Preparation of mono-dispersed titanium oxide–octadecylamine hybrid spherical particles in the submicron size range†
Kota
Shiba
a,
Ken
Onaka
b and
Makoto
Ogawa
*ab
aGraduate School of Creative Science and Engineering, Waseda University, 1-6-1 Nishiwaseda, Shinjuku-ku, Tokyo 169-8050, Japan
bDepartment of Earth Sciences, Waseda University, 1-6-1 Nishiwaseda, Shinjuku-ku, Tokyo 169-8050, Japan. E-mail: waseda.ogawa@gmail.com; Fax: +81-3-3207-4950; Tel: +81-3-5286-1511
First published on 8th December 2011
Abstract
Well-defined titanium oxide–octadecylamine hybrid spherical particles (coefficient of variation (CV) ranged from 4 to 12%) were synthesized by sol–gel reactions with the aid of a flow reactor. The present synthesis consisted of two parts; (1) the initial stage of the reaction, which mainly concerned hydrolysis of titanium tetraisopropoxide (TTIP) and condensation, was conducted in the flow reactor, and then, (2) the solution after passing through the flow reactor was mixed with octadecylamine (ODA) in an aqueous 2-propanol mixture for the growth of titanium oxide–ODA hybrid particles. As for the initial stage, fast flow (2.5 to 40 mL min−1) resulted in the formation of products with narrow particle size distribution (CV 4 to 7%), while slow flow (0.5 mL min−1) led to the polydispersed particle formation (CV 27%) probably due to the growth/aggregation that occurred in the flow reactor. The particle size varied from ca. 70 to 900 nm by varing the composition of the batch reaction (the second step).
1. Introduction
Titanium oxide–surfactant hybrids have attracted much attention because of their variable surface properties which possibly induce unique adsorption/desorption properties and possible conversion to nanoporous titanium oxide which is applicable to photocatalysts, dye sensitized solar cells and so on. Spherical particles with controlled size are one of the attractive morphologies from basic scientific and applied materials viewpoints. Several approaches including those based on emulsion chemistry,1–3 morphology template4 and spray drying5–8 have been reported for the preparation of titanium oxide based hybrid spherical particles. The available particle size ranges from several tens of nanometres to hundreds of microns depending on the synthetic pathways.9–14 Though the size variation has been achieved, the nature of the products may reflect the synthetic pathways, which make it difficult to correlate the properties with the particle size. When the well-defined particles with wide size variation become available from the same synthetic protocol, size dependent phenomena can be discussed in more detail.Recently, we have reported the synthesis of well-defined submicron size titanium oxide–octadecylamine (abbreviated as titanium oxide–ODA) hybrid spherical particles by using a flow reactor to prepare titanium oxide nuclei and subsequently grow as titanium oxide–ODA particles by a conventional batch reaction.15 A flow reactor is composed of a small channel in a range of microns to millimetres and has been known as a synthetic device to realize efficient mixing and heat exchange.16,17 The ODA removal by washing led to the formation of nanoporous spherical particles. The crystallization of the titanium oxide has been achieved by subsequent calcination. Due to the wide range of possible applications, the preparation of well-defined nanoporous spherical particles with wide size range is worth conducting. Parameters such as flow rate, channel length, width etc. can be varied to synthesize particles with controlled size and size distribution. In the present study, we examined the previously reported two step synthesis,15i.e. the initial stage in the flow reactor and the second stage in a conventional batch reaction, to optimize the conditions toward well-defined titanium oxide–ODA hybrid spherical particles with various sizes and narrow particle size distribution.
2. Experimental
2.1 Materials
Titanium tetraisopropoxide (abbreviated as TTIP) was purchased from Tokyo Chemical Industry Co., Ltd. 2-Propanol (abbreviated as IPA) was purchased from Kanto Chemical Co., Ltd. Octadecylamine (abbreviated as ODA) was purchased from Sigma-Aldrich, Inc. All the reagents were used without further purification.2.2 Sample preparation
The titanium oxide–ODA spherical particles were synthesized based on a procedure reported previously.15 The synthetic procedure is schematically shown in Scheme 1. TTIP (0.458 mL)/IPA (9.44 g) solution and IPA (9.74 g) aqueous (0.077 mL) solution were flowed within the tube made of PFA (with the diameter of 1 mm) by the syringe pump (MR2 Pump, provided by YMC, Inc.) at a constant speed (0.5 to 20 mL min−1) and were mixed within the flow reactor made of PTFE and with a flow channel of Y-type junction (KeyChem mixer, Hadar, provided by YMC, Inc.). The channel cross section was ca. 1 mm2. The volume of each solution was 12.5 mL and the molar ratio of TTIP:H2O after the mixing was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.7. The mixture was further flowed within the PFA tube of 70 cm in length. The process in the flow reactor is the first step. Then, the reaction solution was mixed with a solution composed of deionized water (0.30 to 40 mL), IPA (49.32 or 61.65 g) and ODA (0.0684 g, molar ODA/TTIP ratio was 0.18) under magnetic stirring until the addition of the mixture was completed (it took 40 s when the flow rate was 20 mL min−1). This batch reaction is the second step. After aging the solution at room temperature for 24 h, the product was collected by vacuum filtration with a membrane filter (cellulose acetate, pore size; 0.2 μm or PTFE, pore size; 0.1 μm), washed with IPA, and then dried at 60 °C in air for a day.
2.7. The mixture was further flowed within the PFA tube of 70 cm in length. The process in the flow reactor is the first step. Then, the reaction solution was mixed with a solution composed of deionized water (0.30 to 40 mL), IPA (49.32 or 61.65 g) and ODA (0.0684 g, molar ODA/TTIP ratio was 0.18) under magnetic stirring until the addition of the mixture was completed (it took 40 s when the flow rate was 20 mL min−1). This batch reaction is the second step. After aging the solution at room temperature for 24 h, the product was collected by vacuum filtration with a membrane filter (cellulose acetate, pore size; 0.2 μm or PTFE, pore size; 0.1 μm), washed with IPA, and then dried at 60 °C in air for a day.
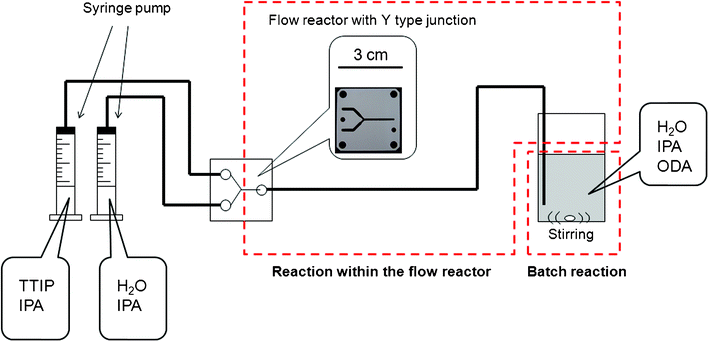 | ||
| Scheme 1 Illustration of the experimental setup used in this study. | ||
2.3 Characterization
The particle size distribution was obtained by a dynamic light scattering (DLS) technique using a Horiba LB-550. The in situ observation of nucleation and growth processes was carried out by correcting a portion of reaction solution and adding it into an excess amount of IPA (200 mL) to eliminate further reaction during DLS measurement. Scanning electron micrographs (SEM) were obtained on a Hitachi S-2380N scanning electron microscope. Prior to the measurements, the samples were coated with a 20 or 30 nm gold layer. Average particle size and coefficient of variation (CV) value were obtained from the SEM images by counting 100 particles. Transmission electron microscopy (TEM) was performed on a JEOL JEM-100CX transmission electron microscope. Thermogravimetric-differential thermal analysis (TG–DTA) curves were recorded on a Rigaku TG-8120 instrument at a heating rate of 10 °C min−1 under air flow and using α-alumina as a standard material. The ODA/titanium oxide ratio in the products was calculated from the weight loss in the TG curve in the temperature range from 150 to 600 °C.3. Results and discussion
3.1 The reaction within the flow reactor: for the formation of titania–ODA particles with narrow particle size distribution
In our previous report on the preparation of monodispersed titanium oxide–ODA spherical particles, we proposed the following reaction mechanism; uniform sized nuclei formed within the flow reactor (the first step) and the nuclei subsequently grew in the aqueous IPA solution containing ODA (the second step).15,18 In the present paper, the hydrolysis and condensation reactions of TTIP in the first step is denoted as ‘the reaction within the flow reactor’ and the growth of pre-formed titanium oxide particles in the second step is denoted as ‘the batch reaction’. A part of TTIP was hydrolyzed within the flow reactor because the molar H2O/TTIP ratio was 2.7, which was not enough to hydrolyze TTIP completely. We performed a DLS measurement to experimentally confirm nucleation process of titanium oxide particles. As shown in Fig. 1, uniform-sized particles with sizes of ca. 2 nm which seem to be titanium oxide nuclei are seen, and they became larger when they were added into the batch reaction solution.18 When the molar H2O/TTIP ratio was 27, the reaction solution in the first step became turbid just after mixing within the flow reactor and irregular shaped particles were obtained after the batch reaction. In order to obtain the narrow particle size distribution, the molar H2O/TTIP ratio was fixed at 2.7 in the present study.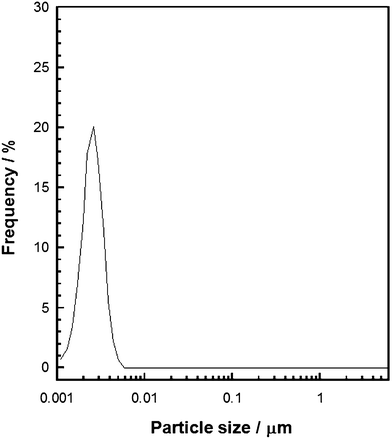 | ||
| Fig. 1 Particle size distribution of titanium oxide particles just after the reaction within the flow reactor. | ||
In order to prepare well-defined titanium oxide–ODA spherical particles, we changed the flow rate (0.5 to 40 mL min−1) in the present study with the expectation that the size of the nuclei varies and this variation affects the particle size and particle size distribution of the resulting titanium oxide–ODA particles. Since the first step occurs within the flow reactor and the second step is the batch reaction, there is a time lag, for the second step, between the first portion and the last portion of the reaction solution added into the batch reaction solution. Thus, we expected that a faster flow rate resulted in the formation of particles with narrower particle size distribution.
Titanium oxide–ODA particles with a broad particle size distribution (particle size: 921 nm and CV: 26.8%, as summarized in Table 1) were obtained (Fig. 2) when the flow rate was 0.5 mL min−1. In this case, the titanium oxide–ODA particle formation and their growth proceeded simultaneously, leading to products with a broad particle size distribution. The solution gradually became translucent when the solution passed through the tube, indicating titanium oxide particles grew/aggregated in the tube to form particles with a broad size distribution.
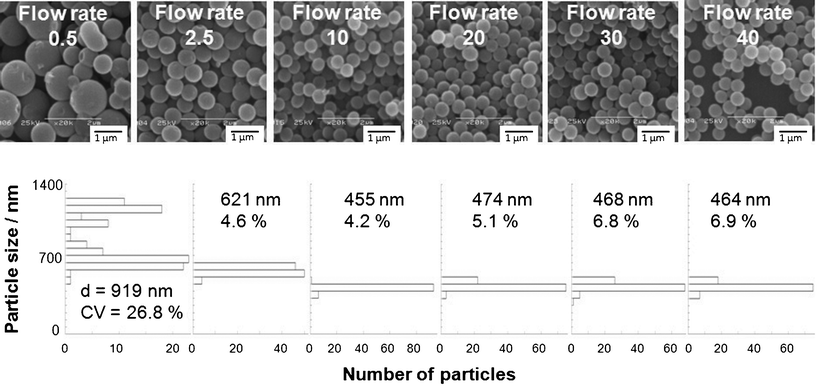 | ||
| Fig. 2 SEM images and particle size distributions of titanium oxide–ODA spherical particles. The amount of water and IPA in the batch reaction was 0.30 mL and 49.32 mL, respectively and flow rate was varied from 0.5 to 40 mL min−1. | ||
| Flow rate/mL min−1 | Amount of H2O / mL | Amount of IPA / g | Average particle size / nm | CV / % | Titanium oxide yield / % | ODA/titanium oxide |
|---|---|---|---|---|---|---|
| 0.5 | 0.30 | 49.32 | 919 | 26.8 | 81 | 0.17 |
| 2.5 | 621 | 4.6 | 86 | 0.17 | ||
| 10 | 455 | 4.2 | 90 | 0.17 | ||
| 20 | 474*1 | 5.1*1 | 90 | 0.17 | ||
| 30 | 468 | 6.8 | 90 | 0.17 | ||
| 40 | 464 | 6.9 | 91 | 0.17 | ||
| 20 | 1.5 | 61.65 | 107 | 7.5 | 98 | 0.17 |
| 3.0 | 68 | 12.3 | 97 | 0.17 |
On the other hand, titanium oxide–ODA hybrid particles with sizes of 455 to 621 nm and relatively narrow particle size distributions (CV 4.2 to 6.9%) were obtained (Fig. 2) when the flow rate ranged from 2.5 to 40 mL min−1. The flow rate was fast enough to avoid the growth/aggregation of irregular shaped titanium oxide–ODA particles, in other words, homogeneous nuclei were supplied by the reaction within the flow reactor for the particle growth that occurred in the batch reaction, leading to products with narrow particle size distributions.
A larger CV value (6.9%) was obtained when the flow rate was 40 mL min−1 (Fig. 3) perhaps due to the insufficient period for the reaction of TTIP and water within the flow reactor and tube. The residence time of the reaction solution within the tube became shorter when the flow rate became faster. A longer tube (280 cm) was used to achieve the same residence time even when the flow was fast (40 mL min−1). SEM images of the product prepared with the tube length of 70 and 280 cm are shown in Fig. 4. A narrower particle size distribution (CV 3.7%) was observed when the 280 cm tube was used and the value was almost same as that obtained at the flow rate of 10 mL min−1 and with the 70 cm tube. Thus, the residence time in the tube is the dominant factor for a narrow particle size distribution. A 700 cm tube led to the formation of particles with CV 3.5% even though particle growth/aggregation perhaps occurred in the tube due to longer residence time. This result indicated that the use of the 700 cm tube and the flow rate faster than 40 mL mL−1 is a possible way towards a lower CV value.
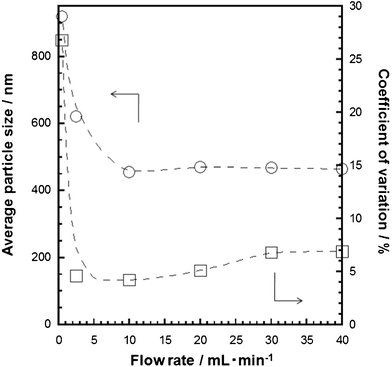 | ||
| Fig. 3 Relationship between average particle size (circle), coefficient of variation (square) and flow rate. The amount of water and IPA in the batch reaction was 0.30 mL and 49.32 mL, respectively and flow rate was varied from 0.5 to 40 mL min−1. | ||
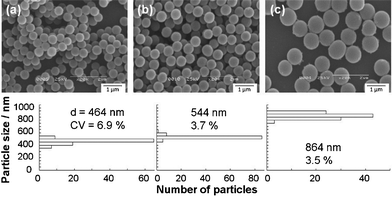 | ||
| Fig. 4 SEM images and particle size distributions of titanium oxide–ODA spherical particles synthesized using a tube with different length, (a) 70, (b) 280 and (c) 700 cm. The amount of water and IPA in the batch reaction was 0.30 mL and 49.32 mL, respectively and flow rate was 40 mL min−1. | ||
In order to achieve a narrower particle size distribution, it is important to shorten the period required for adding all the amount of the solution in the flow reactor into the batch reaction solution (it took 40 s when the flow rate was 20 mL min−1) for the growth of titanium oxide nuclei into titanium oxide–ODA spherical particles as well as to eliminate the influence of the volume difference between the solution coming from the flow reactor and included in the batch reaction because the environment for particle growth chronologically varied more and more when the ratio of the volume of the reaction solution within the flow reactor to that of the batch reaction solution is large (for example, when the volume of the reaction solution within the flow reactor is 25 mL and the volume of the batch reaction solution is 63 mL, total volume is 88 mL. In this case, some particles start their growth in the solution with the volume of ca. 63 mL, while others start their growth in the solution with the volume of 88 mL. This difference probably affects the size and the size distribution of products). We prepared titanium oxide–ODA particles by a sequential reaction using two flow reactors; both a hydrolysis reaction and a titanium oxide–ODA growth reaction occurred in the two flow reactors which were connected with a tube. This sequential reaction within two flow reactors has potential not only to obtain uniform sized particles but also to produce the particles continuously. We have applied the sequential flow reactor set-up to the syntheses of titanium oxide–ODA spherical particles and the details will be reported subsequently.
3.2 The batch reaction: for controlling the particle size of titanium oxide–ODA particles in the size range of several tens of nm to submicron
After the reaction within the flow reactor, we expected that the titanium oxide particles formed within the flow reactor and residual TTIP was further reacted to form titanium oxide–ODA particles in the batch reaction. As many researchers already have been pointed out, TTIP is highly reactive. This means TTIP are hydrolyzed immediately and irregular shaped particles probably form when the amount of water in a reaction solution is large. Based on this assumption, the careful examination of the effect of H2O/TTIP ratio is important to obtain products with well-defined size and shape. We have already reported that uniform sized spherical particles were obtained only by the reactions in the presence of ODA.15 Here, we fixed ODA/TTIP value at 0.18 based on the previous report and varied the relative amount of water to TTIP in the batch reaction.TEM images of titanium oxide–ODA particles prepared from the batch reaction solutions containing various amount of water (1.5 to 40 mL) are shown in Fig. 5. Spherical particles of titanium oxide–ODA with the size of 68 nm formed, while the particle size distribution was relatively broad (CV 12.3%) compared to the submicron sized particles.15 In this case, the reaction solution became turbid within a few seconds after starting the batch reaction. When the amount of water was more than 6.0 mL, translucent suspensions containing smaller-sized and irregular shaped particles were obtained (TEM images and photographs are shown in Fig. 5). The suspension was transparent when the amount of water was 20 mL. The transparency decreased when the added amount of water was 40 mL due to the formation of large particles (compared to those obtained with added water amount of 20 mL) as indicated by TEM image (Fig. 5).
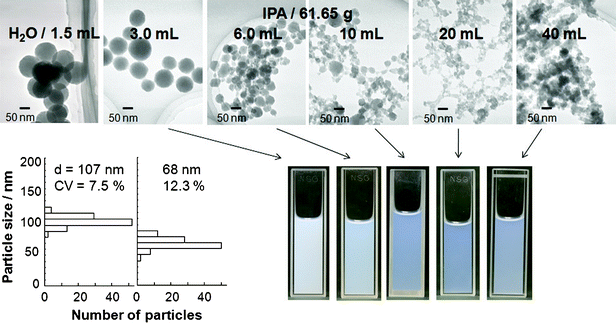 | ||
| Fig. 5 TEM images and particle size distributions of titanium oxide–ODA spherical particles. The amount of water in the batch reaction was varied from 1.5 to 40 mL while the amount of IPA was 61.65 g and flow rate was 20 mL min−1. Photographs show suspensions of titanium oxide–ODA dispersed in aqueous mixture of IPA. | ||
In the present synthesis, the batch reaction solution was aged with magnetic stirring until all solution coming from the flow reactor was added into the batch reaction solution for the formation and growth of titanium oxide–ODA particles. However, depending on the relative amount of water to TTIP in the batch reaction, the rate of magnetic stirring was not enough to make the reaction solution homogeneous, leading to the formation of particles with a broad particle size distribution. Thus, in order to synthesize titanium oxide–ODA particles with a smaller size and CV value, another idea was required, for example, preparing particles in more diluted TTIP concentration and at lower temperature. The hydrolysis of TTIP will proceed within a shorter period when TTIP concentration is more dilute. This is not favorable to achieve the product with uniform size and shape but leads to the formation of relatively a large number of nuclei, which is necessary for smaller size particles. One of the merits of the flow reactor is efficient heat exchange property and designing the reaction pathways in a desired manner. Thus, after the mixing in the flow reactor, it is effective to cool the tube to control the aggregation of nuclei (connecting two flow reactors is also worth trying for eliminating the influence of magnetic stirring). The suspensions prepared with the water amount of 3.0 to 40 mL were stable without precipitation for a month so that their use as a film precursor is worth investigating.
We prepared titanium oxide–ODA particles in a larger size range (submicron) under conditions with a smaller relative amount of water to TTIP in the batch reaction. In fact, larger particles formed when the amount of water to TTIP in the batch reaction was small, while the titanium oxide yield decreased simultaneously. In order to obtain titanium oxide–ODA particles in the larger size range with high titanium oxide yield (90% or higher), we conducted ‘seed growth’ where the preparation of titanium oxide–ODA particles were conducted by employing the batch reaction solution containing titanium oxide–ODA particles which were prepared in advance (this is similar to core–shell particle preparation). Consequently, titanium oxide–ODA particles with the size of ca. 600 nm were obtained (Fig. 6) after the seed growth of titanium oxide–ODA particles with the size of ca. 400 nm, although some particles with the size of ca. 400 nm were seen in the SEM image, indicating the seed growth did not proceed among all the particles or particle formation induced by homogeneous nucleation occurred simultaneously. Further experiments are required to optimize synthetic conditions for larger particles, while we could realize the preparation of core–shell particles by modifying this particle growth procedure.
 | ||
| Fig. 6 SEM images of titanium oxide–ODA spherical particles (a) before and (b) after seed growth. | ||
In the present syntheses, spherical particle formation was thought to be initiated by hydrogen bonding between hydroxyl groups of titanium oxide nuclei and amino groups of ODA molecules because spherical particles did not form in the absence of ODA. Then, titanium oxide–ODA particles assembled together to form secondary particles with a spherical shape and grew also by the reaction with residual TTIP. The reason why the particles are spherical is not clear, the hydrophobic nature of the titanium oxide–ODA particles due to the alkyl chain of ODA is thought to be important. Titanium oxide–ODA particles assembled to minimize surface energy in hydrophilic media, leading to the formation of a spherical shape. The water molecules used in the batch reaction play two roles; one is to assemble titanium oxide–ODA into spherical shape and the other is to hydrolyze residual TTIP for particle growth. As-synthesized titanium oxide–ODA spherical particles dissolved in HCl within a few minutes, indicating that as-sythesized particles were poorly crystallized. Actually, the XRD pattern revealed the amorphous nature of the particles (Fig. S1 in ESI†). When we obtained nanoporous titanium oxide particles, we washed as-synthesized titanium oxide–ODA particles (ODA/titanium oxide 0.17) with HCl/EtOH to remove ODA after collecting and drying the products. Then, titanium oxide particles dissolved ca. 10–15 wt% during the ODA removal process. However, all the particles dissolved when we washed the particles with high ODA loading (ODA/titanium oxide 0.25) even though the particles were once dried and the titanium oxide wall was probably condensed and densified, which might suggest aggregation based growth.
The amount of ODA incorporated in the products is concerned for surface area because ODA acts as a porogen, therefore the amount of ODA in the batch reaction was varied. When titanium oxide–ODA particles with different ODA/titanium oxide ratio were synthesized from the batch reaction solution containing a smaller amount of ODA (SEM images are shown in Fig. 7), larger sized titanium oxide–ODA particles formed and the ODA/titanium oxide ratio of the product reflected the ODA/TTIP ratio in the starting solutions (Table 2). In the case of the syntheses with a smaller amount of ODA, the force to minimize surface energy of titanium oxide–ODA was weaker than the case reported previously15 (but was stronger than the case without ODA), leading to the formation of larger sized particles (892 and 631 nm) and lower sample yield (69 and 82%). ODA removal is possible,15 therefore, titanium oxide–ODA particles with different ODA/titanium oxide ratios may lead to nanoporous titanium oxide with various specific surface area (or porosity).
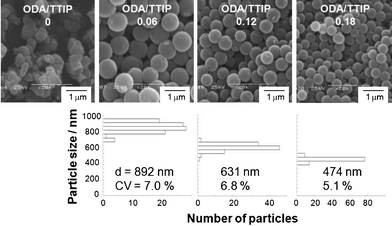 | ||
| Fig. 7 SEM images and particle size distributions of titanium oxide–ODA spherical particles. The amount of water and IPA in the batch reaction was 0.30 mL and 49.32 mL, respectively and molar ODA/TTIP ratio in the starting solutions was varied from 0 to 0.18. | ||
| Flow rate/mL min−1 | Amount of H2O / mL | Amount of IPA / g | Average particle size / nm | CV / % | Titanium oxide yield / % | ODA/titanium oxide (ODA/TTIP) |
|---|---|---|---|---|---|---|
| 20 | 0.30 | 49.32 | — | — | 48 | 0(0) |
| 892 | 7.0 | 69 | 0.07 (0.06) | |||
| 631 | 6.8 | 82 | 0.12 (0.13) | |||
| 474*1 | 5.1*1 | 90 | 0.17 (0.18) |
Titanium oxide–ODA hybrid spherical particles with various sizes from nm to submicron range and narrow particle size distribution are good candidates for preparing films and dispersions that are utilized to investigate particle size dependent phenomena including optical, rheological and other physical/chemical properties. Especially nanometre sized particles may be processed as films, while submicron sized particles are possible candidates for photonic crystal applications. For such applications, the conversion of nanoporous film from the present particle is an important way. Accordingly, conversion to nanoporous materials and processing in a thin film and monolith of the present hybrid particles are being investigated and the results will be reported subsequently.
4. Conclusions
Titanium oxide–octadecylamine hybrid spherical particles with sizes of 70 to 900 nm and a coefficient of variation (CV) of 4 to 12% were synthesized by the sol–gel reaction of titanium tetraisopropoxide (TTIP) in the presence of octadecylamine (ODA). The separation of the nucleation of titanium oxide (within the flow reactor) and their subsequent growth to titanium oxide-octadecylamine hybrid spherical particles (in the batch reaction) is essential for the present preparation. We examined the effect of the flow rate and the composition of starting solutions (H2O/TTIP and ODA/TTIP ratio) on the particle size and the particle size distribution of the products. Fast flow (2.5 to 40 mL min−1) resulted in the formation of titanium oxide–ODA particles with narrow particle size distributions (CV 4 to 7%), while slow flow (0.5 mL min−1) led to the polydispersed particle formation (CV 27%) probably due to the growth/aggregation that occurred in the flow reactor. The titanium oxide–ODA hybrids formed during the batch reaction possessed hydrophobic nature. The high value of H2O/TTIP (the water amount up to 3.0 mL) in the batch reaction led to the formation of small size particles (up to 70 nm) to minimize surface energy. The ODA/TTIP ratio was a factor to vary surface hydrophobicity of titanium oxide–ODA hybrids, leading to the formation of large particles (900 nm) when ODA/TTIP was 0.06.Acknowledgements
The authors are grateful to YMC Inc. for the financial and technical supports through the project. This work was partially supported by the Global COE Program of MEXT and Waseda University Grant for Special Research Projects (2011A-604).References
- B. L. Zhang, B. S. Chen, K. Y. Shi, S. J. He, X. D. Liu, Z. J. Du and K. L. Yang, Appl. Catal., B, 2003, 40, 253–258 Search PubMed.
- J. J. Zhu, H. Wang, J. J. Miao, J. M. Zhu, H. M. Ma and H. Y. Chen, Langmuir, 2004, 20, 11738–11747 CrossRef CAS.
- P. Tartaj, Chem. Commun., 2011, 47, 256–258 RSC.
- J. Chen, Z. Hua, Y. Yan, A. A. Zakhidov, R. H. Baughmand and L. Xu, Chem. Commun., 2010, 46, 1872–1874 RSC.
- F. Iskandar, A. B. D. Nandiyanto, K. M. Yun, C. J. Hogan, Jr., K. Okuyama and P. Biswas, Adv. Mater., 2007, 19, 1408–1412 CrossRef CAS.
- P. Z. Araujo, V. Luca, P. B. Bozzano, H. L. Bianchi, G. J. A. A. Soler-Illia and M. A. Blesa, ACS Appl. Mater. Interfaces, 2010, 2, 1663–1673 Search PubMed.
- Y. Zhang, Y. Shi, Y. H. Liou, A. M. Sawvel, X. Sun, Y. Cai, P. A. Holdenc and G. D. Stucky, J. Mater. Chem., 2010, 20, 4162–4167 RSC.
- H. Oveisi, N. Suzuki, A. Beitollahi and Y. Yamauch, J. Sol-Gel Sci. Technol., 2010, 56, 212–218 Search PubMed.
- S. Eiden-Assmann, J. Widoniak and G. Maret, Chem. Mater., 2004, 16, 6–11 CrossRef CAS.
- S. Eiden-Assmann, J. Widoniak and G. Maret, Colloids Surf., A, 2005, 270–271, 329–334 Search PubMed.
- S. Tanaka, D. Nogami, N. Tsuda and Y. Miyake, J. Colloid Interface Sci., 2009, 334, 188–194 CrossRef CAS.
- D. Chen, L. Cao, F. Huang, P. Imperia, Y. B. Cheng and R. A. Caruso, J. Am. Chem. Soc., 2010, 132, 4438–4444 CrossRef CAS.
- S. Liu, G. Han, M. Shu, L. Han and S. Che, J. Mater. Chem., 2010, 20, 10001–10009 RSC.
- I. G. Yu, Y. J. Kim, H. J. Kim, C. Lee and W. I. Lee, J. Mater. Chem., 2011, 21, 532–538 RSC.
- K. Shiba and M. Ogawa, Chem. Commun., 2009, 6851–6853 RSC.
- P. Watts and C. Wiles, Chem. Commun., 2007, 443–467 RSC.
- Y. Song, J. Hormes and C. S. S. R. Kumar, Small, 2008, 4, 698–711 CrossRef CAS.
- K. Shiba and M. Ogawa, J. Ceram. Soc. Jpn., 2011, 119, 507–512 Search PubMed.
Footnote |
| † Electronic Supplementary Information (ESI) available: XRD pattern of as-synthesized titanium oxide–ODA hybrid particles is provided. See DOI: 10.1039/c1ra00748c/ |
| This journal is © The Royal Society of Chemistry 2012 |
