Heck coupling catalyzed by Pd exchanged supported 12-tunstophosphoric acid—an efficient ligand free, low Pd-loading heterogeneous catalyst†
Soyeb
Pathan
and
Anjali
Patel
*
Department of Chemistry, Faculty of Science, M. S. University of Baroda, Vadodara 390002, India. E-mail: aupatel_chem@yahoo.com
First published on 10th November 2011
Abstract
A stable, efficient ligand free, low Pd-loading heterogeneous catalyst comprising Palladium and supported 12-tunstophosphoric acid was synthesized and characterized by various physico-chemical techniques. The catalytic performance of this Pd-exchanged supported 12-tunstophosphoric acid was evaluated for the Heck coupling of olefins with halobenzene. A catalytic activity for recycled catalyst was also evaluated under optimized conditions. The results demonstrated that the catalyst was truly heterogeneous, stable and very active under the present reaction conditions, and could be reused up to three cycles without any significant leaching.
Introduction
The Pd-catalyzed cross coupling of olefins with aryl halides is known as the Heck reaction.1–3 In recent years, Palladium catalyzed C–C bond formation reactions such as Stille coupling, Suzuki coupling, Heck coupling, have gained immense importance for its use in synthesis of complex organic molecules. Among them, Heck coupling is one of the widely used methods for C–C bond formation,3,4 as well as very useful route for the formation of new C–C bonds in a single operational step.5–7 The reaction catalyzed by palladium (1–5% mole) complexes as pre-catalyst or palladium complexes combined with ligands such as phosphorus ligands,8N-heterocyclic carbenes,9 P,O based ligands,10 thiosemicarbazones11 and bis(thiourea) ligands12 in organic solvents under homogeneous conditions.Even though phosphine ligands stabilize and influence catalytic activity, there is a considerable interest in the development of new phosphorus free palladium catalysts for higher activity, stability and substrate tolerance that allow reactions to be carried out under milder reaction conditions. The number of articles on Heck coupling catalyzed by ligand free palladium salts is available in the literature.5,6,13–19 A literature survey shows that reactions in homogeneous phase, suffer from traditional disadvantages such as that of separation, thus ultimately resulting in environmental problems, especially in the case of toxic ligands. Because of an undesired tendency of precious metal catalysts to remain in organic products, the significant costs associated with them have generated interest for an increase in reactivity and ways to recover and reuse these metals.
The mentioned problems (stabilization without phosphine ligands and separation from reaction mixture) can be overcome by developing cheaper and environmentally benign heterogeneous catalytic systems. A number of studies have been carried by supporting Pd over different supports such as polymer,20 charcoal21 and alumina–silica.22–26 Ligand-free Heck reactions in organic solvents at extremely low Pd-loadings have also been described.5,27 Studies showed that, ligand free catalyst seems to improve upon lowering the Pd-loading as it suppresses the formation of Pd-black, and keep all the metal available for catalysis.5,28 The support not only provides an opportunity to Pd-salts to be dispersed over a large surface area, but also prevents its decomposition into Pd-black.
Recently, Corma et al. reported synthesis of ionic liquid and Pd exchanged polyoxometalate. They partial exchange of the surface protons of H5PO40V2Mo10 by an ionic liquid (IL; butylmethylimidazolium, bmim+) and followed by exchanged of Pd.29 Also, they have successfully used synthesized materials as catalysts for the Heck reaction. The unique results for Heck reactions catalyzed by Pd and ionic liquid exchanged H5PO40V2Mo10 encourage us to study Heck coupling catalyzed by Pd-exchanged supported 12-tungstophosphoric acid (H3PW12O40; TPA) in absence of ionic liquid.
An attempt was made to design a ligand free low Pd loading catalyst possessing advantages of heteropolyacids (HPAs) as well as Pd. For this, we have made use of zirconia (ZrO2) supported TPA as exchanging agent. Palladium was exchanged with available protons of supported 12-tungstophosphoric acid (TPA/ZrO2). The resulting catalyst, Pd exchanged supported 12-tungstophosphoric acid (Pd-TPA/ZrO2), was characterized by various physico-chemical techniques and its catalytic activity for coupling between olefins and halobenzenes was evaluated by varying different parameters. A catalytic activity for recycled catalyst was also evaluated under optimized conditions. In order to see the role of the support, Pd was also supported onto hydrous zirconia (Pd/ZrO2) and its catalytic activity was evaluated under optimized conditions.
Experimental
Preparation of catalysts
The Pd exchanged supported 12-tungstophosphoric acid was synthesized in a two-step process. The first step involves the synthesis of the supported 12-tungstophosphoric acid30 while the second step involves the synthesis of Pd exchanged supported 12- tungstophosphoric acid. 30% 12-tungstophosphoric acid (TPA) supported onto ZrO2 was synthesized by impregnation as reported by us earlier.30 Synthesis of palladium exchanged supported 12-tungstophosphoric acid was followed by the wet impregnation of 1 g of TPA/ZrO2 with 25 mL 0.05 M solution of PdCl2 for 24 h with stirring. The solution was then filtered, washed with distilled water in order to remove the excess of Palladium and dried in air at room temperature. The resulting material was designated as Pd-TPA/ZrO2.The same procedure was followed for the synthesis of Pd supported ZrO2; instead of TPA/ZrO2, 1 gm of ZrO2 was wet impregnated with 25 mL 0.05 M solution of PdCl2 for 24 h with stirring. The resulting material was designated as Pd/ZrO2.
Characterization
The filtrate and solid Pd-TPA/ZrO2 were analyzed for Pd. The analysis of Pd ions in the filtrate was carried by volumetric analysis. The analysis of the product mixtures for any leaching of Pd was carried by using atomic absorption spectrometer AAS GBC-902 instrument. FT-IR spectrum of the sample was obtained by using the KBr wafer on a PerkinElmer instrument. TGA of the samples was carried out on a Mettler Toledo Star SW 7.01 in the temperature range of 50–600 °C under nitrogen atmosphere with a flow rate of 2 mL min−1 and a heating rate of 10 °C min−1. The XRD pattern was obtained by using a Philips PW-1830 diffractometer. The conditions were: Cu-Kα radiation (1.54 Å), scanning angle from 0° to 80°. Elemental analysis and SEM were carried out using JSM 5610 LV combined with INCA instrument for EDX-SEM. Adsorption–desorption isotherms of samples were recorded on a Micromeritics ASAP 2010 surface area analyzer at −196 °C.Catalytic reaction
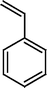
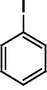
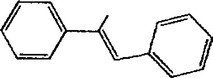
The Heck reaction was carried out in a in a 50 mL glass batch reactor with a magnetic stirrer. The reactor was flushed with N2. In the experiment, aryl halide (0.98 mmol), styrene (1.47 mmol), base (1.96 mmol) and DMF (5 mL) containing Pd-TPA/ZrO2 (100.0 mg, 0.4 mg Pd) were added into the reactor. Then the reactor was heated to the desired temperature in an oil bath under stirring. After reaction, the reactor was cooled, reaction mixture was quenched with water, filter off catalyst. Product was collected by extraction of diethyl ether. The organic phases were then dried with anhydrous magnesium sulfate, filtered and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel with a mixture of ethyl acetate and petroleum ether as eluent. Isolated Product was identified by comparison with the authentic samples and finally by Gas Chromatography-Mass Spectroscopy (GC-MS) and 1H NMR.Results and discussion
The elemental analysis shows that, in the case of Pd/ZrO2, the % of Pd found was 6.05 wt%, while for Pd-TPA/ZrO2, it was 0.4 wt%. In Pd/ZrO2, all protons take part in the exchange with Pd, so the % of Pd was high. In the case of Pd-TPA/ZrO2, if the protons of TPA and of ZrO2 are expected to be exchanged with Pd, the % of Pd would be significantly high. But on the contrary, Pd was found to be in only 0.4 wt%. This indicates the replacement of protons of only TPA by Pd in Pd-TPA/ZrO2.The FT-IR bands for zirconia (ZrO2), TPA/ZrO2 and Pd-TPA/ZrO2 are shown in Fig. S1, ESI.† The FT-IR spectrum for ZrO2 shows two bending vibrations at 1600 and 1370 cm−1 for the O–H–O and H–O–H vibrations. It also shows a weak bending vibration at 600 cm−1 attributed to the Zr–O–H) vibration. The FT-IR spectrum of TPA/ZrO2 (Fig. S1a, ESI†) shows additional stretching vibrational bands for W–O–W, W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and P–O at 812, 964 and 1070 cm−1 respectively. The FT-IR spectrum of Pd-TPA/ZrO2 (Fig. S1b, ESI†) shows bands at 824, 945 and 1056 cm−1 corresponding to the symmetric stretching for W–O–W, W
O and P–O at 812, 964 and 1070 cm−1 respectively. The FT-IR spectrum of Pd-TPA/ZrO2 (Fig. S1b, ESI†) shows bands at 824, 945 and 1056 cm−1 corresponding to the symmetric stretching for W–O–W, W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and P–O. The shift in the band positions as compared to those of TPA/ZrO2 may be due to the change in the environment.
O and P–O. The shift in the band positions as compared to those of TPA/ZrO2 may be due to the change in the environment.
The TGA curve of TPA/ZrO2 shows 12.6% weight loss in the temperature range of 70–100 °C indicating the loss of adsorbed water. Further, there is no weight loss observed up to 450 °C indicating the stabilization of the supported material. TGA of Pd TPA/ZrO2 shows 13% weight loss up to 150 °C due to the loss of adsorbed water. No appreciable weight loss is observed up to 470 °C indicating that the synthesized catalyst is stable up to 470 °C.
The XRD patterns of TPA/ZrO2 and Pd-TPA/ZrO2 are presented in Fig. S2, ESI†. XRD pattern of Pd-TPA/ZrO2 shows the amorphous nature of the catalyst. It does not show any characteristic diffraction line indicating a very high dispersion of solute in a non-crystalline form on the surface of TPA/ZrO2. This observation was further supported by SEM and BET surface area values. SEM of TPA/ZrO2 and Pd-TPA/ZrO2 of are shown in Fig. S3, ESI†. Figure S3a and S3b show a uniform dispersion of TPA in a non-crystalline form on the surface of ZrO2, but after supporting Pd over TPA/ZrO2 (Fig. S3b and S3c), a change in texture of the surface is observed. The increase in surface area for the Pd-TPA/ZrO2 (169 m2 g−1) as compared to that of TPA/ZrO2 (146 m2 g−1) indicates high uniform dispersion of the Pd on the surface of TPA/ZrO2.
A detailed study was carried out on the Heck coupling to optimize the conditions. The effect of concentration of the catalyst on the conversion is shown in Table 1. With an increase in the amount of Pd, the % yield also increases. This suggests that Pd functions as active site for the coupling reaction. From the results, it is clear that very low concentration of Pd (4.7 × 10−6 mole) is sufficient to obtain a yield of stilbene of up to 95%.
| Concentration of Pd × 10−3 (mmole) | % Yield |
|---|---|
| a Iodobenzene (0.98 mmol), styrene (1.47 mmol), K2CO3 (1.96 mmol), DMF (5 mL), 6 h, 120 °C. | |
| 2.35 | 87 |
| 4.7 | 95 |
| 9.4 | 97 |
| 18.8 | 96 |
To study effect of reaction time on the % yield, the Heck reaction was carried out varying the reaction time. The results are shown in Table 2.
| Time (h) | % Yield |
|---|---|
| a Iodobenzene (0.98 mmol), styrene (1.47 mmol), K2CO3 (1.96 mmol), concentration of catalyst (4.7 × 10−6), DMF (5 mL), 120 °C. | |
| 2 | 77.5 |
| 4 | 88.2 |
| 6 | 95.0 |
| 9 | 97.2 |
| 12 | 97.3 |
It is seen from Table 2 that with an increase in the reaction time, the % yield also increases. This is due to the reason that as time increases, the formation of reactive intermediates from the reactant increases, which is finally converted into the products. A 95% yield was observed at 6 h. When the reaction was allowed to continue for 9 h and 12 h, no appreciable change in the % yield was observed.
The Heck reaction was carried out to study effect of different solvent on the % yield of stilbene. The results are shown in Table 3. It is reported that polar, aprotic solvents tend to give the best results for Heck coupling.31–33
As seen from Table 3, the highest activity was obtained with the most polar solvent DMF. DMSO and CH3CN showed lower activity for Heck coupling.
The optimized condition for the maximum yield of stilbene was iodobenzene = 0.98 mmol, styrene=1.47 mmol, K2CO3 = 1.96 mmol, concentration of catalyst = 4.7 × 10−6, DMF = 5 mL, reaction T = 120 °C, reaction time = 6 h.
Control experiments with PdCl2, TPA/ZrO2 and Pd-TPA/ZrO2 were also carried out under optimized conditions. It can be seen from Table 4 that TPA/ZrO2 is inactive towards the coupling of styrene and iodobenzene, indicating the catalytic activity is due to only Pd. The same reaction was carried out by taking an active amount of PdCl2. It was found that the active catalyst gives a 98% yield of trans-stilbene. Almost the same obtained activity for the supported catalyst indicates that Pd is the real active species. Thus, we are successful in supporting Pd onto TPA/ZrO2 without any significant loss in activity and hence in overcoming the traditional problems of homogeneous catalysis. Further, in order to see the effect of the anion, the Heck coupling was carried out using PdCl2 and Pd(OAc)2 as catalysts under optimized condition and the % yield was found almost same for both cases. This indicates that the % yield is not affected by different anions for the present case. So, the small amount of chloride (0.07 wt%) present in the catalyst Pd-TPA/ZrO2, will not play any role and confirms that Pd is the only active site responsible for coupling. In order to see any occlusion of PdCl2 in the present catalyst, estimation of chloride was carried out and small amount of chloride (0.07 wt%) was found in the catalyst Pd-TPA/ZrO2. So, no PdCl2 occlusion is expected. Also, results of control experiments (Table 4) showed there is no significant effect of different anions on % yield and Pd is real active species responsible for coupling reaction. So, if small occlusion of PdCl2 is present, it would not affect the % yield.
The Heck coupling is also carried out with different halobenzenes and olefins under optimized condition (Table 5). The yield obtained by coupling bromobenzene with styrene (59%) was lower compare to the coupling of iodobenzene with styrene (95%). This may be because –I is a better leaving group compared to –Br. Thus, the coupling of iodobenzene with styrene is much easier as compared to the coupling of bromobenzene with styrene to form stilbene. It is also seen from Table 5 that the yield obtained by coupling iodobenzene with α-methyl styrene (49.5%) was lower compared to the coupling of iodobenzene with styrene (95%). This may be attributed to the crowding effect of the methyl group.
The leaching of Pd from Pd-TPA/ZrO2 and Pd/ZrO2 was confirmed by carrying out an analysis of the used catalyst (EDX) as well as the product mixtures (AAS) (Table 6). For Pd-TPA/ZrO2, the analysis of the used catalyst did not show appreciable loss in the Pd content as compared to the fresh catalyst (Table 6). Analysis of the product mixtures showed that if any Pd was present it was below the detection limit, which corresponded to less than 1 ppm. These observations strongly suggest that the present catalyst, Pd-TPA/ZrO2, is truly stable and heterogeneous in nature. While Pd/ZrO2 showed leaching. For Pd/ZrO2, Analysis of the used catalyst showed significant loss in the Pd content (39% of the initial Pd) as compared to the fresh catalyst.
Further, an heterogeneity test was carried out by filtering the catalyst from the reaction mixture at 120 °C after 2 h and the filtrate was allowed to react up to the completion of the reaction (4 h and 6 h). The reaction mixture after 2 h and the filtrate were analyzed. The results of the test showed that no change in the % yield was found for Pd-TPA/ZrO2 indicating the present catalyst is truly heterogeneous. Also, in Pd-TPA/ZrO2, the Pd showed no tendency to agglomerate even after 12 h. i.e. no undesired Pd-black is formed. In contrast, the heterogeneity test (Fig. 1) showed that homogeneous catalysis contributes significantly to the coupling reaction due to leaching of Pd into reaction medium from Pd/ZrO2. Also, because of the higher loading of Pd in Pd/ZrO2, the formation of palladium black is visible during the reaction. The presence of TPA on the surface of ZrO2, the ternary catalyst Pd-TPA/ZrO2 has a higher stabilizing ability to retain Pd as compare to the binary catalyst Pd/ZrO2. It has already been reported that leaching as well as transfer of Pd strongly depends on the nature of the surface of the support materials.34,35 The found observations are in good agreement with the reported ones.
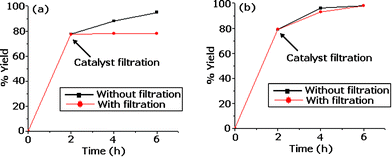 | ||
| Fig. 1 Heterogeneity test (a) Pd-TPA/ZrO2 (b) Pd/ZrO2. | ||
The catalyst remains insoluble in the present reaction conditions and hence can be easily separated by simple filtration followed by washing. The catalyst was washed with dichloromethane and dried at 100 °C.
Heck coupling was carried out with the recycled catalyst under the optimized conditions. The catalyst was recycled in order to test its activity as well as stability. The obtained results are presented in Table 7. As seen from Table 7, the recycled catalyst did not show any appreciable change in the activity up to two cycles, indicating that the catalyst is stable and can be regenerated for repeated use.
| Catalyst | % Yield |
|---|---|
| a Iodobenzene (0.98 mmol), styrene (1.47 mmol), K2CO3 (1.96 mmol), concentration of catalyst (4.7 × 10−6), DMF (5 mL), 120 °C, 6 h. | |
| Pd-TPA/ZrO2 | 95 |
| R 1- Pd-TPA/ZrO2 | 94 |
| R 2- Pd-TPA/ZrO2 | 94 |
| R 3- Pd-TPA/ZrO2 | 93 |
Table 8 shows a comparison of catalytic activity of the present catalyst with reported catalysts. It has been reported that polar solvent causes partial leaching of palladium.36,37 Corma et al.29 have investigated the leaching of palladium and they found that, the catalysts POM-Pd and POM-IL-Pd lose ∼75% and ∼30% of the initial palladium after two catalytic cycles respectively. In the present study, the superiority of the present catalyst lies in its stability. Furthermore, no leaching for fresh as well as recycled catalyst was observed up to three cycles.
Conclusions
In conclusion, we have come up with a new stable, heterogeneous catalyst; Pd exchanged supported in 12-tungstophosphoric acid. The present contribution reports Heck couplings catalyzed by an efficient ligand free, low Pd-loading stable heterogeneous catalyst. The heterogeneous catalyst demonstrates good catalytical performance in coupling reaction without Pd leaching. Moreover, the removal of the catalyst consists of a single filtration and the catalyst can be re-used after a simple work-up. The catalyst is stable under the present reaction conditions and can be used up to three catalytic cycles without significant loss in % yield.Acknowledgements
One of the authors is thankful to University Grants Commission (UGC-RFSMS), New Delhi for financial support.References
- A. de Meijere and F. E. Meyer, Angew. Chem., Int. Ed. Engl., 1994, 33, 2379–2411 CrossRef.
- I. P. Beletskaeya and A. V. Cheprakov, Chem. Rev., 2000, 100, 3009–3066 CrossRef CAS.
- A. B. Dounay and L. E. Overman, Chem. Rev., 2003, 103, 2945–2963 CrossRef CAS.
- M. Lamblin, L. Nassar-Hardy, J. C. Hierso, E. Fouquet and F. X. Felpin, Adv. Synth. Catal., 2010, 352, 33–79 CrossRef CAS.
- M. T. Reetz and J. G. De Vries, Chem. Commun., 2004, 1559–1563 RSC.
- C. Amatore and A. Jutand, Acc. Chem. Res., 2000, 33, 314–321 CrossRef CAS.
- M. Lombardo, M. Chiarucci and C. Trombini, Green Chem., 2009, 11, 574–579 RSC.
- S. D. Walker, T. E. Barder, J. R. Martinelli and S. L. Buchwald, Angew. Chem., Int. Ed., 2004, 43, 1871–1876 CrossRef CAS.
- C. J. O'Brien, E. A. B. Kantchev, C. Valente, N. Hadei, G. A. Chass, A. Lough, A. C. Hopkinson and M. G. Organ, Chem.–Eur. J., 2006, 12, 4743–4748 CrossRef CAS.
- W. M. Dai and Y. Zhang, Tetrahedron Lett., 2005, 46, 1377–1381 CrossRef CAS.
- D. Kovala-Demertzi, N. Kourkoumelis, K. Derlat, J. Michalak, F. J. Andreadaki and I. D. Kostas, Inorg. Chim. Acta, 2008, 361, 1562–1565 CrossRef CAS.
- W. Chen, R. Li, B. Han, B. J. Li, Y. C. Chen, Y. Wu, L. S. Ding and D. Yang, Eur. J. Org. Chem., 2006, 2006, 1177–1184 CrossRef.
- R. F. Heck, Jr. and J. P. Nolley, J. Org. Chem., 1972, 37, 2320–2322 CrossRef CAS.
- P. Fitton, M. P. Johnson and J. E. McKeon, Chem. Commun., 1968, 6 RSC.
- H. A. Dieck and R. F. Heck, J. Am. Chem. Soc., 1974, 96, 1133–1136 CrossRef CAS.
- F. Zhao, M. B. Bhanage, M. Shirai and M. Arai, J. Mol. Catal. A: Chem., 1999, 142, 383–388 CrossRef CAS.
- A. Alimardanov, L. S. V. de Vondervoort, A. H. M. de Vries and J. G. de Vries, Adv. Synth. Catal., 2004, 346, 1812–1817 CrossRef CAS.
- C. Rocaboy and J. A. Gladysz, Org. Lett., 2002, 4, 1993–1196 CrossRef CAS.
- A. F. Schmidt and V. V. Smirnov, J. Mol. Catal. A: Chem., 2003, 203, 75–78 CrossRef CAS.
- J. Lu and P. H. Toy, Chem. Rev., 2009, 109, 815–838 CrossRef CAS.
- L. Yin and J. Liebscher, Chem. Rev., 2007, 107, 133–173 CrossRef CAS.
- R. K. Ramchandani, B. S. Uphade, M. P. Vinod, R. D. Wakharkar, V. R. Choudhary and A. Sudalai, Chem. Commun., 1997, 2071–2072 RSC.
- R. S. Varma, K. P. Naicker and P. J. Liesen, Tetrahedron Lett., 1999, 40, 2075–2078 CrossRef.
- M. K. Richmond, S. L. Scott and H. Alper, J. Am. Chem. Soc., 2001, 123, 10521–10525 CrossRef CAS.
- J. Kim, M. J. Kelly, H. H. Lamb, G. W. Roberts and D. J. Kiserow, J. Phys. Chem., 2008, 112, 10446–10452 Search PubMed.
- V. Polshettiwar, C. Len and A. Fihri, Coord. Chem. Rev., 2009, 253, 2599–2626 CrossRef CAS.
- A. H. M. de Vries, J. M. C. A. Mulders, J. H. M. Mommers, H. J. W. Henderickx and J. G. de Vries, Org. Lett., 2003, 5, 3285–3288 CrossRef CAS.
- M. T. Reetz, E. Westermann, R. Lohmer and G. Lohmer, Tetrahedron Lett., 1998, 39, 8449–8452 CrossRef CAS.
- A. Corma, S. Iborra, F. X. Llabres i Xamena, R. Monton, J. J. Calvino and C. Prestipino, J. Phys. Chem. C, 2010, 114, 8828–8836 CrossRef CAS.
- N. Bhatt, C. Shah and A. Patel, Catal. Lett., 2007, 117, 146–152 CrossRef CAS.
- J. P. Stambuli, S. R. Stauffer, K. H. Shaughnessy and J. F. Hartwig, J. Am. Chem. Soc., 2001, 123, 2677–2678 CrossRef CAS.
- A. Zapf and M. Beller, Chem.–Eur. J., 2001, 7, 2908–2915 CrossRef CAS.
- V. P. W. Böhm and W. A. Herrmann, Chem.–Eur. J., 2001, 7, 4191–4197 CrossRef CAS.
- F. Zhao, K. Murakami, M. Shirai and M. Arai, J. Catal., 2000, 194, 479–483 CrossRef CAS.
- F. Zhao, B. M. Bhanage, M. Shirai and M. Arai, Chem.–Eur. J., 2000, 6, 843–848 CrossRef CAS.
- M. Dams, L. Drijkoningen, B. Pauwels, G. Van Tendeloo, D. E. De Vos and P. A. Jacobs, J. Catal., 2002, 209, 225–236 CrossRef CAS.
- A. Corma and H. Garcia andA. Leyva, J. Mol. Catal. A: Chem., 2005, 230, 97–105 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: FT-IR, XRD, SEM. See DOI: 10.1039/c1ra00687h |
| This journal is © The Royal Society of Chemistry 2012 |






![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: