DOI:
10.1039/C1RA01135A
(Paper)
RSC Adv., 2012,
2, 1827-1834
Microwave-assisted synthesis of hydropyridines and study of the DPPH-scavenging activity†
Received
18th November 2011
, Accepted 18th November 2011
First published on 4th January 2012
Abstract
A series of hydropyridines (HP's) were synthesized using dimedones, ethyl acetoacetate, ammonium acetate and appropriate aldehydes under solvent-free conditions and microwave-irradiation through Hantzsch reaction. All the synthesized HP's were tested in vitro for the 1,1-diphenyl-2-picrylhydrazyl (DPPH) scavenging activity and structure–activity relationship was discussed; only 12 out of the 36 HP's showed antioxidant activity. Polyhydroquinolines (PHQ's) were the most active compounds among the studied HP's, they were characterized by the lack of an ester-like structure substituent at carbon 5.
Introduction
The Hantzsch protocol (Scheme 1) is particularly attractive, since the resulting hydropyridine (HP) scaffold displays a wide range of biological activities and it has been used for the development of a number of lead compounds.1 In general, the standard procedure for the Hantzsch condensation involves a one-pot condensation of the three building blocks in a solvent, such as ethanol, and using a strongly acidic catalyst (e.g., hydrochloric acid).2 One major drawback of this procedure, apart from the long reaction times involving reflux temperatures, is the moderate yields frequently observed when using more complex building blocks. We have recently described a high yielding and rapid microwave-assisted protocol that allows the synthesis of heterocyclic compounds utilizing controlled single-mode microwave irradiation.3
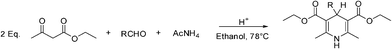 |
| | Scheme 1 Synthesis of hydropyridine derivatives (Hantzsch protocol). | |
Hantzsch 1,4-dihydropyridine (1,4-DHP) and derivatives are widely used as calcium channel blockers for the treatment of cardiovascular disorders including angina, hypertension, and cardiac arrhythmias.4 These compounds are oxidized to pyridine derivatives by the action of cytochrome P-450 in the liver.5 The antioxidant properties of these calcium antagonists may affect their therapeutic action in the course of treatment of some cardiovascular disorders.6 Among the 1,4-DHP derivatives, antioxidant activity was initially found in some 4-unsubstituted compounds and then was also revealed in a group of 4-aryl-1,4-DHP derivatives including nifedipine, nisoldipine, felodipine, nicardipine and lacidipine.7 The aromatization of the HP's is also one of the ubiquitous problems in microwave assisted organic synthesis.8
In this study, HP's were synthesized by microwave irradiation using aldehydes, dimedones, ethyl acetoacetate and ammonium acetate; operational simplicity, low cost, high yields, short reaction times and low toxicity are the key features associated with this protocol. The radical scavenging activity of HP's was studied as well as the structure–activity relationship.
Results and discussion
The reactions of aldehydes 1, dimedones 2, ethyl acetoacetate 3 and ammonium acetate 4, under solvent-free conditions and microwave-irradiations, have been considered as a standard model reaction. The methodology was applied for the synthesis of a variety of HP's (Scheme 2). It is worth noticing that this work is a contribution to the green chemistry protocol since the method is environmentally safe.
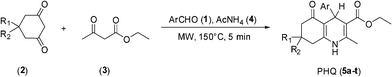 |
| | Scheme 2 Synthesis of polyhydroquinoline (PHQ) derivatives through Hantzsch reaction. | |
Under classical conditions, the model reaction of benzaldehyde, dimedone, ethyl acetoacetate and ammonium acetate, at reflux temperature in ethanol (24 h), produced a solid product (5a), but in low yield (45%); the TLC of reaction mixture indicated the presence of starting materials. However, under solvent-free conditions and microwave-irradiation the yield of product (5a) was improved significantly (96%). The better yield in solvent-free conditions could be explained by a uniform distribution of the eutectic mixture of reactants, being in closer proximity to react than in conditions using ethanol as the solvent. It is clear that microwave-irradiation had an important effect on the reaction and the best results were obtained under solvent-free conditions. In terms of reactivity, it must also be taking into account that microwaves affect the mechanism of reaction; particularly by considering how the system polarity is changing during the progress of the reaction.9 In general, the reactions showed no further progress after 10 min, as evidenced by TLC. The higher yields were obtained at 10 min between 130 and 150 °C; yields were moderate at lower temperatures and product decomposition was registered at higher temperatures. The results of this study are summarized in Table 1.
Table 1 Synthesis of hydropyridines under microwave-irradiation
| Entry |
Ar |
Yields (%)a |
R
1
|
R
2
|
m.p. (°C)b |
m.p. (°C)c |
|
Yields refer to isolated products.
Melting points for the synthesized products.
Melting points of those reported in the literature.
|
| PHQ |
|
|
|
|
|
|
| 5a |
C6H5 |
96 |
CH3 |
CH3 |
227–229 |
225–22710 |
| 5b |
4-MeOC6H4 |
92 |
CH3 |
CH3 |
260–262 |
258–26010 |
| 5c |
4-HO-3-MeOC6H3 |
90 |
CH3 |
CH3 |
200–202 |
208–21010 |
| 5d |
4-EtO-3-HOC6H3 |
95 |
CH3 |
CH3 |
195–197 |
196–19711 |
| 5e |
4-NO2C6H4 |
91 |
CH3 |
CH3 |
240–242 |
240–24210 |
| 5f |
4-(Me)2NC6H4 |
90 |
CH3 |
CH3 |
261–263 |
263–26412 |
| 5g |
iso-propyl |
80 |
CH3 |
CH3 |
144–146 |
147–14813 |
| 5h |
Pentyl |
75 |
CH3 |
CH3 |
150–152 |
153–15414 |
| 5i |
C6H5 |
90 |
H |
H |
241–242 |
240–24115 |
| 5j |
4-MeOC6H4 |
93 |
H |
H |
191–193 |
193–19515 |
| 5k |
4-HOC6H4 |
90 |
H |
H |
219–220 |
220–22215 |
| 5l |
4-HO-3-MeOC6H3 |
90 |
H |
H |
210–212 |
— |
| 5m |
4-EtO-3-HOC6H3 |
92 |
H |
H |
188–190 |
— |
| 5n |
4-NO2C6H4 |
95 |
H |
H |
202–204 |
204–20515 |
| 5o |
iso-propyl |
75 |
H |
H |
177–179 |
180–18216 |
| 5p |
Pentyl |
75 |
H |
H |
129–131 |
220–22216 |
| 5q |
C6H5 |
91 |
CH3 |
H |
204–206 |
— |
| 5r |
4-HO-3-MeOC6H3 |
90 |
CH3 |
H |
239–241 |
— |
| 5s |
4-EtO-3-HOC6H3 |
89 |
CH3 |
H |
146–148 |
— |
| 5t |
CH3 |
80 |
CH3 |
H |
172–174 |
— |
| DHP |
|
|
|
|
|
|
| 6a |
C6H5 |
92 |
|
|
155–157 |
157–15817 |
| 6b |
4-MeOC6H4 |
93 |
|
|
156–158 |
158–16017 |
| 6c |
4-HO-3-MeOC6H3 |
90 |
|
|
152–15417 |
— |
| 6d |
4-EtO-3-HOC6H3 |
96 |
|
|
155–15717 |
— |
| 6e |
4-MeC6H4 |
93 |
|
|
137–139 |
139–14017 |
| 6f |
CH3 |
82 |
|
|
127–129 |
130–13118 |
| 6g |
iso-propyl |
87 |
|
|
92–94 |
95–9718 |
| 6h |
Pentyl |
79 |
|
|
94–9619 |
— |
| PHA |
|
|
|
|
|
|
| 7a |
C6H5 |
98 |
|
|
> 250 |
277–27920 |
| 7b |
4-MeOC6H4 |
95 |
|
|
> 250 |
278–28020 |
| 7c |
4-HO-3-MeOC6H3 |
95 |
|
|
> 250 |
>30021 |
| 7d |
4-EtO-3-HOC6H3 |
90 |
|
|
> 250 |
>30021 |
| 7e |
4-(Me)2NC6H4 |
93 |
|
|
> 250 |
256–25721 |
| 7f |
4-MeC6H4 |
93 |
|
|
> 250 |
279–28120 |
| 7g |
iso-propyl |
85 |
|
|
142–14420 |
— |
| 7h |
H |
83 |
|
|
> 25020 |
— |
The results of the HP synthesis clearly show the specificity of the reaction with respect to aromatic and aliphatic aldehyde reagents (Table 1); the use of aromatic aldehydes produced higher yields (PHQ's, 89–96%) than the obtained with aliphatic aldehydes (75–80%). Moreover, different substituent groups in the aromatic aldehyde reagents did not show any significant effect on the yield of HP's. We investigated the effect of substitution in 1,3-cyclohexanedione system such as 5,5-dimethyl-1,3-cyclohexanedione and 5-methyl-1,3-cyclohexanedione; the methyl group substitution on the dimedone ring did not show any obvious effect on this conversion, and products were obtained in high yields in relatively short reaction times. Only trace amounts of the unwanted dimedone-aldehyde adduct were observed by 1H-NMR and the major isolated product was a polyhydroquinoline (PHQ) derivative.22 To further expand the scope of the reaction, we next examined the reactions of 2 equivalents of diketone with aromatic aldehydes (Scheme 3). As expected, these substrates underwent smooth, one-pot conversion to give the corresponding 1,4-dihydropyridine (6a–h) and 1,8-dioxodecahydroacridines (PHA) (7a–h) (Table 1).
 |
| | Scheme 3 Synthesis of dihydropyridine (DHP) and polyhydroacridine (PHA) derivatives through the Hantzsch reaction. | |
All the products were identified by contrasting their analytical data (IR, 1H-NMR, 13C-NMR and ESI-MS) with those of authentic standards. The isolated products 5a–t were racemic mixtures and not atropisomers.22,23
The HP's were evaluated for their antioxidant activities by the DPPH-scavenging assay; activity was calculated as the intensity decrease (%) in the absorption of the DPPH at 515 nm in the presence of the test compound. The DPPH-scavenging model is extensively used to evaluate antioxidant activities in less time than other methods. The antioxidant activity of our compounds was contrasted with the effect of the BHT and Trolox (Table 2).
Table 2 DPPH-scavenging activity of hydropyridines
| Compoundsa |
% DPPH Scavenging activity (100 μg mL−1)b |
|
By considering all of the synthesized HP's, only those showing antioxidant activity are registered.
Values represent mean ± standard deviation, n = 3.
|
| 5c |
47.5 ± 0.39 |
| 5d |
55.1 ± 0.08 |
| 5k |
48.0 ± 0.25 |
| 5l |
53.5 ± 0.10 |
| 5m |
48.5 ± 0.25 |
| 5r |
47.4 ± 0.19 |
| 5s |
49.0 ± 0.03 |
| 6c |
48.0 ± 0.07 |
| 7c |
25.0 ± 0.30 |
| 7d |
31.0 ± 0.01 |
| 7g |
13.0 ± 0.45 |
| 7h |
45.0 ± 0.10 |
| BHT |
35.0 ± 0.08 |
| Trolox |
80.0 ± 0.03 |
Only 12 of the 36 synthesized HP's showed activity against the DPPH, evaluated at 100 μg mL−1; within this group, most of them were more active than BHT, in decreasing order 5d > 5l > 5s > 5m ≈ 5k ≈ 6c > 5c ≈ 5r > 7h, but the antioxidant activities of all of these 12 HP's were lower than the registered for Trolox (Table 2); thus, PHQ's were the most active compounds, followed by one of the DHP derivatives (6c) and being the PHA derivatives the less active. The presence of an alkyl group at the C-4 reduces the antioxidant activity even though it has been demonstrated that electron-donating substituents at this position improve the reducing power of the proton at this carbon.24 Remarkably, the antioxidant activity of the PHQ's (5c, 5d, 5l, 5m, 5r, 5s) was significantly higher than the activity of other 1,4-DHP's, an specific characteristic of the ortho-hydroxy-alkoxy-phenyl group, when the hydroxyl group is sandwiched between two ortho substituents and at least one them is an alkoxy group, it is possible to observe the formation of an intra-molecular hydrogen bond (Fig. 1).25 Therefore, the physiological activity of HP's could be influenced by their participation on the in vivo redox-reactions.
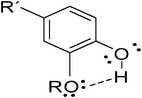 |
| | Fig. 1 Intra-molecular hydrogen bonding. | |
Similar results have been reported by Nakao et al. who analyzed quantitatively the inhibitory activity of various substituted hydroxyphenylureas against lipid peroxidation by aeration of rat brain homogenate; they found that antioxidant activity was mostly governed by the electronic and steric effects of substituents on the phenolic hydroxyl group.26 Moreover, up to this time it has been observed that the activity of derivatives of 1,4-dihydropyridine, as a rule, is either substantially decreased or disappears entirely when a substituent, even an electron donor substituent, was introduced into the 4-position, with the exception of derivatives of 1,4-dihydropyridines possessing weak electron acceptor groups in the 3- and 5-positions. The antioxidant activity of a series of 2,6-dimethyl-3,5-dialkoxycarbonyl-1,4-dihydropyridines possessing various side chain length alkyls (CH3–C16H33) in the ester moiety were evaluated in transition metal-ion catalyzed liposome peroxidation. The compounds 2,6-dimethyl-3,5-diethyloxycarbonyl-1,4-dihydropyridines and 2,6-dimethyl-3,5-dibutyloxycarbonyl-1,4-dihydropyridines exhibit higher antioxidant activities than Trolox and probucol.27 On the other hand, Janero and Burghardt7a suggested that the presence of ethyl ester on C3 and C5 in the DHP ring would result in the complete loss of antiperoxidant activity. This seems to be valid in our test; the PHQ's which displayed good antioxidant activities did not bear ethyl ester substituents at C5 and particularly compound 5d, which was the PHQ with the highest antioxidant activity, did not show such substituents.
Similar studies have been carried out in a series of novel 5-acetyl-2-alkylthio-4-aryl-6-methyl-1,4-dihydropyridine-3-carboxylic acid nitriles. The compound with the highest antioxidant activity has the substituent 3,4-dihydrophenyl at the 4-position, showing the importance of the dihydroxyphenyl substitution at the 4-position.28
Other scientists have studied the antioxidant activity of N-aryl-1,4-dihydropyridines. The compounds 2-methyl-1,4-diphenyl-1,4-dihydropyridine-3-carboxylic acid tert-butyl ester and 1-(4-chlorophenyl)-2-methyl-4-phenyl-1,4-dihydropyridine-3-carboxylic acid tert-butyl ester show significant antioxidant activity because of the presence of a tert-butyl ester group.29
Conclusions
The presented method for the synthesis of hydropyridine derivatives was a good example of a green chemistry protocol. It showed the one-pot reaction of three or four-components coupling dimedones, ethyl acetoacetate, ammonium acetate and appropriate aldehydes under solvent-free conditions and microwave-irradiation through the Hantzsch reaction. The important features of this method include (i) operational simplicity, (ii) no need of any other additive to promote the reaction, (iii) short reaction times, and (iv) high yields of the desired products. All the hydropyridine derivatives were tested in vitro for DPPH activity. Compounds 5c, 5d, 5k, 5l, 5m, 5r, 5s, 6c and 7h showed good DPPH-scavenging activity. This is the first report of the DPPH-scavenging activity of polyhydroquinoline derivatives.
Experimental section
Physical measurements
All reagents were purchased in the highest quality available and were used without further purification. Thin-layer chromatography (TLC) was performed on silica gel F254 plates (Merck). All compounds were detected using UV light. Melting points were obtained on an Electrothermal 88629 apparatus and were uncorrected. Infrared spectra (FTIR) were recorded on a Perkin Elmer FT-IR 1600 spectrophotometer with a KBr disk. 1H and 13C nuclear magnetic resonance spectra at 200 Hz and 50.289 Hz, respectively, were recorded on a Varian Mercury 200 MHz Spectrometer in CDCl3 and DMSO-d6 with TMS as the internal standard. The chemical shifts are expressed as δ values in parts per million (ppm) and the coupling constants (J) are given in hertz (Hz). Electrospray ionization mass spectrometry (ESI-MS) were obtained with an ion trap, and the intensities were reported as a percentage relative to the base peak after the corresponding m/z value. HRMS were obtained in an Agilent LCTOF, a high resolution TOF analyzer with Windows XP based OS and APCI/ESI ionization. The purity was obtained on a High Pressure Liquid Chromatograph 1090 series II, column HPC-18. Microwave equipment was a self-tuning single mode CEM Discover™ Focused Synthesizer. All the spectrophotometric data were acquired using a Spectronic® 20 Genesys™.
General procedure for the preparation of hydropyridine derivatives.
One-pot synthesis of polyhydroquinoline derivatives: aldehydes (1 mmol), ethyl acetoacetate (1 mmol or 2 mmol), dimedones (1 mmol or 2 mmol) and ammonium acetate (1.5 mmol) were properly mixed with the help of a glass rod, placed in a microwave reactor vessel (10 mL) and heated (150 °C/10 min). The progress of the reaction was monitored by TLC (hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate 7
ethyl acetate 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v). After completion of the reaction, the mixture was cooled to RT and dichloromethane (10 mL) was added. Organic solvent was evaporated under reduced pressure and the solid compound was crystallized from absolute ethanol to afford the pure corresponding PHQ derivatives in good yields. All the products were characterized from their spectral data.
3 v/v). After completion of the reaction, the mixture was cooled to RT and dichloromethane (10 mL) was added. Organic solvent was evaporated under reduced pressure and the solid compound was crystallized from absolute ethanol to afford the pure corresponding PHQ derivatives in good yields. All the products were characterized from their spectral data.
Spectroscopic data
2,7,7-Trimethyl-5-oxo-4-phenyl-1,4,5,6,7,8-hexahydroquinoline-3-carboxylic acid ethyl ester (5a).
White solid (815 mg, 2.40 mmol, 96% yield). M.p. 203–205 °C; IR (KBr disk): 3287, 3077, 2964, 1696, 1610 cm−1. 1H-NMR (200 MHz, CDCl3) δ 7.33–7.05 (m, 5H), 6.62 (br s, 1H, NH), 5.05 (s, 1H), 4.06 (q, J = 6.8 Hz, 2H), 2.33 (s, 3H), 2.30–2.08 (m, 4H), 1.20 (t, J = 7.1 Hz, 3H), 1.06 (s, 3H), 0.93 (s, 3H). 13C-NMR (50 MHz, CDCl3) δ 195.7, 167.5, 148.7, 147.1, 143.7, 128.0, 127.9, 126.0, 112.0, 106.0, 59.8, 50.7, 40.9, 36.6, 32.7, 29.4, 27.1, 19.3, 14.2. ESI-MS m/z: 340 [M+H]+, 362 [M+Na]+, 701 [2M+Na]+.
4-(4-Methoxy-phenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylic acid ester (5b).
White solid (850 mg, 2.30 mmol, 92% yield). M.p. 260–262 °C. IR (KBr disk): 3277, 3201, 3078, 2955, 1698, 1606, 1492 cm−1. 1H-NMR (200 MHz, CDCl3) δ 7.17 (d, J = 8.4 Hz, 2H), 6.69 (d, J = 8.4 Hz, 2H), 6.39 (br s, 1H, NH), 4.96 (s, 1H), 4.03 (q, J = 6.9 Hz, 2H), 3.70 (s, 3H), 2.33 (s, 3H), 2.30–2.09 (m, 4), 1.20 (t, J = 6.8 Hz, 3H), 1.04 (s, 3H), 0.91 (s, 3H). 13C-NMR (50 MHz, CDCl3) δ 195.9, 167.6, 157.7, 149.0, 143.6, 139.7, 128.9, 113.2, 111.9, 106.1, 59.8, 55.1, 50.8, 40.7, 35.7, 32.6, 29.5, 27.1, 19.2, 14.3. ESI-MS m/z: 370 [M+H]+, 392 [M+Na]+, 761 [2M+Na]+.
4-(4-Hydroxy-3-methoxy-phenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylic acid ester (5c).
Pale yellow solid (867 mg, 2.25 mmol, 90% yield). M.p. 209–211 °C. IR (KBr disk): 3395, 3290, 3072, 2951, 1697, 1639, 1481 cm−1. 1H-NMR (200 MHz, CDCl3) δ 7.82 (br s, 1H, OH), 6.89 (br s, 1H, NH), 6.68 (d, J = 1.2 Hz, 2H), 6.59 (s, 1H), 4.94 (s, 1H), 4.05 (q, J = 7.0 Hz, 2H), 3.82 (s, 3H), 2.35 (s, 3H), 2.29 (d, J = 6.0 Hz, 2H), 2.16 (d, J = 5.6 Hz, 2H), 1.21 (t, J = 7.0 Hz, 3H), 1.06 (s, 3H), 0.93 (s, 3H). 13C-NMR (50 MHz, CDCl3) δ 195.5, 167.7, 148.9, 146.2, 144.2, 143.9, 139.7, 120.1, 114.2, 111.6, 105.3, 97.0, 59.4, 55.7, 50.8, 40.6, 35.8, 32.5, 29.5, 27.0, 18.9, 14.3. ESI-MS m/z: 386 [M+H]+, 408 [M+Na]+, 793 [2M+Na]+.
4-(4-Ethoxy-3-hydroxy-phenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylic acid ester (5d).
Pale yellow solid (950 mg, 2.37 mmol, 95% yield). M.p. 163–165 °C. IR (KBr disk): 3447, 3279, 3200, 3074, 2960, 1690, 1486 cm−1. 1H-NMR (200 MHz, CDCl3) δ 8.21 (br s, 1H, OH), 6.86 (s, 2H), 6.67 (s, 2H), 4.91 (s, 1H), 4.12–3.97 (m, 4H), 2.43 (s, 3H), 2.30 (d, J = 4.4 Hz, 2H), 2.15 (d, J = 7.0 Hz, 2H), 1.39 (t, J = 7.0 Hz, 3H), 1.21 (t, J = 7.0 Hz, 3H), 1.06 (s, 3H), 0.93 (s, 3H). 13C-NMR (50 MHz, CDCl3) δ 195.4, 167.7, 149.1, 145.4, 144.4, 144.1, 139.6, 120.1, 114.3, 112.8, 111.4, 105.1, 64.1, 59.3, 57.4, 50.8, 40.3, 35.7, 29.5, 26.9, 18.7, 14.9, 14.3. ESI-MS m/z: 400 [M+H]+, 422 [M+Na]+, 821 [2M+Na]+.
2,7,7-Trimethy-4-(4-nitro-phenyl)-5-oxo-1,4,5,6,7,8,hexahydroquinoline-3-carboxylic acid ester (5e).
Pale yellow solid (875 mg, 2.27 mmol, 91% yield). M.p. 241–243 °C. IR (KBr disk): 3506, 3282, 3200, 2964, 1676, 1604, 1489, 1306, 1223, 1166, 870, 755 cm−1. 1H-NMR (200 MHz, CDCl3) δ 8.09 (d, J = 8.8 Hz, 2H), 7.50 (d, J = 8.8 Hz, 2H), 6.56 (br s, 1H, NH), 5.17 (s, 1H), 4.06 (q, J = 7.0 Hz, 2H), 2.39 (s, 3H), 2.31 (d, J = 13.6 Hz, 2H), 2.19 (d, J = 8.8 Hz, 2H), 1.18 (t, J = 7.2 Hz, 3H), 1.08 (s, 3H), 0.91 (s, 1H). 13C-NMR (50 MHz, CDCl3) δ 195.5, 166.9, 154.5, 149.0, 146.2, 144.6, 129.0, 123.3, 111.0, 104.8, 60.1, 50.6, 40.9, 37.2, 32.7, 29.4, 27.0, 19.4, 14.2. ESI-MS m/z: 385 [M+H]+, 407 [M+Na]+, 791 [2M+Na]+.
2,7,7-Trimethyl-4-(4-dimethylamino-phenyl)-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylic acid ester (5f).
Pale yellow solid (860 mg, 2.25 mmol, 90% yield). M.p. 261–263 °C. IR (KBr disk): 3280, 3200, 3076, 2955, 2800, 1686, 1606, 1219 cm−1. 1H-NMR (200 MHz, CDCl3) δ 7.15 (d, J = 8.8 Hz, 2H), 6.57 (d, J = 8.8 Hz, 2H), 4.94 (s, 1H), 4.07 (q, J = 7.1 Hz, 2H), 2.85 (s, 6H), 2.33 (s, 3H), 2.18–2.16 (m, 4H), 1.33 (t, J = 7.1 Hz, 3H), 1.04 (s, 3H), 0.94 (s, 3H). 13C-NMR (50 MHz, CDCl3) δ 195.9, 167.8, 148.9, 143.2, 135.9, 128.6, 112.3, 112.2, 106.4, 59.7, 50.8, 40.7, 35.3, 32.6, 29.5, 27.2, 19.3, 14.3. ESI-MS m/z: 383 [M+H]+, 405 [M+Na]+, 787 [2M+Na]+.
2,7,7-Trimethyl-5-oxo-4-iso-propyl-1,4,5,6,7,8-hexahydroquinoline-3-carboxylic acid ester (5g).
Pale yellow solid (610 mg, 2.00 mmol, 80% yield). M.p. 144–146 °C. IR (KBr disk): 3278, 3208, 3078, 2959, 1682, 1603, 1222 cm−1. 1H-NMR (200 MHz, CDCl3) δ 6.79 (br s, 1H, NH), 4.16 (qq, J1 = J2 = 7.1 Hz, 2H), 4.00 (d, J = 4.4 Hz, 1H), 2.33 (s, 3H), 2.30–20 (m, 4H), 1.73–1.60 (m, 1H), 1.28 (t, J = 7.0 Hz, 3H), 1.12 (s, 3H), 1.09 (s, 1H), 0.76 (d, J = 7.0 Hz, 6H). 13C-NMR (50 MHz, CDCl3) δ 196.4, 168.7, 151.0, 144.4, 109.6, 103.8, 59.7, 51.1, 41.1, 35.5, 35.3, 32.3, 30.0, 27.1, 19.0, 18.8, 18.7, 14.4. ESI-MS m/z: 306 [M+H]+, 328 [M+Na]+, 633 [2M+Na]+.
2,7,7-Trimethyl-5-oxo-4-pentyl-1,4,5,6,7,8-hexahydroquinoline-3-carboxylic acid ester (5h).
White solid (625 mg, 1.87 mmol, 75% yield). M.p. 130–132 °C. IR (KBr disk): 3282, 3210, 2956, 2929, 1697, 1603, 1486, 1221 cm−1. 1H-NMR (200 MHz, CDCl3) δ 6.09 (br s, 1H, NH), 4.20 (qq, J1 = J2 = 7.0 Hz, 2H), 4.02 (t, J = 5.1 Hz, 1H), 2.31 (s, 3H), 2.26 (s, 1H), 2.22 (s, 2H), 1.42–1.18 (m, 8H), 1.28 (t, J = 7.0 Hz, 3H), 1.09 (s, 6H), 0.83 (t, J = 6.6 Hz, 3H). 13C-NMR (50 MHz, CDCl3) δ 196.0, 168.0, 149.7, 144.1, 111.3, 105.4, 59.7, 51.0, 41.1, 36.3, 32.6, 32.2, 30.0, 29.8, 27.0, 24.7, 22.7, 19.3, 14.4, 14.1. ESI-MS m/z: 334 [M+H]+, 356 [M+Na]+, 689 [2M+Na]+.
2-Methyl-5-oxo-4-phenyl-1,4,5,6,7,8-hexahydroquinoline-3-carboxylic acid ester (5i).
White solid (700 mg, 2.25 mmol, 90% yield). M.p. 235–237 °C. IR (KBr disk): 3284, 3210, 3074, 2952, 1692 cm−1. 1H-NMR (200 MHz, DMSO-d6) δ 8.98 (s br, 1H, NH), 7.23–7.00 (m, 5H), 4.94 (s, 1H), 4.00 (q, J = 7.0 Hz, 2H), 2.56–2.46 (m, 2H), 2.31–2.20 (m, 5H), 2.00–1.80 (m, 2H), 1.17 (t, J = 7.0 Hz, 3H). 13C-NMR (50 MHz, DMSO-d6) δ 194.8, 167.1, 151.3, 147.9, 144.8, 127.6, 127.5, 125.5, 111.5, 104.0, 59.0, 36.9, 35.9, 26.4, 21.0, 18.4, 14.2. ESI-MS m/z: 312 [M+H]+, 334 [M+Na]+, 645 [2M+Na]+.
2-Methyl-4-(4-methoxy-phenyl)-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylic acid ester (5j).
White solid (756 mg, 2.32 mmol, 93% yield). M.p. 200–202 °C. IR (KBr disk): 3284, 3214, 3076, 2946, 1692, 1610, 1482, 1228 cm−1. 1H-NMR (200 MHz, CDCl3) δ 7.21 (d, J = 8.8 Hz, 2H), 6.73 (d, J = 8.8 Hz, 2H), 6.41 (br s, 1H, NH), 5.03 (s, 1H), 4.06 (q, J = 7.0 Hz, 2H), 2.40–2.30 (m, 7H), 2.05–1.83 (m, 2H), 1.19 (t, J = 7.0 Hz, 3H). 13C-NMR (50 MHz, CDCl3) δ 196.0, 167.6, 157.8, 149.9, 143.2, 139.8, 128.9, 113.5, 113.3, 106.2, 59.8, 55.1, 37.08, 35.6, 27.4, 21.1, 19.3, 14.2. ESI-MS m/z: 342 [M+H]+, 364 [M+Na]+, 705 [2M+Na]+.
4-(4-Hydroxy-phenyl)-2-methyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylic acid ester (5k).
White solid (736 mg, 2.25 mmol, 90% yield). M.p. 230–232 °C. IR (KBr disk): 3444, 3288, 3210, 3072, 2954, 1680, 1610, 1482, 1222 cm−1. 1H-NMR (200 MHz, CDCl3) δ 8.30 (br s, 1H, OH), 7.97 (br s, 1H, NH), 7.10 (d, J = 8.4 Hz, 2H), 6.65 (d, J = 8.4 Hz, 2H), 5.00 (s br, 1H), 4.04 (q, J = 7.0 Hz, 2H), 2.47–2.25 (m, 7H), 1.96–1.91 (m, 2H), 1.19 (t, J = 7.1 Hz, 3H). 13C-NMR (50 MHz, CDCl3) δ 195.8, 167.8, 155.1, 150.7, 144.1, 139.0, 128.8, 114.7, 112.9, 105.5, 59.4, 37.2, 35.3, 27.0, 21.1, 18.8, 14.3. ESI-MS m/z: 328 [M+H]+, 350 [M+Na]+, 677 [2M+Na]+.
4-(4-Hydroxy-3-methoxy-phenyl)-2-methyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylic acid ester (5l).
White solid (804 mg, 2.25 mmol, 90% yield). M.p. 210–212 °C. IR (KBr disk): 3488, 3296, 3216, 3076, 2948, 1686, 1610, 1484, 1226 cm−1. 1H-NMR (200 MHz, DMSO-d6) δ 9.06 (br s, 1H, OH), 8.62 (br s, 1H, NH), 6.71 (s, 1H), 6.59 (d, J = 8.0 Hz, 1H), 6.50 (d, J = 8.3 Hz, 1H), 4.81 (s, 1H), 4.00 (q, J = 7.0 Hz, 2H), 3.68 (s, 3H), 2.50–2.40 (m, 2H), 2.26–2.17 (m, 5H), 1.90–1.79 (m, 2H), 1.16 (t, J = 7.0 Hz, 3H). 13C-NMR (50 MHz, DMSO-d6) δ 194.8, 167.1, 151.1, 146.7, 144.6, 144.3, 139.1, 119.4, 115.1, 111.9, 111.3, 104.0, 59.0, 55.5, 36.8, 34.8, 26.2, 21.0, 18.2, 14.3. ESI-MS m/z: 358 [M+H]+, 380 [M+Na]+, 737 [2M+Na]+. HRMS: calcd. for [C20H23NO5+H+] 358.1650; found 358.1649.
4-(4-Ethoxy-3-hydroxy-phenyl)-2-methyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylic acid ester (5m).
White solid (854 mg, 2.30 mmol, 92% yield). M.p. 188–190 °C. IR (KBr disk): 3488, 3300, 3220, 3080, 2938, 1690, 1610, 1482, 1228 cm−1. 1H-NMR (200 MHz, CDCl3) δ 8.10 (br s, 1H, OH), 6.90 (d, J = 1.4 Hz, 1H), 6.68–6.66 (m, 2H), 6.52 (s, 1H), 4.96 (s, 1H), 4.10–3.99 (m, 4H), 2.48–2.28 (m, 7H), 2.00–1.80 (m, 2H), 1.40 (t, J = 7.0 Hz, 3H), 1.19 (t, J = 7.0 Hz, 3H). 13C-NMR (50 MHz, CDCl3) δ 195.8, 167.8, 150.7, 145.3, 144.3, 144.1, 139.8, 120.0, 114.2, 112.8, 105.1, 64.2, 59.4, 37.2, 35.6, 26.9, 21.1, 18.8, 14.9, 14.3. ESI-MS m/z: 372 [M+H]+, 394 [M+Na]+, 765 [2M+Na]+. HRMS: calcd. for [C21H25NO5+H+] 372.1805; found 372.1808.
2-Methyl-4-(4-nitro-phenyl)-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylic acid ester (5n).
Pale yellow solid (846 mg, 2.37 mmol, 95% yield). M.p. 167–169 °C. IR (KBr disk): 3294, 3214, 3078, 2948, 1704, 1606, 1480, 1226 cm−1. 1H-NMR (200 MHz, CDCl3) δ 8.08 (d, J = 8.8 Hz, 2H), 7.48 (d, J = 8.8 Hz, 2H), 6.57 (br s, 1H, NH), 5.19 (s, 1H), 4.05 (q, J = 7.0 Hz, 2H), 2.50–2.30 (m, 5H), 2.12–1.88 (m, 2H), 1.17 (t, J = 7.0 Hz, 3H). 13C-NMR (50 MHz, CDCl3) δ 195.7, 166.9, 154.6, 150.6, 146.2, 144.5, 129.0, 127.5, 123.5, 123.4, 115.8, 112.3, 104.8, 60.1, 37.1, 36.9, 33.4, 27.3, 21.0, 20.0, 19.4, 14.2. EM-IES m/z: 357 [M+H]+, 379 [M+Na]+, 735 [2M+Na]+.
2-Methyl-5-oxo-4-iso-propyl-1,4,5,6,7,8-hexahydroquinoline-3-carboxylic acid ester (5o).
White solid (520 mg, 1.87 mmol, 75% yield). M.p. 174–176 °C. IR (KBr disk): 3290, 3212, 3080, 2956, 1694, 1608, 1488, 1226 cm−1. 1H-NMR (200 MHz, CDCl3) δ 5.83 (br s, 1H, NH), 4.26–4.07 (m, 2H), 4.02 (d, J = 4.8 Hz, 1H), 2.47–2.38 (m, 4H), 2.32 (s, 3H), 2.10–1.94 (m, 2H), 1.29 (t, J = 7.0 Hz, 3H), 0.76 (d, J = 2.2 Hz, 3H), 0.72 (d, J = 2.2 Hz, 3H). 13C-NMR (50 MHz, CDCl3) δ 196.5, 168.6, 152.0, 144.0, 111.0, 105.0, 59.7, 37.3, 35.3, 35.2, 27.7, 21.1, 19.1, 18.6, 14.4. ESI-MS m/z: 278 [M+H]+, 300 [M+Na]+, 577 [2M+Na]+.
2-Methy-5-oxo-4-pentyl-1,4,5,6,7,8-hexahydroquinoline-3-carboxylic acid ester (5p).
Pale yellow solid (572 mg, 1.87 mmol, 75% yield). M.p. 129–131 °C. IR (KBr disk): 3292, 3218, 3080, 2930, 2858, 1696, 1604, 1486, 1226 cm−1. 1H-NMR (200 MHz, CDCl3) δ 6.48 (br s, 1H, NH), 4.30–3.10 (m, 2H), 4.03 (t, J = 5.1 Hz, 1H), 2.52–2.35 (m, 4H), 2.31 (s, 3H), 2.06–1.98 (m, 2H), 1.35–1.18 (m, 11H), 0.83 (t, J = 7.0 Hz, 3H). 13C-NMR (50 MHz, CDCl3) δ 196.5, 168.1, 151.6, 144.1, 112.3, 105.5, 59.7, 37.3, 36.5, 32.2, 30.0, 27.4, 24.5, 22.7, 21.3, 19.1, 14.4, 14.1. ESI-MS m/z: 306 [M+H]+, 328 [M+Na]+, 633 [2M+Na]+.
2,7-Dimethyl-5-oxo-4-phenyl-1,4,5,6,7,8-hexahydroquinoline-3-carboxylic acid ester (5q).
Pale yellow solid (740 mg, 2.27 mmol, 91% yield). M.p. 204–206 °C. IR (KBr disk): 3268, 3182, 3062, 2958, 1694, 1602, 1490, 1218 cm−1. 1H-NMR (200 MHz, CDCl3) δ 7.32–7.10 (m, 5H), 6.52 (br s, 1H, NH), 5.10 (s, 1H), 4.06 (q, J = 7.0 Hz, 2H), 2.42–2.00 (m, 8H), 1.20 (t, J = 7.0 Hz, 3H), 1.03 (d, J = 5.0 Hz, 3H). 13C-NMR (50 MHz, CDCl3) δ 196.0, 167.5, 150.0, 147.2, 143.6, 127.9, 127.8, 126.0, 112.9, 106.0, 59.8, 45.4, 36.4, 35.4, 28.3, 20.9, 19.2, 14.2. ESI-MS m/z: 326 [M+H]+, 348 [M+Na]+, 673 [2M+Na]+. HRMS: calcd. for [C20H23NO3+H+] 326.1751; found 326.1755.
4-(4-Hydroxy-3-methoxy-phenyl)-2,7-dimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylic acid ester (5r).
White solid (836 mg, 2.25 mmol, 90% yield). M.p. 239–241 °C. IR (KBr disk): 3314, 3236, 3086, 2960, 1650, 1484, 1224 cm−1. 1H-NMR (200 MHz, DMSO-d6) δ 9.06 (br s, 1H, OH), 8.62 (br s, 1H, NH), 6.70 (d, J = 1.8 Hz, 1H), 6.58 (d, J = 8.4 Hz, 1H), 6.48 (ddd, J1 = 2 Hz, J2 = 8.2 Hz, J3 = 1.8 Hz, 1H), 4.79 (s, 1H), 4.00 (q, J = 7.0 Hz, 2H), 3.67 (s, 3H), 2.25–1.95 (m, 8H), 1.16 (t, J = 7.0 Hz, 3H), 1.00 (d, J = 5.0 Hz, 3H). 13C-NMR (50 MHz, DMSO-d6) δ 194.7, 167.0, 150.6, 146.6, 144.4, 144.2, 138.9, 119.2, 114.9, 111.7, 110.8, 103.9, 58.9, 55.3, 45.4, 34.6, 34.1, 28.0, 20.6, 18.1, 14.2. ESI-MS m/z: 372 [M+H]+, 394 [M+Na]+, 765 [2M+Na]+. HRMS: calcd. for [C21H25NO5+H+] 372.1805; found 372.1808.
4-(4-Ethoxy-3-hydroxy-phenyl)-2,7-dimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylic acid ester (5s).
White solid (857 mg, 2.22 mmol, 89% yield). M.p. 146–148 °C. IR (KBr disk): 3430, 3284, 3208, 3078, 2966, 1690, 1614, 1488, 1218 cm−1. 1H-NMR (200 MHz, DMSO-d6) δ 9.06 (br s, 1H, OH), 8.55 (br s, 1H, NH), 6.69 (d, J = 1.8 Hz, 1H), 6.58 (d, J = 8.4 Hz, 1H), 6.47 (ddd, J1 = J3 = 1.8, J2 = 8.4 Hz, 1H), 4.78 (s, 1H), 4.00 (q, J = 7.0 Hz, 2H), 3.91 (q, J = 7.0 Hz, 2H), 2.26–1.95 (m, 8H), 1.29 (t, J = 7.0 Hz, 3H), 1.15 (t, J = 7.0 Hz, 3H), 1.00 (d, J = 5.2 Hz, 3H). 13C-NMR (50 MHz, DMSO-d6) δ 194.7, 167.0, 150.6, 145.7, 144.8, 144.2, 138.9, 119.3, 115.0, 113.3, 110.8, 103.9, 63.6, 58.9, 45.2, 34.6, 34.1, 28.1, 20.6, 18.1, 14.7, 14.1. ESI-MS m/z: 386 [M+H]+, 408 [M+Na]+, 793 [2M+Na]+. HRMS: calcd. for [C22H27NO5+H+] 386.1962; found 386.1963.
2,4,7-Trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylic acid ester (5t).
Pale yellow solid (526 mg, 2.00 mmol, 80% yield). M.p. 172–175 °C. IR (KBr disk): 3290, 3226, 3084, 2958, 1698, 1610, 1484, 1220 cm−1. 1H-NMR (200 MHz, CDCl3) δ 6.84 (br s, 1H, NH), 4.29–4.05 (m, 2H), 3.92 (q, J = 6.6 Hz, 1H), 2.45–2.01 (m, 5H), 1.20 (t, J = 7.0 Hz, 3), 1.00 (da, J = 4.8 Hz, 3H), 0.79 (d, J = 6.4 Hz, 3H). 13C-NMR (50, MHz, CDCl3) δ 196.6, 167.8, 151.1, 144.0, 113.4, 106.9, 59.6, 45.6, 45.1, 35.4, 28.7, 25.6, 22.7, 20.7, 19.2, 14.4. ESI-MS m/z: 264 [M+H]+, 286 [M+Na]+, 549 [2M+Na]+. HRMS: calcd. for [C15H21NO3+H+] 264.1594; found 264.1595.
2,6-Dimethyl-4-phenyl-1,4-dihydropyridine-3,5-dicarboxylic acid diester (6a).
White solid (757 mg, 2.30 mmol, 92% yield). M.p. 145–147 °C. IR (KBr disk): 3341, 3060, 2979, 1689, 1651, 1210 cm−1. 1H-NMR (200 MHz, CDCl3) δ 7.30–7.06 (m, 5H), 5.83 (br s, 1H, NH), 4.99 (s, 1H), 4.09 (q, J = 7.1 Hz, 2H), 4.08 (q, J = 7.0 Hz, 2H), 2.31 (s, 6H), 1.21 (t, J = 7.1 Hz, 6H). 13C-NMR (50 MHz, CDCl3) δ 167.7, 147.8, 143.9, 128.0, 127.8, 126.1, 104.1, 59.7, 39.6, 19.4, 14.2. ESI-MS m/z: 352 [M+Na]+, 681 [2M+Na]+.
4-(4-methoxy-phenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylic acid diester (6b).
White solid (836 mg, 2.32 mmol, 93% yield). M.p. 144–146 °C. IR (KBr disk): 3342, 3094, 2982, 1690, 1650, 1490, 1210 cm−1. 1H-NMR (200 MHz, CDCl3) δ 7.18 (d, J = 8.7 Hz, 2H), 6.74 (d, J = 8.7 Hz, 2H), 5.71 (br s, 1H), 4.93 (s, 1H), 4.09 (q, J = 7.1 Hz, 2H), 4.08 (q, J = 7.0 Hz, 2H), 3.75 (s, 3H), 2.31 (s, 6H), 1.22 (t, J = 7.1 Hz, 6H). 13C-RMN (50 MHz, CDCl3) δ 168.7, 168.4, 160.3, 155.8, 155.2, 142.7, 132.2, 129.8, 127.9, 126.9, 113.8, 61.6, 61.5, 55.3, 23.1, 16.9, 14.2, 13.8. ESI-MS m/z: 382 [M+Na]+, 741 [2M+Na]+.
4-(4-hydroxy-3-methoxy-phenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylic acid diester (6c).
Pale yellow solid (865 mg, 2.25 mmol, 90% yield). M.p. 152–154 °C. IR (KBr disk): 3350, 2980, 1681, 1651, 1489, 1272 cm−1. 1H-NMR (200 MHz, CDCl3) δ 6.85 (s, 1H), 6.73 (d, J = 1.4 Hz, 2H), 5.75 (s, 1H), 4.92 (s, 1H), 4.10 (q, J = 7.2 Hz, 4H), 3.83 (s, 3H), 2.32 (s, 6H), 1.24 (t, J = 7.0 Hz, 6H). 13C-NMR (50 MHz, CDCl3) δ 167.8, 145.8, 143.8, 143.6, 140.1, 120.4, 113.8, 110.8, 104.2, 59.7, 55.7, 39.1, 19.6, 14.3. ESI-MS m/z: 398 [M+Na]+, 773 [2M+Na]+.
4-(3-Ethoxy-4-hydroxy-phenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylic acid diester (6d).
Pale yellow solid (935 mg, 2.40 mmol, 96% yield). M.p. 155–157 °C. IR (KBr disk): 3495, 3316, 2979, 1688, 1644, 1493, 1207 cm−1. 1H-NMR (200 MHz, CDCl3) δ 6.83 (d, J = 1.4 Hz, 1H), 6.73 (dd, J1 = 0.8, J2 = 2.2 Hz, 2H), 5.66 (br s, 1H), 5.53 (s, 1H), 4.90 (s, 1H), 4.08 (q, J = 7.0 Hz, 6H), 2.32 (s, 6H), 1.40 (t, J = 7.0 Hz, 3H), 1.23 (t, J = 7.2 Hz, 6H). 13C-NMR (50 MHz, CDCl3) δ 167.7, 145.0, 144.0, 143.5, 140.0, 120.3, 113.8, 111.8, 104.3, 64.2, 59.7, 39.0, 19.6, 14.9, 14.3. ESI-MS m/z: 412 [M+Na]+, 801 [2M+Na]+, 428 [M+K]+.
4-(4-Methyl-phenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylic acid diester (6e).
White solid (798 mg, 2.32 mmol, 93% yield). M.p. 127–129 °C. IR (KBr disk): 3357, 3087, 2985, 1695, 1652, 1487, 1213 cm−1. 1H-NMR (200 MHz, CDCl3) δ 7.14 (d, J = 8.1 Hz, 2H), 6.98 (d, J = 8.1 Hz, 2H), 5.75 (br s, 1H), 4.93 (s, 1H), 4.06 (q, J = 7.1 Hz, 4H), 2.28 (s, 6H), 2.25 (s, 3H), 1.20 (t, J = 7.1 Hz, 6H). 13C-NMR (50 MHz, CDCl3) δ 167.7, 145.0, 143.8, 135.5, 128.6, 127.8, 104.2, 59.7, 39.1, 21.1, 19.6, 14.3. ESI-MS m/z: 366 [M+Na]+, 709 [2M+Na]+.
2,4,6-Trimethyl-1,4-dihydropyridine-3,5-dicarboxylic acid diester (6f).
White solid (548 mg, 2.05 mmol, 82% yield). M.p. 117-119 °C. IR (KBr disk): 3344, 3104, 2964, 1697, 1644, 1491, 1215 cm−1. 1H-NMR (200 MHz, CDCl3) δ 4.27–4.10 (m, 4H), 3.83 (q, J = 6.6 Hz, 1H), 2.26 (s, 6H), 1.29 (t, J = 7.0 Hz, 6H), 0.97 (d, J = 6.6 Hz, 3H). 13C-NMR (50 MHz, CDCl3) δ 167.9, 144.4, 104.7, 59.6, 28.5, 22.3, 19.4, 14.5. ESI-MS m/z: 290 [M+Na]+, 557 [2M+Na]+.
2,6-Dimethyl-4-iso-propyl-1,4-dihydropyridine-3,5-dicarboxylic acid diester (6g).
White solid (642 mg, 2.17 mmol, 87% yield). M.p. 90–92 °C. IR (KBr disk): 3344, 3090, 2960, 1696, 1651, 1484, 1215 cm−1. 1H-NMR (200 MHz, CDCl3) δ 4.31–4.08 (m, 4H), 3.92 (d, J = 5.6 Hz, 1H), 2.30 (s, 6H), 1.68–1.50 (m, 1H), 1.29 (t, J = 7.1 Hz, 6H), 0.74 (d, J = 6.8 Hz, 6H). 13C-NMR (50 MHz, CDCl3) δ 168.8, 144.7, 101.6, 59.6, 38.8, 35.5, 19.3, 18.5, 14.4. ESI-MS m/z: 318 [M+Na]+, 613 [2M+Na]+.
2,6-Dimethyl-4-pentyl-1,4-dihydropyridine-3,5-dicarboxylic acid diester (6h).
White solid (640 mg, 1.97 mmol, 79% yield). M.p. 94–96 °C. IR (KBr disk): 3336, 3092, 2926, 1696, 1649, 1485, 1213 cm−1. 1H-NMR (200 MHz, CDCl3) δ 4.26–4.09 (m, 4H), 3.92 (t, J = 5.3 H, 1H), 2.20 (s, 6H), 1.33–1.21 (m, 14H), 0.84 (t, J = 7.0 Hz, 3H). 13C-NMR (50 MHz, CDCl3) δ 168.2, 144.7, 103.2, 59.5, 36.8, 32.8, 32.1, 24.5, 22.7, 19.4, 14.4, 14.1. ESI-MS m/z: 346 [M+Na]+, 669 [2M+Na]+.
3,3,6,6-Tetramethyl-4-Phenyl-3,4,6,7,9,10-hexahydro-2H,5H-acridine-1,8-dione (7a).
White solid (856 mg, 2.45 mmol, 98% yield) M.p. 180–182 °C. IR (KBr disk): 3027, 2961, 2873, 2634, 1592, 1372 1246 cm−1. 1H-NMR (200 MHz, CDCl3) δ 11.9 (s, 1H), 7.30–7.07 (m, 5H), 5.54 (s, 1H), 2.43–2.34 (m, 8H), 1.23 (s, 6H), 1.09 (s, 6H). 13C-NMR (50 MHz, CDCl3) δ 190.4, 189.3, 138.0, 128.2, 126.7, 125.8, 115.5, 47.0, 46.4, 32.7, 31.3, 29.6, 27.4. ESI-MS m/z: 350 [M+H]+, 372 [M+Na]+.
3,3,6,6-Tetramethyl-9-(4-methoxy-phenyl)-3,4,6,7,9,10-hexahydro-2H,5H-acridine-1,8-dione (7b).
Pale yellow solid (900 mg, 2.37 mmol, 95% yield). M.p. > 250 °C. IR (KBr disk): 3275, 3204, 3069, 2955, 1643, 1607, 1481, 1224 cm−1. 1H-NMR (200 MHz, CDCl3) δ 8.26 (s, 1H), 7.25 (d, J = 8.8 Hz, 2H), 6.72 (d, J = 8.8 Hz, 2H), 5.04 (s, 1H), 3.66 (s, 3H), 2.30–2.10 (m, 8H), 1.06 (s, 6H), 0.95 (s, 6H). 13C-NMR (50 MHz, CDCl3) δ 196.2, 157.6, 149.4, 139.2, 128.9, 113.2, 55.0, 50.9, 40.5, 32.7, 32.6, 29.6, 27.0. ESI-MS m/z: 380 [M+H]+, 781 [2M+Na]+.
9-(4-Hydroxy-3-methoxy-phenyl)-3,3,6,6-tetramethyl-3,4,6,7,9,10-hexahydro-2H,5H-acridine-1,8-dione (7c).
White solid (940 mg, 2.37 mmol, 95% yield). P.f. > 250 °C. IR (KBr disk): 3274, 3168, 3048, 2956, 1642, 1626, 1490 cm−1. 1H-NMR (200 MHz, CDCl3) δ 8.59 (s, 1H), 7.28 (s, 1H), 6.92 (s, 1H), 6.65 (s, 2H), 4.92 (s, 1H), 3.81 (s, 3H), 2.34 (d, J = 4.4 Hz, 4H), 2.16 (d, J = 6.2 Hz, 4H), 1.07 (s, 6H), 0.96 (s, 6H). 13C-NMR (50 MHz, CDCl3) δ 195.3, 148.6, 146.4, 143.9, 138.9, 119.9, 114.4, 112.9, 111.9, 55.7, 50.8, 40.5, 32.7, 32.4, 29.6, 26.9. ESI-MS m/z: 396 [M+H]+, 434 [M+K]+, 813 [2M+Na]+.
9-(3-Ethoxy-4-hydroxy-phenyl)-3,3,6,6-tetramethyl-3,4,6,7,9,10-hexahydro-2H,5H-acridine-1,8-dione (7d).
White solid (920 mg, 2.25 mmol, 90% yield). M.p. > 250 °C. IR (KBr disk): 3276, 3202, 3078, 2964, 1620, 1514, 1222 cm−1. 1H-NMR (200 MHz, CDCl3) δ 9.07 (s, 1H), 8.25 (s, 1H), 6.75 (s, 1H), 6.55 (s, 2H), 4.74 (s, 1H), 3.94 (q, J = 7.0 Hz, 2H), 3.33 (s, 3H), 2.46–1.97 (m, 8H), 1.33 (t, J = 7.0 Hz, 3H), 1.05 (s, 6H), 0.92 (s, 6H). 13C-NMR (50 MHz, CDCl3) δ 194.4, 148.7, 145.6, 144.5, 138.5, 119.9, 114.7, 113.7, 112.1, 63.8, 50.5, 40.1, 32.1, 29.3, 26.6, 14.8. ESI-MS m/z: 410 [M+H]+, 432 [M+Na]+, 841 [2M+Na]+.
9-(4-N,N-Dimethyl-phenyl)-3,3,6,6-tetramethyl-3,4,6,7,9,10-hexahydro-2H,5H-acridine-1,8-dione (7e).
Pale yellow solid (911 mg, 2.32 mmol, 93% yield). M.p. > 250 °C. IR (KBr disk): 3268, 3184, 3066, 2956, 1630, 1600, 1222 cm−1. 1H-NMR (200 MHz, CDCl3) δ 8.10 (s, 1H), 7.18 (d, J = 8.4 Hz, 2H), 6.54 (d, J = 8.4 Hz, 2H), 4.99 (s, 1H), 2.80 (s, 6H), 2.28–2.07 (m, 8H), 1.05 (s, 6H), 0.95 (s, 6H). 13C-NMR (50 MHz, CDCl3) δ 196.1, 149.1, 148.7, 135.4, 128.6, 113.5, 112.2, 50.9, 40.6, 32.5, 32.4, 29.6, 27.2. ESI-MS m/z: 393 [M+H]+, 807 [2M+Na]+.
9-(4-Methyl-phenyl)-3,3,6,6-tetramethyl-3,4,6,7,9,10-hexahydro-2H,5H-acridine-1,8-dione (7f).
Pale orange solid (845 mg, 2.32 mmol, 93% yield). M.p. >250 °C. IR (KBr disk): 3278, 3183, 3066, 2956, 1649, 1606, 1490, 1366, 1221 cm−1. 1H-NMR (200 MHz, CDCl3) δ 8.22 (br s, 1H), 7.22 (d, J = 7.8 Hz, 2H), 6.99 (d, J = 7.6 Hz, 2H), 5.06 (s, 1H), 2.22–2.17 (m, 11H), 1.05 (s, 6H), 0.95 (s, 6H). 13C-NMR (50 MHz, CDCl3) δ 196.0, 149.4, 143.8, 135.2, 128.7, 127.9, 113.2, 50.9, 40.5, 33.2, 32.5, 29.5, 27.0, 21.1. EM-IES m/z: 727 [2M+H]+, 749 [2M+Na]+, 766 [2M+K]+.
9-iso-propyl-3,3,6,6-tetramethyl-3,4,6,7,9,10-hexahydro-2H,5H-acridine-1,8-dione (7g).
White solid (670 mg, 2.12 mmol, 85% yield). M.p. 142–144 °C. IR (KBr disk): 3036, 2964, 1582, 1248 cm−1. 1H-NMR (200 MHz, CDCl3) δ 12.4 (s, 1H), 3.10–2.82 (m, 1H), 2.30 (s, 8H), 1.08 (s, 6H), 1.06 (s, 6H), 0.84 (d, J = 6.2 Hz, 6H). 13C-NMR (50 MHz, CDCl3) δ 190.4, 189.4, 116.4, 46.9, 46.2, 38.0, 31.0, 30.0, 26.8, 25.7, 22.3. EM-IES m/z: 338 [M+Na]+, 653 [2M+Na]+.
3,3,6,6,-Tetramethyl-3,4,6,7,9,10-hexahydro-2H,5H-acridine-1,8-dione (7h).
White solid (567 mg, 2.07 mmol, 83% yield). M.p. 127-129 °C. IR (KBr disk): 3236, 3082, 2950, 1720, 1690, 1608 cm−1. 1H-NMR (200 MHz, CDCl3) δ 5.02 (br s, 1H), 3.51 (d, J = 3.0 Hz, 2H), 3.07 (d, J = 13.6 Hz, 2H), 2.88 (s, 2H), 2.29 (d, J = 14.0 Hz, 2H), 2.17 (d, J = 6.6 Hz, 2H), 1.20 (s, 3H), 1.00 (s, 6H), 0.81 (s, 3H). 13C-NMR (50 MHz, CDCl3) 206.5, 192.8, 157.4, 98.4, 97.0, 60.4, 50.7, 49.9, 42.6, 41.3, 32.5, 31.1, 30.4, 29.7, 28.3, 26.5. EM-IES m/z: 296 [M+Na]+, 569 [2M+Na]+.
DPPH-scavenging activity
Scavenging activities of the hydropyridine derivatives towards DPPH were assessed by the method described by Scherer and Godoy30 with slight modifications. Briefly, a solution of DPPH (0.15 mM) in methanol was prepared. Hydropyridine derivatives at 100 μg mL−1 (0.2 mL) were mixed with the DPPH solution (1.8 mL); the mixture was vigorously shaken, incubated in dark conditions (37 °C/30 min), and the absorbance was measured at 515 nm by using a UV-visible spectrophotometer, Spectronic® 20 Genesys™. The DPPH-scavenging activity of the hydropyridines was calculated as follows:
DPPH-scavenging effect (%) = [(A0 − A1)/A0] × 100
Where: A0 was the absorbance of control; and A1 was the absorbance in the presence of standard (BHT or Trolox) or of the hydropyridine derivatives at 100 μg mL−1. BHT and Trolox were used as positive controls. All the tests were performed in triplicate.
Acknowledgements
Authors acknowledgements the financial support from Consejo Nacional de Ciencia y Tecnología (CONACyT, GRANT No SEP-2004-CO1-47835) and from PROMEP (Project UAS-PTC-040, PROMEP/103.5/11/4214).
References
-
(a) A. Stadler and C. O. Kappe, J. Comb. Chem., 2001, 3, 624 CrossRef CAS;
(b) J. E. Biggs-Houck, A. Younai and J. T. Shaw, Curr. Opin. Chem. Biol., 2010, 14, 371 CrossRef CAS;
(c) J. V. Johnson, B. S. Rauckman, D. P. Baccanari and B. Roth, J. Med. Chem., 1989, 32, 1942 CrossRef CAS.
- A. Hantzsch, Ann. Chem., 1881, 1 Search PubMed.
- I. A. Rivero, E. A. Reynoso-Soto and A. Ochoa-Terán, Arkivoc, 2011,(ii), 260 CAS.
-
(a)
C. Hansch, P. G. Sammes and J. B. Taylor, Comprehensive medicinal chemistry : the rational design, mechanistic study & therapeutic application of chemical compounds; 1st ed.; Pergamon Press: Oxford ; New York, 1990 Search PubMed;
(b) K. H. Lee and K. Y. Ko, Bull. Korean Chem. Soc., 2002, 23, 1505 CrossRef CAS.
-
(a) R. H. Böcker and F. P. Guengerich, J. Med. Chem., 1986, 29, 1596 CrossRef;
(b) F. P. Guengerich, W. R. Brian, M. Iwasaki, M. A. Sari, C. Baeaernhielm and P. Berntsson, J. Med. Chem., 1991, 34, 1838 CrossRef CAS.
- W. B. Weglicki, I. T. Mak and M. G. Simic, J. Mol. Cell. Cardiol., 1990, 22, 1199 CrossRef CAS.
-
(a) D. R. Janero and B. Burghardt, Biochem. Pharmacol., 1989, 38, 4344 CrossRef CAS;
(b) F. T. van Amsterdam, A. Roveri, M. Maiorino, E. Ratti and F. Ursini, Free Radical Biol. Med., 1992, 12, 183 CrossRef CAS.
- I. C. Cotterill, A. Y. Usyatinsky, J. M. Arnold, D. S. Clark, J. S. Dordick, P. C. Michels and Y. L. Khmelnitsky, Tetrahedron Lett., 1998, 39, 1117 CrossRef CAS.
- R. A. Abramovitch, Org. Prep. Proced. Int., 1991, 23, 685 CrossRef.
- V. M. Markhele, S. A. Sadaphal and M. S. Shingare, Bull. Catal. Soc. India, 2007, 6, 125 Search PubMed.
- A. Kumar and R. A. Maurya, Tetrahedron Lett., 2007, 48, 3887 CrossRef CAS.
- M. Maheswara, V. Siddaiah, G. L. V. Damu and C. V. Rao, Arkivoc, 2006, 201 CAS.
- L. M. Wang, J. Sheng, L. Zhang, J. W. Han, Z. Y. Fan, H. Tian and C. T. Qian, Tetrahedron, 2005, 61, 1539 CrossRef CAS.
- S. H. Ji, Z. Q. Jiang, J. Lu and T. P. Loh, Synlett, 2004, 0831 CrossRef CAS.
- S. Ko and C. F. Yao, Tetrahedron, 2006, 62, 7293 CrossRef CAS.
- S. Ko, M. N. V. Lin, C. Sastry and C. F. Yao, Tetrahedron Lett., 2005, 46, 5771 CrossRef CAS.
- P. R. Gómez, R. Osnaya, I. Zamora, B. B. Velasco, G. Arroyo, S. J. E. Ramírez, J. Trujillo, F. Delgado and R. Miranda, J. Mex. Chem. Soc., 2007, 4, 181 Search PubMed.
- M. C. Bagley and M. C. Lubinu, Synthesis, 2006, 1283 CrossRef CAS.
- G. V. M. Sharma, K. L. Reddy, P. S. Lakshmi and R. K. Palakodety, Synthesis, 2006, 0055 CrossRef CAS.
- G. M. Ziarani, A. Badiei, M. Hassanzadeh and S. Mousavi, Arabian J. Chem., 2011 DOI:10.1016/j.arabjc.2011.01.037.
- S. K. Singh and K. N. Singh, J. Heterocycl. Chem., 2011, 48, 69 CrossRef CAS.
- S. R. Cherkupally and R. Mekala, Chem. Pharm. Bull., 2008, 56, 1002 CrossRef CAS.
- A. Kumar and R. A. Maurya, Tetrahedron, 2007, 63, 1946 CrossRef CAS.
- D. Tirzite, G. Tirzitis and D. Antipova, Chem. Heterocycl. Compd., 1999, 35, 592 CrossRef CAS.
- C. Yamagami, N. Motohashi, T. Emoto, A. Hamasaki, T. Tanahashi, N. Nagakura and Y. Takeuchi, Bioorg. Med. Chem. Lett., 2004, 14, 5629 CrossRef CAS.
- K. Nakao, R. Shimizu, H. Kubota, M. Yasuhara, Y. Hashimura, T. Suzuki, T. Fujita and H. Ohmizu, Bioorg. Med. Chem., 1998, 6, 849 CrossRef CAS.
- G. Tirzitis, D. Tirzitis and Z. Hyvonen, Czech. J. Food Sci., 2001, 3, 81 Search PubMed.
- D. Tirzitis, A. Krauze, G. Zubareva, G. Tirzitis and G. Duburs, Chem. Heterocycl. Compd., 2002, 38, 795 CrossRef.
- A. Kumar, R. A. Maurya, S. Sharma, M. Kumar and G. Bhatia, Eur. J. Med. Chem., 2010, 45, 501 CrossRef CAS.
- R. Scherer and H. T. Godoy, Food Chem., 2009, 112, 654 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2012 |
Click here to see how this site uses Cookies. View our privacy policy here. 



![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate 7
ethyl acetate 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v). After completion of the reaction, the mixture was cooled to RT and dichloromethane (10 mL) was added. Organic solvent was evaporated under reduced pressure and the solid compound was crystallized from absolute ethanol to afford the pure corresponding PHQ derivatives in good yields. All the products were characterized from their spectral data.
3 v/v). After completion of the reaction, the mixture was cooled to RT and dichloromethane (10 mL) was added. Organic solvent was evaporated under reduced pressure and the solid compound was crystallized from absolute ethanol to afford the pure corresponding PHQ derivatives in good yields. All the products were characterized from their spectral data.
