Design of highly sensitive volatile organic compound sensors by controlling NiO loading on ZnO nanowire networks†
Chan Woong
Na
,
Hyung-Sik
Woo
and
Jong-Heun
Lee
*
Department of Materials Science and Engineering, Korea University, Anam-Dong, Seongbuk-Gu, Seoul 136-713, Republic of Korea. E-mail: jongheun@korea.ac.kr; Fax: +82-2-928-3584; Tel: +82-2-3290-3282
First published on 17th November 2011
Abstract
Highly sensitive detection of volatile organic compounds, such as C2H5OH and HCHO, has been achieved by decorating p-type NiO nanoparticles on n-type ZnO nanowire networks, whereas the incorporation of Ni into the lattice of the ZnO nanowires deteriorated the gas sensing characteristics.
Crystalline oxide nanowire (NW) networks are promising chemoresistive materials with a high gas response, good stability and fast response kinetics on account of their large surface area, high crystallinity and gas accessible networked structures.1 The gas sensing characteristics can be enhanced further or tuned using additives. Additives for gas sensor design have been reported to have a range of roles, including catalytic promotion of the gas sensing reaction,2 selective gas detection by manipulating the acid–base properties,3 control of the donor density,4 and extension of the electron depletion layer by the formation of a p-n junction.5 These studies suggest that the loading amount, configuration, phase and incorporation into the host lattice of additives are the key parameters, and should be controlled carefully to achieve high-performance oxide semiconductor gas sensors.
Nickel oxide (NiO) is a p-type semiconductor with versatile applications, such as in resistive switching devices,6acatalysts6b and gas sensors.6cNiO can also be used to modify or tune the chemoresistive sensing characteristics of n-type oxide semiconductors, such as SnO2 and ZnO.7 Although the enhancement of gas response,8a gas response/recovery kinetics8b and selectivity8c by the addition of NiO has been reported, the precise role of NiO in the gas sensing characteristics remains unclear because the incorporation of Ni-species into the lattice of an n-type semiconductor and the formation of a secondary NiO phase will have a completely different impact on the gas sensing behavior, i.e., a change in donor density (and its consequent effect on the electron depletion layer thickness and sensor resistance) and an extension of the electron depletion layer near the interface of the p-n junction, respectively.
In this study, to determine the role of NiO in the gas sensing reaction of n-type ZnO NWs, three different ZnO NW network sensors, (1) a ‘ZnO sensor’ (pristine ZnO NW networks), (2) a ‘NiO-decorated ZnO sensor’ (ZnO NW networks decorated with a discrete form of p-type NiO particles, p-n junction) and (3) a ‘Ni-doped ZnO sensor’ (Ni-doped ZnO networks, incorporation of Ni into ZnO lattice), were prepared and the gas sensing mechanisms were studied in relation to the phase of the Ni-based additives.
To the best of the authors' knowledge, control of the gas sensing mechanism in ZnO NW networks according to the phase and configuration of NiO additives has never been reported and will provide general insight into the role of additives for the design of oxide semiconductor gas sensors.
Pristine ZnO NW networks were grown on an alumina substrate with two gold electrodes (‘ZnO sensor’, Fig. S1a in ESI†) by thermal evaporation. The ZnO NWs were identified as single crystalline ones with clean surfaces (Fig. S1b–d in ESI†). Subsequently, the decorative NiO particles were formed on the as-grown ZnO NWs by the thermal evaporation of NiCl2 powders (99.99%, Aldrich) in Ar gas (Ar: 200 sccm) at 500 °C (Fig. 1a). (‘NiO-decorated ZnO sensor’). The Ni-doped ZnO NWs were grown on alumina substrates with two gold electrodes by a thermal reaction of NiCl2 and a mixture of ZnO and graphite powders in Ar–O2 gas (Ar: 100 sccm, O2: 1 sccm) using a two-temperature-zone reactor (temperature of Ni-source: 600 °C, temperature of Zn-source and substrate: 900 °C). (‘Ni-doped ZnO sensor’, Fig. S2 in ESI†). In all 3 different sensors, high-density NWs were grown homogeneously between two gold electrodes (Fig. S1a in ESI†, Fig. 1a–c, Fig. S2a–b in ESI†). The diameters of the NiO-decorated ZnO NWs ranged from 50 to 80 nm (Fig. 1d and 1e), and were similar to those of ZnO NWs (Fig. S1b–c in ESI†) and Ni-doped ZnO NWs (Fig. S2c–d in ESI†). In the lattice-resolved TEM image (Fig. 1f) of the NiO-decorated ZnO NWs, the (01![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) fringes of ZnO and the (220) fringes of the NiO particles were separated by 2.81 Å and 1.47 Å, respectively. The fast Fourier transform (FFT) pattern confirms that a NiO nanoparticle is single crystalline fcc NiO phase (Fig. 1g) and highly crystalline ZnO NW are grown along the [01
0) fringes of ZnO and the (220) fringes of the NiO particles were separated by 2.81 Å and 1.47 Å, respectively. The fast Fourier transform (FFT) pattern confirms that a NiO nanoparticle is single crystalline fcc NiO phase (Fig. 1g) and highly crystalline ZnO NW are grown along the [01![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0] direction (Fig. 1h). This shows that the crystalline NiO nanoparticles are coated uniformly on single crystalline ZnO NWs. Besides the lenticular morphology of the NiO nanoparticles (Fig. 1e), an angular configuration of the NiO nanoparticles was also observed (Fig. 1i), which were identified as NiO nanoparticles by elemental mapping of Zn, Ni, and O using energy dispersive X-ray spectroscopy (EDS). The incorporation of Ni into the lattice of ZnO NWs in Ni-doped ZnO NWs was confirmed from the crystalline lattice image, the presence of a uniform Ni concentration profile across the single NW with clean surface morphology in the EDS line scan profile (Fig. S2d–f in ESI†) and EDS analyses of different spots (data not shown). The near band edge (NBE) peak of the Ni-doped ZnO NWs determined from photoluminescence (PL) analysis was observed at 3.21 eV, which was substantially lower than both pristine ZnO NWs (3.25 eV) and NiO-decorated ZnO NWs (3.25 eV) (Fig. S3 in ESI†). The red shift in the Ni-doped ZnO NWs was attributed to the incorporation of Ni into the lattice of ZnO NWs.9 This is supported by the same NBE peak positions of pristine and NiO-decorated ZnO NWs. Finally, the single phase of Ni-doped ZnO NWs and two phases (NiO + ZnO) of NiO-decorated ZnO NWs were clearly confirmed by X-ray diffraction (Fig. S4 in ESI†).
0] direction (Fig. 1h). This shows that the crystalline NiO nanoparticles are coated uniformly on single crystalline ZnO NWs. Besides the lenticular morphology of the NiO nanoparticles (Fig. 1e), an angular configuration of the NiO nanoparticles was also observed (Fig. 1i), which were identified as NiO nanoparticles by elemental mapping of Zn, Ni, and O using energy dispersive X-ray spectroscopy (EDS). The incorporation of Ni into the lattice of ZnO NWs in Ni-doped ZnO NWs was confirmed from the crystalline lattice image, the presence of a uniform Ni concentration profile across the single NW with clean surface morphology in the EDS line scan profile (Fig. S2d–f in ESI†) and EDS analyses of different spots (data not shown). The near band edge (NBE) peak of the Ni-doped ZnO NWs determined from photoluminescence (PL) analysis was observed at 3.21 eV, which was substantially lower than both pristine ZnO NWs (3.25 eV) and NiO-decorated ZnO NWs (3.25 eV) (Fig. S3 in ESI†). The red shift in the Ni-doped ZnO NWs was attributed to the incorporation of Ni into the lattice of ZnO NWs.9 This is supported by the same NBE peak positions of pristine and NiO-decorated ZnO NWs. Finally, the single phase of Ni-doped ZnO NWs and two phases (NiO + ZnO) of NiO-decorated ZnO NWs were clearly confirmed by X-ray diffraction (Fig. S4 in ESI†).
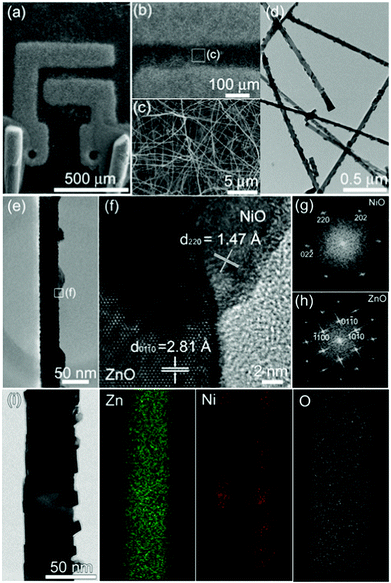 | ||
| Fig. 1 Morphology and crystal structures of the NiO-decorated ZnO NWs: (a), (b), and (c) SEM images of NiO-decorated ZnO NWs grown on alumina substrates with two Au electrodes; (d), (e), and (f) TEM images of ZnO NWs decorated with lenticular NiO nanoparticles; (g) and (h) FFT of electron diffraction patterns of NiO and ZnO; (i) TEM image of ZnO NW decorated with angular NiO nanoparticles and EDS elemental mapping of Zn, Ni, and O. | ||
To understand the role of NiO in the gas sensing of NiO-loaded ZnO NW networks, the gas responses (S = Ra/Rg, Ra: resistance in air, Rg: resistance in gas) of pristine ZnO, NiO-decorated ZnO and Ni-doped ZnO NWs to different volatile organic compounds (5 ppm C2H5OH, HCHO, CH3CHO, CO, C3H8, H2, benzene, toluene, and p-xylene) were measured over the temperature range of 300–450 °C (Fig. 2a–c). All these sensors showed typical n-type gas sensing behavior, i.e., a decrease in resistance by reducing gases. In particular, n-type gas sensing in NiO-decorated ZnO NWs confirms that conduction occurs along continuous n-type ZnO NWs rather than the discrete configuration of p-type NiO nanoparticles. In general, the sensors showed strong responses to C2H5OH and HCHO over a wide range of sensing temperatures, and the maximum gas responses were obtained at 450 °C. On the other hand, the absolute gas responses were completely different according to the sensor configurations. The response to 5 ppm C2H5OH of NiO-decorated ZnO NWs at 450 °C was 29.04, which was 7.96 times higher than that of pristine ZnO NWs (3.65). Interestingly, Ni doping deteriorated the response to 5 ppm C2H5OH down to 2.18.
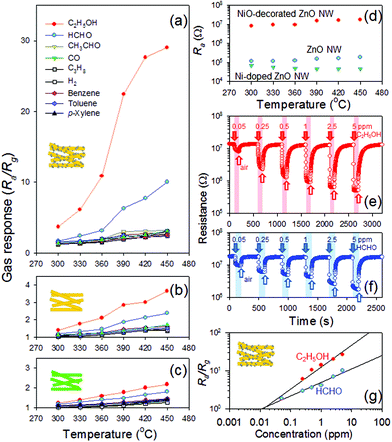 | ||
| Fig. 2 Gas responses (Ra/Rg, Ra: resistance in air and Rg: resistance in gas) to C2H5OH, HCHO, CH3CHO, CO, C3H8, H2, benzene, toluene, and p-xylene over the temperature range of 300 to 450 °C: (a) gas response of NiO-decorated ZnO NW sensor; (b) gas response of ZnO NW sensor; (c) gas response of Ni-doped ZnO NW sensor; (d) the resistances of ZnO, Ni-doped ZnO, and NiO-decorated ZnO NW sensors in air; (e) and (f) dynamic sensing transients and (g) gas responses of NiO-decorated ZnO NW sensor to 0.05–5 ppm C2H5OH and HCHO at 450 °C. | ||
The effect of NiO loading on the Ra values of the sensors was measured (Fig. 2d). The Ra values of the NiO-decorated ZnO NW sensor (8348–17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 kΩ) were ∼2 orders of magnitude higher than those of the pristine ZnO NW sensor (116–192 kΩ), which was attributed to the extension of the electron depletion layer at the interface between p-type NiO and n-type ZnO NWs and its consequent narrowing of the semiconducting path along the inner parts of the ZnO NWs. In contrast, the Ra values were decreased substantially to 47–71 kΩ by Ni doping. The change in Ra for the 5 different ZnO and Ni-doped ZnO NW sensors prepared using the same experimental procedure was <10%. This shows that the decrease in Ra emanates not from sample variation but from Ni doping. He et al.9 reported similar data demonstrating that the electrical conductivity of Ni-doped ZnO NWs was 10–20 times higher than that of pure ZnO NWs and explained this by the co-existence of Ni3+ and Ni2+. X-Ray photoelectron spectroscopy (XPS) was performed to examine the concentration and oxidation state of the Ni component (Fig. S5 in ESI†). The Ni concentrations of NiO-decorated ZnO NWs and Ni-doped ZnO NWs were determined by XPS to be 4.85 and 19.12 at%. Both Ni2+ and Ni3+ bonding structures were observed in Ni 2p3/2spectra and the Ni3+ area of the Ni-doped ZnO NW was larger than that of the NiO-decorated ZnO NWs. This suggests that the incorporation of Ni3+ into the ZnO lattice is responsible for the Ni-induced decrease in resistance. The increase in donor density is known to reduce the gas response to reducing gases in n-type oxide semiconductors,4 which is consistent with the deterioration of gas responses by Ni doping.
400 kΩ) were ∼2 orders of magnitude higher than those of the pristine ZnO NW sensor (116–192 kΩ), which was attributed to the extension of the electron depletion layer at the interface between p-type NiO and n-type ZnO NWs and its consequent narrowing of the semiconducting path along the inner parts of the ZnO NWs. In contrast, the Ra values were decreased substantially to 47–71 kΩ by Ni doping. The change in Ra for the 5 different ZnO and Ni-doped ZnO NW sensors prepared using the same experimental procedure was <10%. This shows that the decrease in Ra emanates not from sample variation but from Ni doping. He et al.9 reported similar data demonstrating that the electrical conductivity of Ni-doped ZnO NWs was 10–20 times higher than that of pure ZnO NWs and explained this by the co-existence of Ni3+ and Ni2+. X-Ray photoelectron spectroscopy (XPS) was performed to examine the concentration and oxidation state of the Ni component (Fig. S5 in ESI†). The Ni concentrations of NiO-decorated ZnO NWs and Ni-doped ZnO NWs were determined by XPS to be 4.85 and 19.12 at%. Both Ni2+ and Ni3+ bonding structures were observed in Ni 2p3/2spectra and the Ni3+ area of the Ni-doped ZnO NW was larger than that of the NiO-decorated ZnO NWs. This suggests that the incorporation of Ni3+ into the ZnO lattice is responsible for the Ni-induced decrease in resistance. The increase in donor density is known to reduce the gas response to reducing gases in n-type oxide semiconductors,4 which is consistent with the deterioration of gas responses by Ni doping.
As a measure for selective detection, the selectivity for 5 ppm C2H5OH (or HCHO) was defined as the response ratio between the gas responses to 5 ppm C2H5OH (or HCHO) and those to other interference gases (SLethanol/gas = Sethanol/Sgas, SLHCHO/gas = SHCHO/Sgas). The SLethanol/gas values for the interference gases (5 ppm CH3CHO, CO, C3H8, H2, benzene, toluene, and p-xylene) of the NiO-decorated ZnO sensor at 450 °C ranged from 9.1 to 12.1 (Fig. S6 in ESI†), which are significantly higher than those of pristine ZnO (2.1–2.6) and Ni-doped ZnO (1.5–1.7) sensors. The C2H5OH gas can be either dehydrogenated (C2H5OH (g) → CH3CHO (g) + H2(g)) at the surface of basic oxide or dehydrated (C2H5OH(g) → C2H4(g) + H2O(g)) at the surface of acidic oxide.10 In the literature, the enhancement of C2H5OH response by the addition of basic oxides to SnO2 gas sensors has been explained by the dissociation of C2H5OH into more reactive reducing gases (CH3CHO and H2).10 Considering that NiO is classified as a basic metal oxide, the selective detection of C2H5OH in NiO-decorated ZnO NWs can be understood from a similar viewpoint. The SLHCHO/gas values for interference gases of the NiO-decorated ZnO sensor ranged from 3.1 to 4.2, which are significantly higher than those of the pristine ZnO (1.4–1.7) and Ni-doped ZnO (1.2–1.4) sensors. This demonstrates that the decoration of NiO is very promising for enhancing the selectivity for C2H5OH and HCHO. Significant differences in the gas responses, Ra values and selectivity between the NiO-decorated ZnO and Ni-doped ZnO sensors clearly suggest that the sensing mechanisms of these two sensors should be understood in completely different frameworks.
The sensing transients to C2H5OH and HCHO of the NiO-decorated ZnO NWs sensor were measured at 450 °C, which showed stable sensing and recovery characteristics (Fig. 2e–f). The gas responses to 0.05–5 ppm C2H5OH ranged from 1.82 to 26.15 at 450 °C. The low detection limit of C2H5OH was calculated to be 0.017 ppm (Fig. 2g) when Ra/Rg ≥ 1.2 was used as the criterion for reliable gas sensing. This is markedly lower than that needed for screening intoxicated drivers ([C2H5OH] > 200 ppm).11 Accordingly, this sensor can be used as a ppb-level alcohol sensor and a breath alcohol detector.
The gas responses of the NiO-decorated ZnO, ZnO, and Ni-doped ZnO NWs to 5 ppm HCHO at 450 °C were 10.03, 2.38 and 1.81, respectively (Fig. 2a–c). The tendency of the HCHO responses according to the NiO configuration was similar to that of the C2H5OH responses. Note that the response to HCHO is also significantly higher than those of all other interference gases, even though the cross-response to C2H5OH should be suppressed further. In particular, the HCHO response of the NiO-decorated ZnO sensor at 450 °C was 3.1–4.2 times higher than those of CH3CHO, CO, C3H8, H2, benzene, toluene and p-xylene (Fig. S6 in ESI†). The gas responses to 0.05–5 ppm of HCHO ranged from 1.89 to 10.03. This is one of the highest responses to 0.05 to 10 ppm HCHO reported in the literature.12 The low detection limit of HCHO was calculated to be 0.019 ppm at the same criterion (Ra/Rg ≥ 1.2). According to the occupational safety and health administration of the USA,13 the permissible exposure limit (PEL) for HCHO in the workplace is 2 ppm in the case of short-term exposure (15 min) and becomes lower (0.75 ppm) when the 8 h time weighted average value is considered. Therefore, the present NiO-decorated ZnO NW network sensor with ppb-level sensitivity can be applied to high-performance HCHO sensors with negligible cross-responses to benzene, toluene and xylene.
In summary, ZnO, NiO-decorated ZnO, and Ni-doped ZnO NW networks were prepared and their gas sensing characteristics were examined and compared to determine the role of NiO additives in the chemoresistive sensing of ZnO NWs. The formation of a p-n junction in the NiO-decorated ZnO NWs and the incorporation of Ni into the lattice of the ZnO NWs were confirmed by SEM, TEM, EDS, PL, XPS and XRD. The gas responses, Ra values, and selectivity for C2H5OH and HCHO of the NiO-decorated ZnO and Ni-doped ZnO sensors were significantly different from each other, which clearly indicates that the sensing mechanisms of these two sensors should be understood in a completely different framework. The doping of Ni in the ZnO NWs decreased the Ra value and deteriorated the gas sensing characteristics. In contrast, the decoration of p-type NiO nanoparticles on n-type ZnO NWs increased the Ra value by two orders of magnitude due to the extension of the electron depletion layer near the p-n junction, and greatly enhanced the responses and selectivity for C2H5OH and HCHO. The NiO-decorated ZnO NWs provide a promising material platform for the highly sensitive and selective detection of C2H5OH and HCHO with a ppb-level response.
Acknowledgements
This work was supported by the KOSEF NRL Program (R0A-2008-000-20032-0).References
- (a) A. Kolmakov and M. Moskovits, Annu. Rev. Mater. Res., 2004, 34, 151 CrossRef CAS; (b) E. Comini, C. Baratto, G. Faglia, M. Ferroni, A. Vomiero and G. Sberveglieri, Prog. Mater. Sci., 2009, 54, 1 CrossRef CAS.
- (a) A. Kolmakov, D. O. Klenov, Y. Lilach, S. Stemmer and M. Moskovits, Nano Lett., 2005, 5, 667 CrossRef CAS; (b) G. De, R. Köhn, G. Xomeritakis and C. J. Brinker, Chem. Commun., 2007, 1840–1842 RSC; (c) H. J. Kim, K. I. Choi, A. Pan, I. D. Kim, H. R. Kim, K. M. Kim, C. W. Na, G. Cao and J. H. Lee, J. Mater. Chem., 2011, 21, 6549 RSC.
- T. Jinkawa, G. Sakai, J. Tamaki, N. Miura and N. Yamazoe, J. Mol. Catal. A: Chem., 2000, 155, 193 CrossRef CAS.
- N. Yamazoe and K. Shimanoe, Sens. Actuators, B, 2009, 138, 100 CrossRef.
- (a) M.-G. Willinger, G. Neri, E. Rauwel, A. Bonavita, G. Micali and N. Pinna, Nano Lett., 2008, 8, 4201 CrossRef CAS; (b) C. Marichy, N. Donato, M.-G. Willinger, M. Latino, D. Karpinsky, S.-H. Yu, G. Neri and N. Pinna, Adv. Funct. Mater., 2011, 21, 658 CrossRef CAS; (c) I. S. Hwang, J. K. Choi, S. J. Kim, K. Y. Dong, J. H. Kwon, B. K. Ju and J. H. Lee, Sens. Actuators, B, 2009, 142, 105 CrossRef.
- (a) R. Waser, R. Dittmann, G. Staikov and K. Szot, Adv. Mater., 2009, 21, 2632 CrossRef CAS; (b) E. Ruckenstein and Y. H. Hu, Appl. Catal., A, 1995, 133, 149 CrossRef CAS; (c) N. G. Cho, I. S. Hwang, H. G. Kim, J. H. Lee and I. D. Kim, Sens. Actuators, B, 2011, 155, 366 CrossRef.
- (a) X. Liu, J. Zhang, X. Guo and S. Wang, Sens. Actuators, B, 2011, 152, 162 CrossRef; (b) L. Liu, L. Wang, C. Guo, Q. Dong and W. Li, J. Am. Ceram. Soc., 2011, 94, 771 CrossRef CAS; (c) P. Lv, Z. A. Tang, T. Yu, F. T. Zhang, G. F. Wei, Z. X. Huang and Y. Hu, Sens. Actuators, B, 2008, 132, 74 CrossRef.
- (a) Z. Wang, Z. Li, J. Sun, H. Zhang, W. Wang, W. Zheng and C. Wang, J. Phys. Chem. C, 2010, 114, 6100 CrossRef CAS; (b) H. R. Kim, K. I. Kim, K. M. Kim, I. D. Kim, G. Cao and J. H. Lee, Chem. Commun., 2010, 46, 5061 RSC; (c) V. V. Sysoev, B. K. Button, K. Wepsiec, S. Dmitriev and A. Kolmakov, Nano Lett., 2006, 6, 1584 CrossRef CAS.
- H. He, C. S. Lao, L. J. Chen, D. Davidovic and Z. L. Wang, J. Am. Chem. Soc., 2005, 127, 16376 CrossRef CAS.
- T. Jinkawa, G. Sakai, J. Tamaki, N. Miura and N. Yamazoe, J. Mol. Catal. A: Chem., 2000, 155, 193 CrossRef CAS.
- Y. Liu, E. Koep and M. Liu, Chem. Mater., 2005, 17, 3997 CrossRef CAS.
- (a) D. Mao, J. Yao, X. Lai, M. Yang, J. Du and D. Wang, Small, 2011, 7, 578 CrossRef CAS; (b) Z. Bai, C. Xie, M. Hu and S. Zhang, Phys. E., 2008, 41, 235 CrossRef CAS; (c) Y. Zheng, J. Wang and P. Yao, Sens. Actuators, B, 2011, 156, 723 CrossRef; (d) T. Itoh, I. Matsubara, W. Shin, N. Izu and M. Nishibori, Sens. Actuators, B, 2008, 128, 512 CrossRef; (e) J. Wang, P. Zhang, J. Q. Qi and P. J. Yao, Sens. Actuators, B, 2009, 136, 399 CrossRef.
- http://www.osha.gov/ .
Footnote |
| † Electronic supplementary information (ESI) available: Detailed preparation and characterization section. See DOI: 10.1039/c1ra01001h |
| This journal is © The Royal Society of Chemistry 2012 |
