Chemiluminescence from the biomimetic reaction of 1,2,4-trioxolanes and 1,2,4,5-tetroxanes with ferrous ions†
D. V.
Kazakov
*a,
A. R.
Timerbaev
a,
F. E.
Safarov
a,
T. I.
Nazirov
a,
O. B.
Kazakova
a,
G. Y.
Ishmuratov
a,
A. O.
Terent'ev
b,
D. A.
Borisov
b,
A. G.
Tolstikov
c,
G. A.
Tolstikov
a and
W.
Adam
de
aInstitute of Organic Chemistry, Ufa Scientific Center of the RAS, 71 Pr. Oktyabrya, 450054, Ufa, Russia. E-mail: dmitri_kazakov@mail.ru; Fax: +7 3472 356066; Tel: +7 3472 356111
bZelinsky Institute of Organic Chemistry of the RAS, 47 Leninskiy prospekt, 119991, Moscow, Russia
cInstitute of Petrochemistry and Catalysis of the RAS, 141 Prospect Oktyabrya, 450075, Ufa, Russia
dDepartment of Chemistry, Facundo Bueso 110, University of Puerto Rico, Rio Piedras, Puerto Rico, 00931, USA
eInstitut für Organische Chemie, Universität Würzburg, Am Hubland, Wurzburg, 97074, Germany
First published on 11th November 2011
Abstract
The 1,2,4-trioxolane and 1,2,4,5-tetroxane pharmacophores are currently considered as the next generation of synthetic antimalarial drugs. This fact has stimulated the exploration of the chemiluminescence displayed by these cyclic peroxides in biomimetic reactions with Fe(II). The CL has been induced by FeSO4 and/or the FeCl3/L-cysteine/Rhodamine G system in aqueous (50%) acetonitrile. The light emission in the visible spectral region has been recorded for triterpenoid-based 1,2,4-trioxolanes, purely synthetic ozonide OZ03, bicyclic 1,2,4,5-tetroxanes, a tetroxane derived from deoxycholic acid, the diperoxide of trifluoroacetone, as well as the natural artemisinin. The herein discovered CL provides a promising perspective for the study of pharmacologically active peroxides in biomedical applications.
The discovery of new antimalarial drugs based on the natural trioxane artemisinin1 has expanded during the past decades of intensive research activity in the chemistry of such cyclic peroxides. Understandably, artemisinin-based combination therapy is presently recommended by the WHO for the treatment of malaria.1 The connection between the peroxide functionality and antimalarial activity has incited the development of more effective peroxide-based antimalarial medicaments. Once it was recognized that purely synthetic 1,2,4-trioxolanes and 1,2,4,5-tetraoxanes possess biological activity comparable or even superior to natural artemisinin, research along these lines received much attention.2 Since this discovery, considerable work has been focused on the synthesis of new peroxide derivatives to explore their biological activity and their mechanism of action.3 Through these efforts, the 1,2,4-trioxolane arterolane (OZ277) drug was developed, which is now in phase III clinical trials (Fig. 1).3a,4
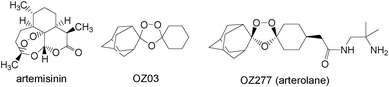 | ||
| Fig. 1 Structures of the natural peroxide artemisinin, the purely synthetic antimalarial drug candidate arterolane, and its precursor OZ03. | ||
Moreover, the tetroxane RKA182 was recently identified as a drug development candidate with superior antimalarial activity over the semisynthetic artemisinin.2d Because of their outstanding antimalarial properties, these simple cyclic peroxides are nowadays considered as the next generation of synthetic drugs for their deployment in the control and eradication of malaria. In this context, we report on the light emission of 1,2,4-trioxolanes and 1,2,4,5-tetroxanes in their reactions with Fe(II), an unprecedented property of this intriguing class of biologically active peroxides. This constitutes a biomedically important discovery since the antimalarial activity of antimalarial peroxides is presumed to be mediated by Fe(II)-induced cleavage of the peroxide bond.3b–d,5 Whether the currently observed chemiluminescence (CL)6 in the biomimetic transformations of the artemisinin-related peroxides is linked to their antimalarial properties is a worthwhile supposition to pursue. It should, however, be emphasized that the involvement of electronically excited states (singlets as well as triplets) in cellular processes of biomedical significance is well documented.7 Also well known are applications of CL in antioxidant assays and in oxidative stress studies.6c,8 Consequently, our freshly discovered CL phenomenon paves the way towards studying the mechanism of antimalarial action of such cyclic peroxides under biomimetic conditions or directly in cellular systems, to assess their biomedical importance. Apart from this fundamental scientific significance, our CL findings provide promising opportunities for the in vivo detection and clinical monitoring of the behavior of peroxide-based medicaments under ultra-sensitive analytical conditions.
For the CL study in question, the hitherto unknown and potentially biologically active triterpenoid-based 1,2,4-trioxolanes 1 and 2 have been synthesized,9 as well as the dioxazolidine 3. These cyclic peroxides contain two possible pharmacophores, namely the antimalaria-active peroxy group and the natural terpenoid allobetulin structure. The latter is known to possess a wide range of biological activities.10 Also the 1,2,4,5-tetroxanes 4–7 have been prepared for our exploratory study (Fig. 2), which include the symmetrical trifluoroacetone-derived tetroxane,411a the bicyclic tetroxanes 5 and 6,11b as well as the steroidal 1,2,4,5-tetroxane 7. The latter resembles structurally the family of biologically active tetroxanes derived from cholic and deoxycholic acids.12
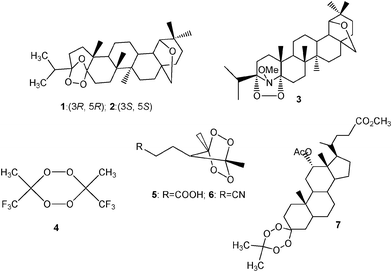 | ||
| Fig. 2 Structures of the 1,2,4-trioxolanes 1 and 2, the 1,2-dioxazolidine 3, and the 1,2,4,5-tetroxanes 4–7. | ||
In an analogy of the Fe(II)-mediated cleavage of the antimalarial peroxides that produce the reactive species responsible for parasite killing,3b–d,5 we conducted the chemical transformations of the ozonides 1 and 2, and dioxazolidine 3 with ferrous ions, to scrutinize possible light emission. Indeed, we found that the biomimetic reactions of cyclic peroxides 1–3 with FeCl3/L-cysteine hydrochloride (a source of ferrous ions) in the presence of Rhodamine G (a CL enhancer) in CH3CN/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 70 °C emit light in the visible region 550–650 nm [Fig. S-1, ESI†].
1) at 70 °C emit light in the visible region 550–650 nm [Fig. S-1, ESI†].
Under an oxygen atmosphere, administered by passing a slow stream of oxygen gas through the CH3CN/H2O solution, the total amount of light evolved in the reaction was considerably increased compared to the process conducted under argon. TLC analysis of the reaction mixture made after CL decay (Fig. 3) disclosed consumption of the peroxides 1 and 2. This observation is in line with earlier findings that artemisinin, trioxolanes and tetroxanes readily react with ferrous ions produced from inorganic ferric salt or from hemin by reaction with reducing agents.3b–d,5 These experimental facts, combined with the now observed oxygen effect on the light emission, imply that peroxy radicals mediate the CL in the present peroxide reactions. According to this established mechanism,8 the alkyl radicals generated in the reactions of the peroxides 1–3 with the FeCl3/L-cysteine reductant react with molecular oxygen to form peroxy radicals. Recombination of the latter affords electronically excited carbonyl species, followed by light emission through energy transfer to Rhodamine G, the luminescence enhancer. The direct involvement of the dye in this CL reaction cannot be excluded.
![Time profile (solid line) of the CL decay for the reaction of the trioxolane 1 with FeCl3/l-cysteine hydrochloride in the presence of Rhodamine G ([peroxide 1] = [FeCl3] = [Rhodamine G] = 1.5 × 10−3 M, [l-cysteine] = 3 × 10−3 M, CH3CN : H2O (1 : 1), 70 °C, O2 atmosphere). Dotted line shows emission recorded under the same conditions, but in the absence of FeCl3. The arrow marks the moment of mixing the reagents.](/image/article/2012/RA/c1ra00784j/c1ra00784j-f3.gif) | ||
Fig. 3 Time profile (solid line) of the CL decay for the reaction of the trioxolane 1 with FeCl3/L-cysteine hydrochloride in the presence of Rhodamine G ([peroxide 1] = [FeCl3] = [Rhodamine G] = 1.5 × 10−3 M, [L-cysteine] = 3 × 10−3 M, CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1 H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 70 °C, O2 atmosphere). Dotted line shows emission recorded under the same conditions, but in the absence of FeCl3. The arrow marks the moment of mixing the reagents. 1), 70 °C, O2 atmosphere). Dotted line shows emission recorded under the same conditions, but in the absence of FeCl3. The arrow marks the moment of mixing the reagents. | ||
Furthermore, we have found that the reaction of artemisinin, as well as the biologically active trioxolane OZ03—the precursor of arterolane—with either FeSO4 or FeCl3/L-cysteine in the presence of Rhodamine G in aqueous (50%) acetonitrile is accompanied by light emission from the excited dye (see ESI for the experimental details†). Similar to the trioxolanes 1 and 2, the observed CL was found to be oxygen dependent. These unprecedented findings do not only widen the range of light-emitting 1,2,4-trioxolane reactions, but also testify that CL is not limited to natural peroxides, but also may be solicited from synthetic trioxolanes such as OZ03.
The results of the chemiluminescence characteristics for the reactions of the peroxides 1 or 2 with the FeCl3/L-cysteine hydrochloride/Rhodamine G system are presented in Table 1.
| Peroxide | Conditions | CL characteristics |
|---|---|---|
a All experiments were carried out in a CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1 H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture. The CL yields were estimated from the S/[peroxide] ratio. 1) mixture. The CL yields were estimated from the S/[peroxide] ratio.
|
||
| 1 or 2 | [Peroxide 1 or 2] = [FeCl3] = [Rhodamine G] = 1.5 × 10−3 M, [L-cysteine] = 3 × 10−3 M, 70 °C, O2 atmosphere. | ϕ CL = 1.5 × 10−9 |
| S = 1.3 × 109 | ||
| OZ03 | [OZ03] = 2 × 10−3 M, [FeSO4] = 1 × 10−3 M, [Rhodamine G] = 5 × 10−4 M, 70 °C, O2 atmosphere | ϕ CL = 8 × 10−8 |
| S = 3.6 × 1010 | ||
| 4 | [Peroxide 4] = [FeSO4] = 2 × 10−3 M, [Rhodamine G] = 3 × 10−3 M, 30 °C, air atmosphere | ϕ CL = 4.9 × 10−8 |
| S = 5.8 × 1010 | ||
| I CL = 4.9 × 1010 | ||
| 6 | [Peroxide 6] = 2 × 10−3 M, [FeSO4] = 1 × 10−3 M, [Rhodamine G] = 5 × 10−4 M, 50 °C, Ar atmosphere | ϕ CL = 1.4 × 10−8 |
| S = 1.6 × 1010 | ||
| I CL = 1.8 × 109 |
As already mentioned, 1,2,4,5-tetroxanes may serve as good candidates for the development of effective antimalarial drugs.2a,d,3,12 It was, therefore, of interest to assess whether CL could also be observed in the reactions of ferrous iron with this promising class of biologically active peroxides. As anticipated, the tetroxanes 4–7 readily react with Fe(II) ions to produce CL in the visible region with or without Rhodamine G as enhancer.
On mixing the tetroxane 4 and the FeSO4 solution ([peroxide 4] = [FeSO4] = 2 × 10−3 M, CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1
H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 30 °C), the reaction mixture changed from colorless to brown, which indicates that the Fe2+ ions were oxidized instantly by the peroxide. Analysis of the visible luminescence emission by means of cut-off filters revealed that the CL lies in the spectral region 380–480 nm. This corresponds to the fluorescence of 1,1,1-trifluoroacetone in aqueous (50%) acetonitrile (Fig. S-2, ESI†). Hence, singlet-excited ketone is the emitter of the observed visible CL. Also mechanistically significant is the fact that a catalytic amount of the ferrous salt does not induce the decomposition of the tetroxane; thus, this chemiluminescent reaction is a stoichiometric process.
1), 30 °C), the reaction mixture changed from colorless to brown, which indicates that the Fe2+ ions were oxidized instantly by the peroxide. Analysis of the visible luminescence emission by means of cut-off filters revealed that the CL lies in the spectral region 380–480 nm. This corresponds to the fluorescence of 1,1,1-trifluoroacetone in aqueous (50%) acetonitrile (Fig. S-2, ESI†). Hence, singlet-excited ketone is the emitter of the observed visible CL. Also mechanistically significant is the fact that a catalytic amount of the ferrous salt does not induce the decomposition of the tetroxane; thus, this chemiluminescent reaction is a stoichiometric process.
With an excess of FeSO4 salt ([peroxide 4] = 2 × 10−4 M, [FeSO4] = 4 × 10−3 M, 30 °C), linear plots of the natural logarithm of the CL intensity versus time were obtained (Fig. S-3, ESI†). Reproducible values of the pseudo-first-order rate constants were obtained, which were independent of whether an oxygen (k = 0.38 ± 0.04 s−1) or an argon (k = 0.41 ± 0.02 s−1) atmosphere was used. Evidently, the CL behavior exhibited in the reduction of the trifluoroacetone tetroxane (4) by FeSO4 differs significantly from that observed for the ozonides 1–3: for example, replacing Ar by O2 does not affect the kinetics or the CL intensity of the tetroxane but of the ozonide reaction.
Also the related bicyclic tetroxanes 5 and 6 released CL in their reactions with Fe(II) salts. When a solution of peroxide 5 or 6 was treated with an equal amount of FeSO4 ([peroxide 5 or 6] = 2 × 10−3 M or 1 × 10−3 M, [FeSO4] = 2 × 10−3 M or 1 × 10−3 M, CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1
H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 60 °C), CL was instantly observed (Fig. 4). Notably, no light emission was recorded during thermolysis of the peroxides under the same conditions but without ferrous ions. The CL emission spectra for the reactions of FeSO4 with tetroxanes 5 and 6 are quite similar; both display several emission bands at λ = 460–500, 500–570 and >570 nm. The mechanistically more significant difference in the CL between trifluoroacetone diperoxide and the bicyclic tetroxanes is the quenching effect by molecular oxygen on the light emission. A substantial increase in the luminescence intensity was noticed when the reactions of tetroxanes 5 and 6 with FeSO4 were carried out under an argon atmosphere, whereas the kinetics of luminescence decay were not affected. This oxygen effect suggests that triplet states are involved in the observed CL. As the spectra of the light emissions under argon and oxygen atmospheres manifest, molecular oxygen suppresses the luminescence at 500–570 nm substantially (Fig. S-4, ESI†).
1), 60 °C), CL was instantly observed (Fig. 4). Notably, no light emission was recorded during thermolysis of the peroxides under the same conditions but without ferrous ions. The CL emission spectra for the reactions of FeSO4 with tetroxanes 5 and 6 are quite similar; both display several emission bands at λ = 460–500, 500–570 and >570 nm. The mechanistically more significant difference in the CL between trifluoroacetone diperoxide and the bicyclic tetroxanes is the quenching effect by molecular oxygen on the light emission. A substantial increase in the luminescence intensity was noticed when the reactions of tetroxanes 5 and 6 with FeSO4 were carried out under an argon atmosphere, whereas the kinetics of luminescence decay were not affected. This oxygen effect suggests that triplet states are involved in the observed CL. As the spectra of the light emissions under argon and oxygen atmospheres manifest, molecular oxygen suppresses the luminescence at 500–570 nm substantially (Fig. S-4, ESI†).
![Time profile of the CL decay for the reaction of the tetroxane 5 with FeSO4 [peroxide 5] = [FeSO4] = 2 × 10−3 M, CH3CN : H2O (1 : 1), 60 °C, Ar atmosphere). The arrow marks the moment of mixing the reagents.](/image/article/2012/RA/c1ra00784j/c1ra00784j-f4.gif) | ||
Fig. 4 Time profile of the CL decay for the reaction of the tetroxane 5 with FeSO4 [peroxide 5] = [FeSO4] = 2 × 10−3 M, CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1 H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 60 °C, Ar atmosphere). The arrow marks the moment of mixing the reagents. 1), 60 °C, Ar atmosphere). The arrow marks the moment of mixing the reagents. | ||
It should be noted that the CL observed in the reactions of the tetroxanes 4–6 with FeSO4 was considerably enhanced in the presence of Rhodamine G (Fig. S-5, ESI†). This dye magnifies the emission intensity by energy transfer from the excited carbonyl species produced in the reduction of peroxides by Fe2+ ions. Alternatively, Rhodamine G may be excited in the reaction with the oxygen radicals generated in this FeSO4/tetroxane 4–6 reaction. Indeed, CL arising from the direct oxidation of dyes (e.g. Rhodamine B) has been described in the literature.13
The final tetroxane we examined was the steroid-based peroxide 7. Analogous to the tetroxanes 4–6, on heating (60 °C) in the presence of the FeCl3/L-cysteine/Rhodamine G mixture in acetonitrile, CL was displayed in the visible region.
The chemiluminescence characteristics of the tetroxane 4–6 reactions were found to be similar to those determined for the trioxolanes 1 and 2 (see Table 1). The rather low values are typical for oxidation processes and some biological reactions.8a A careful choice, however, of an appropriate CL activator and/or design of tetroxanes and trioxolanes with chromophore-containing groups will considerably enhance the chemiluminescence intensity of these peroxides.
What is the CL mechanism of the presently discovered reaction of tetroxanes with ferrous ions? A mechanistic scenario is proposed in Scheme 1 for the reaction of tetroxanes with ferrous ions.5d Presumably, light emission may be attributed to the decomposition of the peroxy intermediates A and/or B, in which electronically excited ketone is formed.
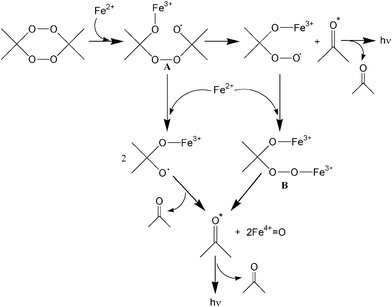 | ||
| Scheme 1 Proposed mechanism for the chemiluminescence of the reaction of tetroxanes with ferrous ions. | ||
In conclusion, we have shown that synthetic 1,2,4-trioxolanes and 1,2,4,5-tetroxanes of structural diversity, as well as the natural artemisinin, produce electronically excited states in their biomimetic reduction by ferrous ions. The light emission is not only important for the chemiluminescence of liquid-phase organic reactions, but also provides a convenient strategy for the use of the discovered phenomenon in the analytical detection of biologically active cyclic peroxides. Therewith, the elucidation of the mechanism of antimalarial action under biomimetic conditions, or possibly even in living organisms, becomes feasible by means of the herein reported CL method.
The research was supported by the Russian Foundation for Basic Research (Grant No 09-03-00831-a), Grant of President of RF for Support of Young Doctors of Science (Grant No MD-3852.2009.3), and the Branch of Chemistry and Material Sciences of the RAS (№1-OKh). WA thanks the Deutsche Forschungsgemeinschaft (DFG), Fonds der Chemischen Industrie, and the Alexander von Humboldt-Stiftung for many years of generous financial support, and the University of Puerto Rico, Rio Piedras Campus for the ‘Professor Emerito’ distinction. This research work is dedicated as a token of appreciation to Professor Gerhard Bringmann for his 60th birthday.
References
- (a) G. A. Biagini, P. M. O'Neill, P. G. Bray and S. A. Ward, Curr. Opin. Pharmacol., 2005, 5, 473 CrossRef CAS; (b) N. J. White, Science, 2008, 320, 330 CrossRef CAS; (c) C. W. Jefford, Drug Discovery Today, 2007, 12, 487 CrossRef CAS; (d) World Health Organization: http://www.who.int/topics/malaria/en/.
- (a) J. L.Vennerstrom, H.-N. Fu, W. Y. Ellis, A. L. Ager, Jr, J. K. Wood, S. L. Andersen, L. Gerena and W. K. Milhoust, J. Med. Chem., 1992, 35, 3023 CrossRef; (b) J. L. Vennerstrom, S. Arbe-Barnes, R. Brun, S. A. Charman, F. C. K. Chiu, J. Chollet, Y. Dong, A. Dorn, D. Hunziker, H. Matile, K. McIntosh, M. Padmanilayam, J. S. Tomas, C. Scheurer, B. Scorneaux, Y. Tang, H. Urwyler, S. Wittlin and W. N. Charman, Nature, 2004, 430, 900 CrossRef CAS; (c) J. L. Vennerstrom, Y. Dong, S. Wittlin, K. Sriraghavan, J. Chollet, S. A. Charman, W. N. Charman, C. Scheurer, H. Urwyler, J. S. Tomas, C. Snyder, D. J. Creek, J. Morizzi, M. Koltun, H. Matile, X. Wang, M. Padmanilayam, Y. Tang, A. Dorn and R. Brun, J. Med. Chem., 2010, 53, 481 CrossRef; (d) P. M. O'Neill, R. K. Amewu, G. L. Nixon, F. B. ElGarah, M. Mungthin, J. Chadwick, A. E. Shone, L. Vivas, H. Lander, V. Barton, S. Muangnoicharoen, P. G. Bray, J. Davies, B. Kevin Park, S. Wittlin, R. Brun, M. Preschel, K. Zhang and S. A. Ward, Angew. Chem., Int. Ed., 2010, 49, 5693 CrossRef CAS.
- For recent reviews on antimalarial peroxides, see: (a) K. M. Muraleedharan and M. A. Avery, Drug Discovery Today, 2009, 14, 793 CrossRef CAS; (b) D. M. Opsenica and B. A. Šolaja, J. Serb. Chem. Soc., 2009, 74, 1155 CrossRef CAS; (c) P. M. O'Neill, J. Chadwick and S. L. Rawe, in The Chemistry of Peroxides, ed. Z. Rappoport, Wiley, Chichester, 2006, vol. 2, part 1, ch. 17, pp. 1279 Search PubMed; (d) B. Meunier and A. Robert, Acc. Chem. Res., 2010, 43, 1444 CrossRef CAS.
- Ranbaxy Laboratories Limited: http://www.ranbaxy.com/researchndevelopment/overview.aspx..
- (a) D. J. Creek, W. N. Charman, F. C. K. Chiu, R. J. Prankerd, Y. Dong, J. L. Vennerstrom and S. A. Charman, Antimicrob. Agents Chemother., 2008, 52, 1291 CrossRef CAS; (b) G. H. Posner and P. M. O'Neill, Acc. Chem. Res., 2004, 37, 397 CrossRef CAS; (c) A. Robert, F. Benoit-Vical and B. Meunier, Coord. Chem. Rev., 2005, 249, 1927 CrossRef CAS; (d) I. Opsenica, N. Terzić, D. Opsenica, G. Angelovski, M. Lehnig, P. Eilbracht, B. Tinant, Z. Juranić, K. S. Smith, Y. S. Yang, D. S. Diaz, P. L. Smith, W. K. Milhous, D. Doković and B. A. Šolaja, J. Med. Chem., 2006, 49, 3790 CrossRef CAS.
- For reviews on chemiluminescence phenomenon, see: (a) W. Adam, D. V. Kazakov and V. P. Kazakov, Chem. Rev., 2005, 105, 3371 CrossRef CAS; (b) W. Adam, D. Reinhardt and C. R. Saha-Möller, Analyst, 1996, 121, 1527 RSC; (c) A. Roda, M. Guardigli, E. Michelini, M. Mirasoli and P. Pasini, Anal. Chem., 2003, 75, 462 A CrossRef CAS.
- (a) S. Miyamoto, G. R. Martinez, A. P. B. Martins, M. H. G. Medeiros and P. Di Mascio, J. Am. Chem. Soc., 2003, 125, 4510 CrossRef CAS; (b) A. C. Velosa, W. J. Baader, C. V. Stevani, C. M. Mano and E. J. H. Bechara, Chem. Res. Toxicol., 2007, 20, 1162 CrossRef CAS; (c) G. E. Ronsein, M. C. B. Oliveira, S. Miyamoto, M. H. G. Medeiros and P. Di Mascio, Chem. Res. Toxicol., 2008, 21, 1271 CrossRef CAS.
- (a) G. F. Fedorova, A. V. Trofimov, R. F. Vasil'ev and T. L. Veprintsev, Arkivoc, 2007, VIII, 163 Search PubMed; (b) G. F. Fedorova, V. A. Menshov, A. V. Trofimov and R. F. Vasil'ev, Analyst, 2009, 134, 2128 RSC.
- Detailed synthesis of peroxides1–3: O. B. Kazakova, D. V. Kazakov, E. Yu. Yamansarov, N. I. Medvedeva, G. A. Tolstikov, K. Yu. Suponitsky and D. E. Arkhipov, Tetrahedron Lett., 2011, 52, 976 CrossRef CAS.
- (a) O. B. Kazakova, G. V. Giniyatullina, E. Yu. Yamansarov and G. A. Tolstikov, Bioorg. Med. Chem. Lett., 2010, 20, 4088 CrossRef CAS; (b) C. Genet, A. Strehle, C. Schmidt, G. Boudjelal, A. Lobstein, K. Schoonjans, M. Souchet, J. Auwerx, R. Saladin and A. Wagner, J. Med. Chem., 2010, 53, 178 CrossRef CAS; (c) L. A. Baltina, O. B. Flekhter, L. R. Nigmatullina, E. I. Boreko, N. I. Pavlova, S. N. Nikolaeva, O. V. Savinova and G. A. Tolstikov, Bioorg. Med. Chem. Lett., 2003, 13, 3549 CrossRef CAS.
- (a) W. Adam, G. Asensio, R. Curci, J. A. Marco, M. E. González-Núňez and R. Mello, Tetrahedron Lett., 1992, 33, 5833 CrossRef CAS; (b) A. O. Terent'ev, D. A. Borisov, V. V. Chernyshev and G. I. Nikishin, J. Org. Chem., 2009, 74, 3335 CrossRef CAS.
- D. M. Opsenica, N. Terzić, P. L. Smith, Y. Yang, L. Anova, K. S. Smith and B. A. Šolaja, Bioorg. Med. Chem., 2008, 16, 7039 CrossRef CAS.
- Y. Ma, X. Jin, M. Zhou, Z. Zhang, X. Teng and H. Chen, Anal. Chim. Acta, 2003, 489, 173 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Chemiluminescence procedure for the reaction of the cyclic peroxides with ferrous ions, the fluorescence and chemiluminescence spectra recorded in the peroxide reactions, and the kinetics of the chemiluminescence decay. See DOI: 10.1039/c1ra00784j |
| This journal is © The Royal Society of Chemistry 2012 |
