig. 6, which confirms that the redox of Ni(OH)2 is a proton diffusion-controlled reaction.41 According to the literature, the increase in the rate of proton diffusion will cause the decrease of electrode polarization during the charge–discharge process.38,39 The rate of proton diffusion is decided by the proton diffusion coefficient. The proton diffusion coefficient of the Ni(OH)2 electrode is evaluated from the slope of plots. In the case of semi-infinite diffusion, the peak current, ip, in the CV can be expressed by the Sevick equation,42
| ip = 2.65 × 105 × n3/2 × A × D1/2 × C0 × v1/2 | (2) |
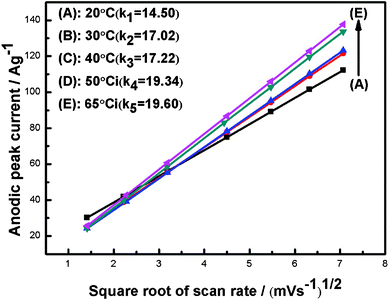 | ||
| Fig. 6 Plots of the anodic peak current (ip) and the square root of the scan rate (v1/2). | ||
where n is the number of the electrons transferred, A is the surface area of the electrode, D is the proton diffusion coefficient, C0 is the proton concentration, and v is the scanning rate. For the samples of NH20, NH30, NH40, NH50 and NH65, the n, A, C0 and v can be considered as the same value. In this way, based on the classical eqn 2, the proton diffusion coefficient only depends on the slope of ipvs.v1/2 in Fig. 6. It is noteworthy that the slopes of ipvs.v1/2 in Fig. 6 are in the order of K65 °C > K50 °C > K40 °C > K30 °C > K20 °C. Therefore, the proton diffusion coefficient of samples from highest to lowest is D65 °C > D50 °C > D40 °C > D30 °C > D20 °C. That is to say, the proton diffusion coefficient increases when the synthesized temperature changes from 20 to 65 °C. The increase of the proton diffusion means that the sample has a short ion diffusion path. That indicates the active materials have much more opportunity to contact the electrolyte solution, which in turn will favour the fast ionic transportation, accelerate the electrode reaction and decrease the polarization of the electrode during the charge–discharge process.28,38,43
Fig. 7 shows the discharge curves of Ni(OH)2 electrodes prepared at various electrodeposition temperatures and the bare nickel foam. The curves clearly exhibit two variation ranges, a linear variation of the time dependence of the potential (below about 0.25 V) indicates the double-layer capacitance behavior, which resulted from the charge separation taking place between the electrode and electrolyte interface, and a slope variation of the time dependence of the potential (from 0.25 to 0.55 V) suggests a typical pseudocapacitance behavior, which is caused by the electrochemical adsorption–desorption or redox reaction at an interface between the electrode and electrolyte.44,45 In a word, the shape of the discharge curves does not only show the characteristics of a pure double-layer capacitor but also pseudocapacitance. The corresponding specific capacitance of the as-deposited Ni(OH)2 electrodes can be evaluated from the discharge curves according to the following equation:46
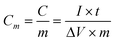 | (3) |
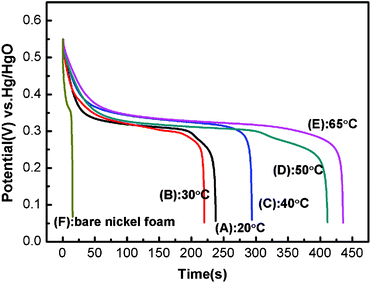 | ||
| Fig. 7 Discharge curves of Ni(OH)2 electrodes obtained at different electrodeposition temperatures within a −0.05 to 0.55 V potential range at a current density of 4 A g−1 in 1 M KOH electrolyte: (A) 20 °C; (B) 30 °C; (C) 40 °C; (D) 50 °C; (E) 65 °C; (F) bare nickel foam. | ||
where Cm (F g−1) is the specific capacitance of the electrode, I (A) is the current of discharge, t (s) is the time of discharge, ΔV (V) is the total potential drop (0.5 V) during discharge, and m (g) is the amount of active material of Ni(OH)2 within the electrode. It can be found that when the bare nickel foam isn't the electrodeposited active material, the specific capacitance of the bare nickel foam used as the current collector is 0.087 F. The specific capacitances of NH20, NH30, NH40, NH50 and NH65 samples at 4 A g−1 are calculated to be 1901, 1763, 2199, 3291, and 3357 F g−1, respectively. Evidently, with increasing temperature, the specific capacitances of these samples at 4 A g−1 maintain an increase tendency except the NH30 sample. The surprising enhancement of the specific capacitance with the temperature increase can be mainly attributable to the electrochemically accessible and effective utilization of Ni(OH)2, which is in good agreement with the results of oxygen evolution overpotentials and proton diffusion coefficients. However, the specific capacitance for the NH30 sample slightly dropped maybe due to the tendency to aggregate and the growth of the particle size, as already mentioned in the preceding analysis by FESEM (Fig. 2). Moreover, the discharge potential plateau of the NH65 sample is the highest among all samples indicating that the NH65 sample has a better discharge capacity than any other samples during the charge–discharge process. Furthermore, the discharge potential is also prolonged during the discharge process as the temperature increases except for the NH30 sample.
The variation of the specific capacitance for the NH65 sample with current density is shown in Fig. 8 and the inset is the charge and discharge curves of NH65 at different current densities (Fig. 8 inset). The impressive specific capacitance of the NH65 sample at 4, 8, 16, and 32 A g−1 were 3357, 2630, 2077, and 1692 F g−1, respectively. Around 50.4% initial capacitance retention can be retained even when the current density increases by as much as eight times, thus indicating good rate capability.47 Therefore, the Ni65 sample has superior electrochemical properties, making them promising electrode materials for practical applications.48
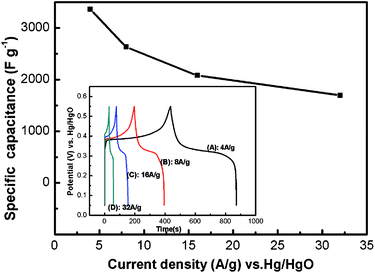 | ||
| Fig. 8 The variation of the specific capacitance for NH65 samples with the current density; inset: charge and discharge curves of NH65 sample within −0.05 to 0.55 V potential range in 1 M KOH electrolyte at different current densities: (A) 4 A g−1; (B) 8 A g−1; (C) 16 A g−1; (D) 32 A g−1. | ||
In order to investigate the effect of electrodeposition temperature on the electrochemical performance of Ni(OH)2 electrode in depth, the electrochemical impedance spectra in the form of Nyquist plots for Ni(OH)2 samples are given in Fig. 9, where Z' and Z′′ are the real and imaginary parts of the impedance, respectively. As can be seen in Fig. 9, the Nyquist plot for each sample displays a linear part in the low-frequency region and a small semicircle in the high frequency region. The slope of a straight line in the low frequency region represents the diffusive resistance (Warburg impedence) resulting from the diffusion of ions from the bulk solution to the electrode surface.49 The more vertical the line, the more closely the supercapacitor behaves as an ideal capacitor.50 The slopes of all electrodes along the imaginary axis (Z′′) were clearly found to be similar to each other and that of the NH65 sample is the highest, which indicates that the diffusive resistance of NH65 is the lowest. Moreover, the conspicuous semicircle in the high-frequency range is associated with the surface properties of the porous electrode structure,51 which is in accordance with the faradic charge transfer resistance (Rct). It is notable the diameter of the half circle for NH65 is the smallest among all samples. Additionally, the intercept at the real axis (Z') of the plot is the internal resistance (Rb), which includes the intrinsic resistance of active materials, the entire resistances of the ionic resistance of the electrolyte, and the contact resistance at the active material and current collector interface.15 It is can be seen that the internal resistances of NH20, NH30, NH40, NH50 and NH65 are as little as approximately 0.85, 0.90, 0.91, 1.05, and 0.84 Ω, respectively, and they are in the order of NH65 ≈ NH20 < NH30 ≈ NH40 < NH50. In brief, at the deposition temperature of 65 °C, the diffusive resistance, the faradic charge transfer resistance and the internal resistance of the synthesized sample are all the lowest.
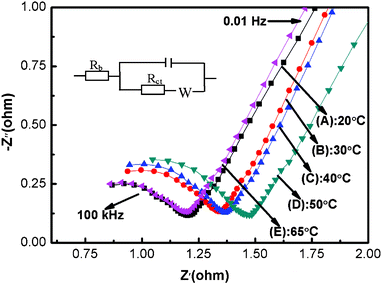 | ||
| Fig. 9 Nyquist plots of the Ni(OH)2 electrodes prepared at different electrodeposition temperatures (0.25 V; electrolyte: 1 M KOH; the frequency range: 10−2 to 105 Hz): (A) 20 °C; (B) 30 °C; (C) 40 °C; (D) 50 °C; (E) 65 °C. | ||
4. Conclusions
In summary, the electrochemical performance of the Ni(OH)2 samples synthesized in a temperature window ranging from 20 to 65 °C was systematically studied in 1 M KOH electrolyte. All the test results indicate that the deposition temperature had significantly influenced the crystalline structure, morphology, specific surface area and electrochemical characteristics, such as capacitance change, the reaction reversibility, and the proton diffuse coefficient, of the prepared Ni(OH)2 material. When the Ni(OH)2 sample was deposited at a temperature of 65 °C, it revealed that the porous honeycomb-like surface morphology, the short ion diffusion path, and especially the highly improved BET surface area should be responsible for the enhanced electrochemical performance.Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (NNSFC NO.20903050) and the Fundamental Research Funds for the Central University (Lzujbky-2009-28).References
- Y. F. Liu, H. G. Pan, M. X. Gao and Q. D. Wang, J. Mater. Chem., 2011, 21, 4743 RSC.
- L. X. Lei, M. Hu, X. R. Gao and Y. M. Sun, Electrochim. Acta, 2008, 54, 671 CrossRef CAS.
- A. K. Shukla, S. Venugopalan and B. Hariprakash, J. Power Sources, 2001, 100, 125 CrossRef CAS.
- B. Hariprakash, S. K. Martha, M. S. Hegde and A. K. Shukla, J. Appl. Electrochem., 2005, 35, 27 CrossRef CAS.
- H. L. Wang, H. S. Casalongue, Y. Y. Liang and H. J. Dai, J. Am. Chem. Soc., 2010, 132, 7472 CrossRef CAS.
- S. K. Meher, P. Justin and G. R. Rao, ACS Appl. Mater. Interfaces, 2011, 3, 2063 CAS.
- H. Pang, Q. Y. Lu, Y. Z. Zhang, Y. C. Li and F. Gao, Nanoscale, 2010, 2, 920 RSC.
- S. Chen, J. W. Zhu, H. Zhou and X. Wang, RSC Adv., 2011, 1, 484 RSC.
- C. Y. Yan, H. Jang, T. Zhao, C. Z. Li, J. Ma and P. S. Lee, J. Mater. Chem., 2011, 21, 10482 RSC.
- W. Xing, S. Z. Qiao, X. Z. Wu, X. L. Gao, J. Zhou and S. P. Zhou, J. Power Sources, 2011, 196, 4123 CrossRef CAS.
- S. K. Meher, P. Justin and G. Ranga Rao, Nanoscale, 2011, 3, 683 RSC.
- J. W. Xiao and S. H. Yang, RSC Adv., 2011, 1, 588 RSC.
- I. Jung, J. Choi and Y. Tak, J. Mater. Chem., 2010, 20, 6164 RSC.
- J. Cheng, G. P. Cao and Y. S. Yang, J. Power Sources, 2006, 159, 734 CrossRef CAS.
- J. W. Lang, L. B. Kong, W. J. Wu, M. Liu, Y. C. Luo and L. Kang, J. Solid State Electrochem., 2009, 13, 333 CrossRef CAS.
- G. X. Hu, C. X. Li and H. Gong, J. Power Sources, 2010, 195, 6977 CrossRef CAS.
- D. D. Zhao, W. J. Zhou and H. L. Li, Chem. Mater., 2007, 19, 3882 CrossRef CAS.
- J. Y. Park, P. Dutta and R. Advincula, Soft Matter, 2011, 7, 3775 RSC.
- J. E. Hujdic, A. P. Sargisian, J. Shao, T. Ye and E. J. Menke, Nanoscale, 2011, 3, 2697 RSC.
- S. Dalgleish, H. Yoshikawa, M. M. Matsushita, K. Awaga and N. Robertson, Chem. Sci., 2011, 2, 316 RSC.
- D. C. Yang, G. W. Meng, C. H. Zhu and X. G. Zhu, Chem. Commun., 2009, 7110 RSC.
- G. W. Yang, C. L. Xu and H. L. Li, Chem. Commun., 2008, 6537 RSC.
- G. R. Fu, Z. A. Hu, L. J. Xie, X. Q. Jin, Y. L. Xie, Y. X. Wang, Z. Y. Zhang, Y. Y. Yang and H. Y. Wu, Int. J. Electrochem. Sci., 2009, 4, 1052 CAS.
- L. A. Hutton, M. Vidotti, A. N. Patel, M. E. Newton, P. R. Unwin and J. V. Macpherson, J. Phys. Chem. C, 2011, 115, 1649 CAS.
- Y. W. Tan, S. S. Srinivasan and K. S. Choi, J. Am. Chem. Soc., 2005, 127, 3596 CrossRef CAS.
- L. P. Liu, Z. T. Zhou and C. H. Peng, Electrochim. Acta, 2008, 54, 434 CrossRef CAS.
- X. Y. Wang, H. A. Luo, P.V. Parkhutik, A. C. Millan and E. Matveeva, J. Power Sources, 2003, 115, 153 CrossRef CAS.
- T. A. Han, J. P. Tu, J. B. Wu, Y. Li and Y. F. Yuan, J. Electrochem. Soc., 2006, 153, A738 CrossRef CAS.
- F. C. Luo, Q. Y. Chen and Z. L. Yin, Trans. Nonferrous Met. Soc. China, 2007, 17, 654 CrossRef.
- X. J. Zhang, W. H. Shi, J. X. Zhu, W. Y. Zhao, J. Ma, S. Mhaisalkar, T. L. Maria, Y. H. Yang, H. Zhang, H. H. Hng and Q. Y. Yan, Nano Res., 2010, 3, 643 CrossRef CAS.
- L. Z. Wang, K. Takada, A. Kajiyama, M. Onoda, Y. Michiue, L. Q. Zhang, M. Watanabe and T. Sasaki, Chem. Mater., 2003, 15, 4508 CrossRef CAS.
- C. Lin, L. B. Kong, Y. Y. Liang and H. L. Li, Chem. Commun., 2004, 9, 1646 Search PubMed.
- S. Ida, D. Shiga, M. Koinuma and Y. Matsumoto, J. Am. Chem. Soc., 2008, 130, 14038 CrossRef CAS.
- N. Sac-Epée, M. R. Palacìn, A. Delahaye-Vidal, Y. Chabre and J. M. Tarâscon, J. Electrochem. Soc., 1998, 145, 1434 CrossRef.
- Q. F. Yi, J. J. Zhang, W. Huang and X. P. Liu, Catal. Commun., 2007, 8, 1017 CrossRef CAS.
- B. Liu, H. T. Yuan, Z. X. Zhou and D. Y. Song, J. Power Sources, 1999, 79, 277 CrossRef.
- E. Shuangguan, Z. R. Chang, H. W. Tang, X. Z. Yuan and H. J. Wang, Int. J. Hydrogen Energy, 2010, 35, 9716 CrossRef.
- M. A. Kiani, M. F. Mousavi and S. Ghasemi, J. Power Sources, 2010, 195, 5794 CrossRef CAS.
- W. Y. Li, S. Y. Zhang and J. Chen, J. Phys. Chem. B, 2005, 109, 14025 CrossRef CAS.
- W. G. Zhang, W. Q. Jiang, L. M. Yu, Z. Z. Fu, W. Xia and M. L. Yang, Int. J. Hydrogen Energy, 2009, 34, 473 CrossRef CAS.
- P. Elumalai, H. N. Vasan and N. Munichandraiah, J. Power Sources, 2001, 93, 201 CrossRef CAS.
- W. Sugimoto, H. Iwata, Y. Yasunaga, Y. Murakami and Y. Takasu, Angew. Chem., Int. Ed., 2003, 42, 4092 CrossRef CAS.
- H. Wei, Y. Y. Lv, L. Han, B. Tu and D. Y. Zhao, Chem. Mater., 2011, 23, 2353 CrossRef CAS.
- D. D. Zhao, S. J. Bao, W. J. Zhou and H. L. Li, Electrochem. Commun., 2007, 9, 869 CrossRef CAS.
- W. Sugimoto, H. Iwata, Y. Yasunaga, Y. Murakami and Y. Takasu, Angew. Chem., Int. Ed., 2003, 42, 4092 CrossRef CAS.
- Y. G. Wang and Y. Y. Xia, J. Electrochem. Soc., 2006, 153, A450 CrossRef CAS.
- H. Xia, J. K. Feng, H. L. Wang, M. O. Lai and L. Lu, J. Power Sources, 2010, 195, 4410 CrossRef CAS.
- H. Jiang, T. Zhao, C. Z. Li and J. Ma, J. Mater. Chem., 2011, 21, 3818 RSC.
- J. Wang, Analytical Electrochemisitry: Controlled-Potential Techniques; John Wiley/Sons: Hoboken, 2006, chapter373 Search PubMed.
- C. G. Liu, Z. N. Yu, D. Neff, A. Zhamu and B. Z. Jang, Nano Lett., 2010, 10, 4863 CrossRef CAS.
- Y. K. Zhou, B. L. He, W. J. Zhou, J. E. Huang, X. H. Li and B. Wu, Electrochim. Acta, 2004, 49, 257 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
