Introducing carbon nanotubes into living walled plant cells through cellulase-induced nanoholes†
Maged F.
Serag
*ab,
Noritada
Kaji
ac,
Manabu
Tokeshi
ac and
Yoshinobu
Baba
acd
aDepartment of Applied Chemistry, Graduate School of Engineering, Nagoya University, Nagoya, Japan. E-mail: magedserag@yahoo.com; Fax: +81-52-789-4666; Tel: +81-52-789-3560
bDepartment of Pharmacognosy, Faculty of Pharmacy, Zagazig University, Egypt
cFIRST Research Centre for Innovative Nanodevices, Nagoya University, Japan
dNational Institute of Advanced Industrial Science and Technology (AIST), Takamatsu, Japan
First published on 10th November 2011
Abstract
Carbon nanotubes can intracellularly transport through different cellular barriers. However, their use in plant cells is limited by the cellulosic cell wall surrounding these cells. Here we show that cup-stacked carbon nanotubes with cellulase immobilized on their sidewalls and tips penetrate the cell wall and transport intracellularly through cellulase-induce nanoholes.
Because of their extraordinary physical, chemical and mechanical properties, carbon nanotubes (CNT) have been proven to be a useful tool in the field of cell nanobiotechnology. CNT have an extraordinary ability to host a wide spectrum of molecules including DNA, proteins and other chemicals either inside holes or covalently or non-covalently attached to their outer surfaces.1–7 Therefore, a wide spectrum of molecules is possible to be co-delivered into a wide variety of living cells.8 Also, an increasing number of reports have studied the toxicological impact and safety profile of carbon nanomaterials on both plant9 and mammalian cells,10–13 indicating that a high degree of CNT functionalization leads to a dramatic reduction in toxic effects.14
Cup-stacked carbon nanotubes (CSCNT) consisting of truncated conical graphene layers are of particular interest because whereas conventional carbon nanotubes are made of seamless cylinders of hexagonal carbon networks, the stacked cup structure provides a hollow tubular morphology. This truncated-cone morphology provides a large portion of exposed and reactive edges in the outer and inner surfaces of the hollow tubes and permits a high degree of functionalization.15–18
The plant cell wall is a unique cellular barrier that surrounds plant cells. The most characteristic component of plant cell wall is cellulose, which largely determines their shape. Together with hemicelluloses and pectin, such thick cellulosic barriers impede the passage of macromolecules into the cell. Therefore plant protoplasts (plant cells made devoid of cell wallviacellulase treatment) have previously been used to study the internalization of nanomaterials such as mesoporous silica nanoparticles,19quantum dots and polystyrene nanospheres.20
CNT uptake studies are, indeed, essential for the development of nanotransporters for plant cells which in turn has a particular importance for plant cell transformation. Recently, we have shown the ability of multi-walled carbon nanotubes to traverse through the cell membrane of plant protoplasts;21,22 however, introducing carbon nanotubes through the cell wall of walled cells remains challenging. In protoplast-based transformation methods, the entire plant cell wall is removed to make the DNA/DNA-vector accessible to cell transcription machinery. Meanwhile, the viability of protoplasts and their capability of dividing are strongly reduced by chemicals applied to disorganize the cell wall.23 Here, we introduce a unique strategy to deliver CSCNT into walled plant cells by immobilizing cellulase (an enzyme that catalyzes cellulose hydrolysis) on their tips and walls. The immobilized cellulase is proposed to induce local lesions in the cell wall, through which carbon nanotubes can transport into the interior of the cell.
Results and discussion
Experimental approach
In solution, cellulase subunits adopt a tadpole conformation. The length of each tadpole-like structure is about 21.5 nm.24 This fact indicated that the diameter of the carbon nanomaterial should be wide enough to properly hold the immobilized enzyme molecules. Because CSCNT are characterized by a unique wide diameter of about 60–100 nm, we employed such kind of tubes to achieve our aim.The resolution of the stacked-cups of CSCNT was difficult using the atomic force microscope (AFM), because the thickness of the AFM cantilever was 200 nm. However, in very rare cases, we were able to resolve the stacked cup structure (Fig. 1a). Functionalization of CSCNT in this study was performed via sulphuric/nitric acid oxidation. This approach allowed shortening of the CSCNT and insertion of carboxylate groups around tips and walls of the stacked cups (Fig. 1b, see Supplementary Information, Fig. S1†). Cellulase was immobilized on CSCNT via a carbodiimide reaction under controlled conditions (see Supplementary Methods†).
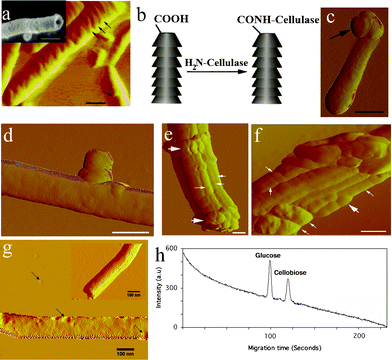 | ||
| Fig. 1 Characterization of cellulase-immobilized CSCNT. (a) Amplitude AFM image of CSCNT (Scale bar 100 nm). Inset image is a SEM image of a short-type CSCNT (Scale bar: 250 nm). (b) Cellulase immobilization on CSCNT tips. (c) Amplitude AFM image of cellulase (arrow) immobilized CSCNT (scale bar: 100 nm). (d) Amplitude AFM image of cellulase attached to the sidewall of a single CSCNT (Scale bar 70 nm). (e), (f) Amplitude AFM images of aggregated CSCNT-cellulase. Thin white arrows indicate CSCNT while thick white arrows indicate cellulase. Scale bars 100 nm. (g) Amplitude AFM image of a single CSCNT dispersed in 0.1% OG in comparison with a CSCNT in absence of OG (Inset). Black arrows indicate small aggregated micelles of OG. (h) Microchip capillary electrophoresis-based detection of cellulose microfibrils hydrolysis products (glucose and cellobiose) by CSCNT-cellulase. | ||
Characterization of cellulase-immobilized CSCNT
AFM imaging of cellulase-immobilized CSCNT revealed two types of hybrid structures, either a single CSCNT carrying a cluster of enzyme molecules (Fig. 1c, d) or an aggregation of several nanotubes linked to many enzyme molecules (Fig. 1e, f).Under our experimental conditions, CSCNT-cellulase tended to aggregate in the plant cell medium. To overcome this, we included 0.1% of the non-ionic surfactant β-D-octyl glucoside (OG) in the cell medium. OG stabilized CSCNT solution for 3 months with no obvious precipitation. In addition, atomic force microscopy (AFM) images revealed that the surfactant adsorbs onto the oxidized CSCNT surface in a random fashion and heterogeneous density (Fig. 1g). It is important to underline that OG molecules do not form organized micelles around the cup-stacked tubes because the graphitic cylindrical structure is not homogenous and continuous like in the case of conventional types of carbon nanotubes. Therefore, the functionalized edges likely hamper the formation of organized micelles around this type of tubes. Furthermore, because CSCNT contain carboxylate groups with a density depending on the degree of functionalization, OG molecules adsorb onto CSCNT with a heterogeneous density.
The activity of the immobilized cellulase in CSCNT-cellulase was assessed against cellulose microfibrils and compared with that of free cellulase (see Supplementary Methods†). Cellulose incubated with either free cellulase or cellulase-immobilized carbon nanotubes showed two hydrolysis products (Fig. 1h) attributable to glucose and cellobiose which are the major hydrolysis products of cellulose.25 Under our experimental conditions, one mg of cellulase-immobilized CSCNT yielded an activity equivalent to 2.5 μg of free cellulase (see Supplementary methods†).
Uptake of CSCNT by Arabidopsis thaliana walled cells
We investigated the uptake of CSCNT-cellulase by Arabidopsis thalianacells. To avoid any dysfunction that might happens to the attached cellulase molecules during fluorescence labeling of either cellulase or CSCNT, we decided to physically label the nanotubes with core quantum dots (photoluminescence emission maximum: 567 nm) embedded in Tri-octyl phosphine oxide (TOPO) (see Supplementary Methods†). AFM images showed that quantum dots adsorb to the surface of CSCNT (see Supplementary Information, Fig. S2†), and therefore provided a platform to track nanotubes outside and inside A. thalianacells using epifluorescence microscopy. Because nanotubes aggregation would disturb the stability of the physically attached quantum dots and cause their release, we performed our experiments using the freshly prepared conjugates.First, A. thalianacells were incubated for 3 h with CSCNTCdSe without cellulase attachment. After washing the cells with fresh nutrient medium, the cells were imaged by epifluorescence and confocal microscopy where no sign of internalization was observed (Fig. 2a). CSCNTCdSe-cellulase was, then, allowed to interact with A. thalianacells in suspension culture for 3 h. CSCNT-cellulase microparticles (∼1 μm in size) were observed penetrating the cell wall (see Supplementary Information, Movie 1†). Epifluorescence and confocal images showed that CSCNT-cellulase transported into the cell (Fig. 2b–d, see Supplementary Information Fig. S3†). Transportation is believed to occur through local contact sites between CSCNT-cellulase and the cell wall where local hydrolysis of cellulose assists their internalization (Fig. 2b, see Supplementary Information, Movie 2 and related supplementary text†).
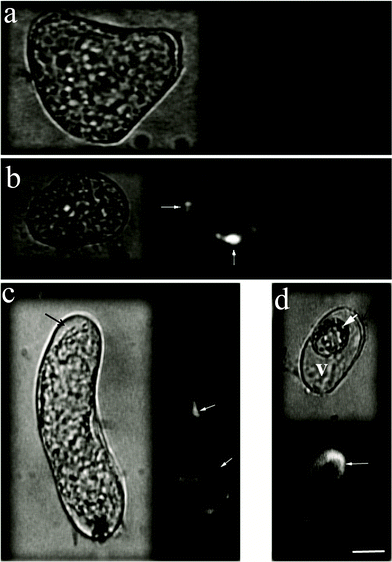 | ||
| Fig. 2 Epifluorescence images of A. thalianacells after uptake of CSCNT-cellulase. Cells were incubated for 3 h with CdSe-labeled CSCNT-cellulase. The left images represent bright-field imaging of cells while the right images represent the fluorescence imaging of the same cells. (a) A. thalianacells incubated with CdSe-labeled CSCNT (no cellulase). (b) A cell showing CSCNT-cellulase leaking into the interior of the cell through local contact sites between CSCNT-cellulase and the cell wall, where internalization is assisted by local hydrolysis of cellulose (thin arrows). (c, d) A thick white arrow indicates a cell containing crescent-shape aggregations of CSCNT-cellulase microparticles, while thin arrows indicate fluorescence of CdSe-labeled CSCNT-cellulase inside cells (V: cell vacuole). The black thick arrows indicate the cell wall. (scale bar: 10 μm). | ||
To investigate the potential toxicity of CSCNT-Cellulase on plant cells, we incubated A. thalianacells in cell medium containing such kind of material. In three independent experiments, A. thaliana suspension cells, treated with CSCNT-cellulase, showed no signs of toxicity (see Supplementary information, Fig. S4†). CSCNT was observed to slightly increase the viability of cultured Arabidopsiscells in suspension culture. One possible reason is the antioxidant property of carbon nanotubes in solution.26 Also, activated carbon has been proven to stimulate cell growth in suspension culture through adsorption of polyphenolic toxic compounds secreted by the plant cells.27,28
Conclusions
The major premise of this communication was to link carbon nanotubes as a technically very important nanomaterial for plant cell molecular delivery science. We illustrated the ability of cellulase-immobilized CSCNT to penetrate the thick cellulosic cell wall of the plant cell and transport into the cell. The strategy of cellulase immobilization circumvented the complete removal of cell wall that might affect cell viability. This could represent a new, exciting direction that may open up new opportunities in plant cell genetic transformation.References
- A. Bianco, Expert Opin. Drug Delivery, 2004, 1, 57–65 Search PubMed.
- D. Cai, Nat. Methods, 2005, 2, 449–454 CrossRef CAS.
- D. Pantarotto, J. P. Briand, M. Prato and A. Bianco, Chemm. Commun., 2004, 43, 5242–5246 Search PubMed.
- L. Gao, ChemBioChem, 2006, 7, 239–242 CrossRef CAS.
- N. W. Shi Kam and H. Dai, J. Am. Chem. Soc., 2005, 127, 6021–6026 CrossRef CAS.
- N. W. Shi Kam, M. O'Connell, J. A. Wisdom and H. Dai, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 11600–11605 CrossRef CAS.
- A. Bianco, K. Kostarelos and M. Prato, Curr. Opin. Chem. Biol., 2005, 9, 674–679 CrossRef CAS.
- K. Kostarelos, Nat. Nanotechnol., 2007, 2, 108–113 CrossRef.
- D. Lin and B. Xing, Environ. Pollut., 2007, 150, 243–250 CrossRef CAS.
- Y. Sato, Mol. BioSyst., 2005, 1, 176–182 RSC.
- V. E. Kagan, Toxicol. Lett., 2006, 165, 88–100 CrossRef CAS.
- K. Pulskamp, S. Diabate and H. F. Krug, Toxicol. Lett., 2006, 168, 58–74.
- H. Dumortier, Nano Lett., 2006, 6, 1522–1528 CrossRef CAS.
- C. M. Sayes, Toxicol. Lett., 2006, 161, 135–142 CrossRef CAS.
- M. Endo, Appl. Phys. Lett., 2002, 80, 1267–1269 CrossRef CAS.
- C. Kim, J. Appl. Phys., 2004, 96, 5903–5905 CrossRef CAS.
- T. Hasobe, S. Fukusumi and P. V. Kamat, Angew. Chem., Int. Ed., 2006, 45, 755–759 CrossRef CAS.
- K. Saito, M. Ohtani and S. Fukusumi, J. Am. Chem. Soc., 2006, 128, 14216–14217 CrossRef CAS.
- F. Torney, B. G. Trewyn, V. S.-Y. Lin and K. Wang, Nat. Nanotechnol., 2007, 2, 295–300 CrossRef CAS.
- E. Etxberria, P. Gonzalez, E. Baroja-Fernandez and J. Pozueta-Romero, Plant Signaling Behav., 2006, 1, 196–200 Search PubMed.
- M. F. Serag, ACS Nano, 2011, 5, 493–499 CrossRef CAS.
- M. F. Serag, ACS Nano DOI:10.1021/nn2035654.
- M. Rakoczy-Trojanowska, Cell. Mol. Biol. Lett., 2002, 7, 849–858 Search PubMed.
- I. Pilz, Biochem. J., 1990, 271, 277–280 CAS.
- W. D. Murray, Biotechnol. Bioeng., 1987, 29, 1151–1154 CrossRef CAS.
- P. Watt, J. Mater. Chem., 2003, 13, 491–495 RSC.
- S. Winkle and G. Pullman, Plant Cell Rep., 2005, 24, 201–208 CrossRef CAS.
- M. J. Pan and J. Stadel, Plant Growth Regul., 1998, 26, 155–163 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: supplementary methods, additional figures and movies. See DOI: 10.1039/c1ra00760b/ |
| This journal is © The Royal Society of Chemistry 2012 |
