Extraction of radioactive cesium using innovative functionalized porous materials†
Carole
Delchet
ab,
Alexei
Tokarev
b,
Xavier
Dumail
b,
Guillaume
Toquer
a,
Yves
Barré
c,
Yannick
Guari
*b,
Christian
Guerin
b,
Joulia
Larionova
b and
Agnès
Grandjean
*a
aInstitut de Chimie Séparative de Marcoule, UMR5257 CEA-CNRS-UM2-ENSCM, BP17171, 30207, Bagnols sur Cèze, France. E-mail: agnes.grandjean@cea.fr; Fax: +33 4667 97611; Tel: +33 4667 96622
bInstitut Charles Gerhardt Montpellier, UMR 5253 CNRS-UM2-ENSCM-UM1, Chimie Moléculaire et Organisation du Solide, Université Montpellier II, Place E. Bataillon, 34095, Montpellier cedex 5, France. E-mail: yannick.guari@um2.fr; Fax: +33 4671 43852; Tel: +33 4671 44224
cCEA/DEN/DTCD/SPDE/Laboratoire Des Procédés Avancés de Décontamination Centre de Marcoule, BP17171, Bagnols sur Ceze, France
First published on 22nd May 2012
Abstract
A new approach to an efficient and selective extraction of Cs+ ions from water, sea water enriched with Cs+ and a radioactive solution simulating the effluents of the Fukushima reactors (137Cs, 29 kBq L−1) was developed by using porous silica- or glass-based nanocomposites containing Prussian blue type nanoparticles, Co2+/[Fe(CN)6]3−, with sizes below 10 nm. A particular emphasis is given on the kinetics of cesium sorption fitted by using the classical reaction order model as well as a diffusion model in order to better understand the sorption mechanism. Compared to the amount of Co2+/[Fe(CN)6]3− nanoparticles, the sorption capacities of studied nanocomposites are more than three times higher than the ones observed for the respective bulk materials. These nanocomposites present a high selectivity to Cs+ and extract it in trace amounts.
Introduction
Numerous processes from nuclear facilities (fuel processing, power plants, laboratories, remediation or removal and others) generate important volumes of radioactive effluents which should be treated in order to minimize their impact on environment. Among those, radioactive cesium isotopes are ones of the most abundant fission products of uranium which are dangerous to health. Gamma-emitter 134Cs and 137Cs with a half-life of 2 and 30 years, respectively, are mainly present in these fission products. Both isotopes are radiotoxic because they are an analogue of potassium and thus may be quickly assimilated in the body. They accumulate in the food chain and persist in the environment for hundreds of years. For these reasons, the problem of selective cesium effluents decontamination has attracted a great deal of attention in recent years.However, the use of classical inorganic sorbents such as manganese oxide, zeolites, iron hydroxide or barium sulfate usually employed for extraction of various radioactive elements such as Sr, Co, Ni, or actinides1,2 is inefficient in the case of cesium due to their low affinity. In the case of the Fukushima disaster, one of the major problems now is to rapidly clean up areas that have been heavily contaminated by radioactivity. Cesium is one of the major radioactive elements present in water (essentially sea water) that was used for cooling down the damaged reactor in the first days of the disaster. Thus the elaboration of innovative materials able to remove radioactive cesium with a continuous process (such as a column process) and minimize the waste volume, matching with the classical waste confinement matrix such as cement or glass, is a challenge for the cleanup of the Fukushima site.
Cyano-bridged coordination polymers based on hexacyanometallates and transition metal ions, also called Prussian blue analogues, present a high affinity for the capture of cesium ions over a wide range of pH and salinity due to a selective insertion of Cs+ into the crystalline structures of cyanometallates.3–5 The pioneering employment of cyano-bridged coordination polymers for cesium decontamination was proposed around ten years ago by Lehto's group by using granular potassium cobalt hexacyanoferrate (CsTreat) similar to K2[CoFe(CN)6].6,7 Note that this bulk Prussian blue analogue is actually used at industrial scale for selective extraction of Cs+ from contaminated effluents. This coordination polymer is selective to the cesium ion extraction, however due to its low mechanical hardness and its fine powder shape leading to a slow filtration rate and also a clogging problem, only a small volume of effluent may be treated. For this reason, the use of the bulk compound in column process decontamination is limited. In order to avoid these problems, several composite materials with mainly silica supports loaded with cyano-bridged coordination polymers particles have been proposed.8–12 The first kind of materials were K2M[Fe(CN)6]/SiO2 composites (M = Ni, Cu, Co) obtained by successive impregnation of porous silica13 or polymer modified silica14,15 by bivalent transition metal ions and a hexacyanoferrate precursor. The second kind of composites were synthesized by direct incorporation of coordination polymer particles (K2(CoFe(CN)6 or K2(NiFe(CN)6)) into the silica gel9 or into hydrated zirconia16 during the synthesis. However, all these composite materials suffer several drawbacks: their final composition are not well controlled and thus are relatively poorly reproducible. In addition, the cyano-bridged coordination polymer particles are only weakly linked to the inorganic support and may be removed during the stage of the cesium extraction.
An alternative promising synthetic route developed in the recent few years concerns the covalent grafting of specific functions on porous silica in order to anchor the cyano-bridged complexes or cyano-bridged metallic particles into the silica pores or on the silica surface. Two composite materials containing self-assembled monolayers on mesoporous silica functionalized with ethylenediamine covalently coordinated to Cu[Fe(CN)6]2− complex in the first case12 and silica (as powder or as films) functionalized with ethylenediamine tetracetic acid derivative coordinated to Ni2+/[Fe(CN)6]3− particles have recently been reported.11,12 In both cases an increase of “selective” sorption of Cs+ has been obtained in comparison with bulk Prussian blue.
Using this line of thought, some of us have recently used a mesoporous silica matrix functionalized with pyridine groups in order to synthesize cyano-bridged coordination polymer nanoparticles of various compositions and various sizes (ranging from 3 to 6 nm) covalently attached into the silica pores.17 In this system a good control of the nanoparticles' composition, nanoparticles' size and nanoparticles' quantity inserted into the silica has been obtained that give an excellent opportunity to investigate the cesium ion extraction by using these nanocomposite materials. Recently we performed the preliminary study of a cesium extraction by using such nanocomposites which show a good and selective Cs+ sorption in pure water.18 These results encouraged us to extend this approach to the synthesis of cyano-bridged coordination polymer nanoparticles inserted into a porous glass matrix instead of silica. Even if porous glasses have a smaller surface area than silica, they present better thermal and chemical stability, mechanical hardness and irradiation damage resistance.19 In addition, they are available in very flexible forms, for instance as pearls, that permits the utilization of the obtained nanocomposites for a cesium extraction in column or cartridge processes. Today, on an industrial scale, selective extraction of Cs+ from contaminated effluents is performed by using bulk Prussian blue analogous in which the maximum extraction capacity is never reached. In addition, this extraction generates a large quantity of sludge which should be confined and stored thereafter. In the present work we propose innovative nanocomposite materials consisting of coordination polymer nanoparticles covalently grafted to matrixes specifically designed for column or cartridge process decontamination. This allows us to achieve the maximum extraction capacity and significantly reduce the amount of waste.
The present manuscript describes the synthesis of both, silica- and glass-based nanocomposites containing small sized coordination polymer nanoparticles covalently linked to pore walls of the support and the cesium extraction with these nanocomposites from pure water, sea water and radioactive solution simulating the effluents of Fukushima reactors. A particular emphasis is given on the kinetics of cesium sorption fitted by using the classical reaction order model as well as a diffusion model in order to better understand the sorption mechanism. Adsorption capacities and distribution coefficients for these materials in pure water and in radioactive sea water solutions are also studied to evaluate the potential of these nanocomposites for an industrial process.
Experimental part
All the chemical and reagents are of Analytical grade.Syntheses
The synthesis of the bulk Prussian blue analogues Co3[Fe(CN)6]2·H2O (CoFC), used for comparison of Cs+ adsorption with the nanocomposites was performed by using previously described procedures from Co(BF4)2 and TBA3[Fe(CN)6].17Synthesis of functionalized matrices
The grafting of the organic functionality –(CH2)2C5H4N into the glasses’ pores was performed by using the same procedure as in the case of the SBA-15 type silica matrix. An hybrid glass NC5H4(CH2)2SiO1.5/SiO2 (PG-Py) was obtained. The content of pyridine groups was also determined by elemental analysis. Elemental analysis found: Si, 39.2; N, 0.40 i.e. an organic loading of 0.3 mmol g−1.
Synthesis of nanocomposite materials Co2+/[Fe(CN)6]3−/matrices-Py
The intrapore growth of cyano-bridged coordination polymer nanoparticles Co2+/[Fe(CN)6]3− into the silica or glass pores was performed by using a previously described simplified procedure.17 Firstly, a matrix-Py as a powder for silica or pearls for glass (1 g) was added to 150 mL of a 10−2 mol L−1 methanolic solution of Co(BF4)2 under air. The mixture was stirred for 2 h at room temperature. After filtration, the silica powder or glass pearls were thoroughly washed 3 times with methanol. Secondly, the as-obtained powder or pearls was added to a 10−2 mol L−1 methanolic solution of [N(C4H9)4]3[Fe(CN)6]. The mixture was stirred again for 2 h, the product was filtered off and thoroughly washed with methanol. Such consecutive treatments with metal salts and cyanometallate precursors were repeated 5 times. The resulting nanocomposites were then dried in air over night. The elemental analyses of the obtained nanocomposites Co2+/[Fe(CN)6]3−/Silica-Py (CoFC/Silica-Py) and Co2+/[Fe(CN)6]3−/PG-Py (CoFC/PG-Py) are given in Table 1.| Sample | Co/Si | Fe/Si | Co/Fe | wt% CoCF | IR (cm−1) |
|---|---|---|---|---|---|
| CoFC | — | — | 1.64 | — | 2087, 2159 |
| CoFC@Silica-Py | 0.027 | 0.021 | 1.28 | 10 | 2117, 2159 |
| CoFC@PG-Py | 0.006 | 0.005 | 1.17 | 3 | 2121, 2159 |
Cesium sorption experiments
Cs+ sorption experiments on both nanocomposites as well as on the analogous bulk Co3[Fe(CN)6]2·H2O compound were performed firstly by using non-radioactive Cs+ containing aqueous solutions in order to investigate the sorption kinetics and secondly with non-radioactive and radioactive Cs+ containing aqueous saline solutions (sea water). The saline solutions contain Na (9.6 g L−1), Mg (1.28 g L−1), Ca (0.4 g L−1), K (0.5 g L−1) and Sr (0.0088 g L−1). Sorption isotherms were plotted from data obtained at reaction equilibrium. So equilibrium studies in pure water were performed before isotherm sorption experiments also in pure water. All Cs+ extraction experiments were performed in batch solution under shaking at room temperature.Equilibrium studies
For the equilibrium studies, 10 mg of the nanocomposite were shaken in 20 mL of a 0.5 mmol L−1 of CsNO3 solution in deionized water. Experiments were performed from 5 min to 2 days. Then the nanocomposite was separated from the liquid phase by filtration through a 0.2 μm cellulose acetate membrane and the remaining Cs+ concentration of the supernatant was measured using ionic chromatography. To determine the time required to reach equilibrium, the time dependence of the adsorbed cesium quantity, Qt, (mmol g−1) was used and is defined as: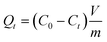 | (1) |
Isotherm studies
For adsorption isotherm studies, a first parent solution of 2 mmol L−1 of Cs+ was prepared by dissolving CsNO3 in deionized water. Solutions with Cs+ concentrations ranging from 0.01 mmol L−1 to 2 mmol L−1 were prepared by diluting the parent solution with deionized water. In each experiment, 10 mg of sorbent nanocomposite were added to 20 mL of investigated solutions for 24 h, the time at which the equilibrium is always reached. Then the nanocomposite was separated from the liquid phase by filtration through a 0.2 μm cellulose acetate membrane. The remaining Cs+ concentration of the liquid phase was then analyzed by ionic chromatography. To establish the adsorption isotherm, the remaining solute concentration of the cesium at equilibrium, Ce, (mmol L−1) was compared with the quantity of the cesium retained on solid particles (nanocomposite or bulk materials), Qe, (mmol g−1). The relationship Q = f(C) is known as a “sorption isotherm”. The cesium concentration retained in the solid particles at equilibrium is given by the equation: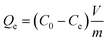 | (2) |
Sorption experiments with simulated radioactive sea water
The third kind of sorption experiments concerns an effect of the additional salts presented in the sea water on the sorption properties of nanocomposites by using both, the Cs+ enriched sea water and sea water solutions simulating the Fukushima reactors' effluents containing radioactive 137Cs. On the one hand, 10 mg of the sorbent nanocomposite were added to 20 mL of the enriched sea water solution containing 10−4 mol L−1 of CsCl and mixed for 24 h (until equilibrium). Then the nanocomposite was separated from the liquid phase by filtration through a 0.2 μm cellulose acetate membrane and the Cs+ concentration of the liquid phase was analyzed by the ionic chromatography. On the other hand, 10 mg of the nanocomposite was shaken with 20 mL of the enriched sea water solution containing 137Cs (29 kBq L−1) over 24 h. Then the solid was filtered off and the remaining Cs+ in solution was analysed by gamma spectrometry.Physical measurements
Thermal analysis was performed with a Setaram Instrument under nitrogen flow with a heating rate of 5 °C min−1. IR spectra were recorded on a Perkin Elmer 1600 spectrometer with a 4 cm−1 resolution. UV-Vis spectra were recorded in KBr disks on a Cary 5E spectrometer. Elemental analyses were performed by the Service Central d'Analyse (CNRS, Vernaison, France). Nitrogen and carbon content in the samples was determined using a LECO instrument. The samples were heated at 3000 °C under oxygen. Nitrogen and carbon were transformed respectively into NOx and CO and detected by using an IR detector. Cesium species concentrations in solution for sorption experiments in inactive conditions were measured using ionic chromatography (from Metrohm). Solutions were injected in a column as mobile phase. Cs species are retained on the stationary phase and are then eluted after 20 min and analyzed by conductivity. Cobalt and Iron species concentrations in solution for sorption experiments were measured using coupled plasma atomic emission spectroscopy from SPECTRO. Powder X-ray diffraction patterns and Small Angle X-Ray Scattering (SAXS) were measured on a Bruker® D8 advanced Diffractometer in Bragg-Bentano geometry with Ni-filtered Cu-Kα radiation. The measurement parameters are: step size 0.02008; counting time 15 s. Samples for Transmission Electron Microscopy (TEM) measurements were prepared using extractive replicas or ultramicrotomy techniques. Extractive replica technique consists of deposition of the nanocomposite from ethanolic suspension on a freshly cleaved mica plate. After evaporation of the ethanol, a carbon film is deposited onto the mica plate. Then, the latter is immersed in a dilute HF solution. The carbon film is then detached from the mica plate and floats on the surface of the solution which allows dissolution of the silica part of the nanocomposite keeping the cyano-bridged coordination polymer nanoparticles stuck to the carbon film. After washing the carbon film twice, it is deposited onto copper grids for TEM observation. Thus, this technique allows visualization of the cyano-bridged coordination polymer nanoparticles after the removal of silica. Ultramicrotomy technique consists of suspension of the material in a resin which is polymerized at low temperature (i.e. 70 °C), then slices of ca. 60 to 100 nm are cut with an ultramicrotome apparatus equipped with a diamond knife. This technique allows visualization of both the cyano-bridged coordination polymer nanoparticles and the matrix. TEM measurements were carried out at 200 kV with a microscope JEOL CX200. The nanoparticles size distribution histograms were determined using enlarged TEM micrographs taken at magnification of x50 K. A large number of nanoparticles (400–600) were counted in order to obtain a size distribution with good statistics. Scanning Electron Microscopy measurements were performed with a Philips Quanta 200 operating at 15 kV equipped with a Bruker detector. System software for EDX analysis was developed by Bruker. Surface area was obtained using nitrogen adsorption isotherms on an ASAP2020 analyser from Micromeritics. Samples were degassed under vacuum at 60 °C over night prior to analysis. Surface area was determined using Brunauer-Emmett-Teller (BET) method.Results and discussion
Synthesis of the nanocomposite materials
For the synthesis of nanocomposites containing cyano-bridged coordination polymer nanoparticles, we used two different matrices: (i) SBA-15 mesostructured silica with 10 nm pore size as a powder. This material was used as a model for investigations of different synthetic steps and sorption mechanisms; and (ii) porous glass pearls presenting higher chemical and thermal stability than silica which can be used for cesium extraction in column or cartridge processes. Co3[Fe(CN)6]2 (CoFC) nanoparticles were chosen to insert into both matrices due to the high cesium adsorption capacity of the bulk Co3[Fe(CN)6]2 compound.25 Note that cobalt ferrocyanides are most often used in low radioactive waste treatment26,27 and are also mentioned in the literature as the most selective compounds for Cs+ extraction stable under γ irradiation.4Fig. 1 represents schematically the method that we used in order to form the nanocomposite materials containing Co2+/[Fe(CN)6]3− coordination polymer nanoparticles into the porous silica or glass matrices functionalized by –(CH2)2C5H4N. It consists of the intrapore growth of the cyano-bridged networks at specific sites of the matrices performed by consecutive coordination of Co2+ and [Fe(CN)6]3− using the following typical procedure. The pyridine-functionalized matrices (powder Silica-py or glass-py pearls) were consecutively treated 5 times first with a methanolic solution of Co2+ and then with a methanolic solution of [N(C4H9)4]3[Fe(CN)6]. At each step of the treatment, the obtained powder (or pearls) was thoroughly washed with methanol and the final materials Co2+/[Fe(CN)6]3−/Silica-Pyridine (CoFC/Silica-Py) and Co2+/[Fe(CN)6]3−/PG-Pyridine (CoFC/PG-Py) were dried at 110 °C in vacuo. The elemental analysis of these nanocomposites and the weight % (wt%) of inserted Co2+/[Fe(CN)6]3− nanoparticles are given in Table 1. The content of Co2+/[Fe(CN)6]3− particles inserted in the pores of Silica-Py and Glass-Py matrixes can be estimated to about 10 wt% and 3 wt%, respectively (Table 1). Compared to the specific surface of each support, the amount of Co-ferrocyanide based nanoparticles is proportional to the available surface of the porous materials (Table 2).
![Schematic representation of the intrapore growth of cyano-bridged coordination polymer nanoparticles Co2+/[Fe(CN)6]3− by using mesostructured silica or porous glass as matrix.](/image/article/2012/RA/c2ra00012a/c2ra00012a-f1.gif) | ||
| Fig. 1 Schematic representation of the intrapore growth of cyano-bridged coordination polymer nanoparticles Co2+/[Fe(CN)6]3− by using mesostructured silica or porous glass as matrix. | ||
| Samples | S BET/m2 g−1 | Porous Volume/cm3 g−1 | Pore filling (%) | d 100/nm | Pore Diameter/nm | d a/nm |
|---|---|---|---|---|---|---|
| a Nanoparticle diameter obtained from TEM measurements. b From BJH method on the nitrogen adsorption curve. | ||||||
| Silica | 540 | 0.83 | — | 9.72 | 9.4 | — |
| CoFC@ Silica-Py | 231 | 0.41 | 50 | 9.83 | 8.1 | 5.8 |
| PG | 113 | 1.1 | — | 30b | — | |
| CoFC@ PG-Py | 94 | 1.0 | 10 | — | 28b | 2.7 |
Textural characterizations of the nanocomposites
The porous structure of the samples was characterized by using nitrogen adsorption isotherm and SAXS measurements. The nitrogen adsorption isotherms allow the determination of the specific surface by BET method, and the total pore volume Vp (cm3 g−1). The nitrogen physisorption isotherms of the pristine mesoporous matrices silica and PG as well as the corresponding nanocomposites CoFC@Silica-Py and CoFC@PG-Py are shown on Fig. 2a. The mesostructured silica matrix exhibits a typical adsorption–desorption isotherm for a mesoporous structure of type IV with an H1 hysteresis loop. A similar isotherm and mesoporosity are obtained for the corresponding nanocomposite CoFC@Silica-Py, that proves the preservation of the cylindrical pore system after intrapore growth of the cyano-bridged metallic coordination polymer. The amount of the adsorbed nitrogen as well as the BET surface were reduced after formation of the nanoparticles. The decrease of the mesoporous volume and the pore diameter after formation of the nanoparticles, clearly demonstrate a filling of the pores with the guest species leading to the conclusion that the nanoparticles are formed inside the pores. The total pore volume calculated at p/p0 = 0.9 is equal to 0.83 cm3 g−1 for the pristine mesoporous silica and to 0.41 cm3 g−1 for CoFC@Silica-Py (Table 2) that indicates a degree of pore filling of ca. 50%. The nitrogen physisorption isotherms and pore-size distributions at the adsorption branch of the pristine mesoporous glass PG and its respective nanocomposite CoFC@PG-Py (Fig. 2a, Table 2) present a filling of the pores with the cyano-bridged polymer nanoparticles of ca. 10%.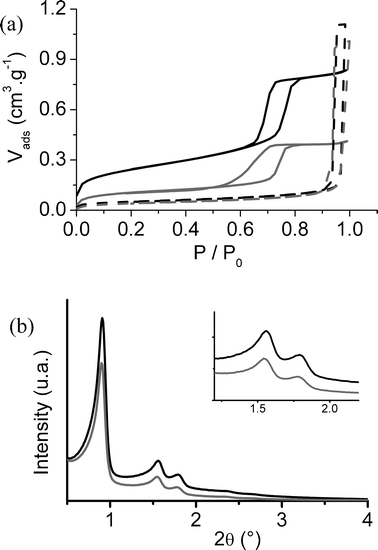 | ||
| Fig. 2 (a) Nitrogen adsorption isotherms for the pristine mesoporous silica(- - -) and glass PG(—) as well as for the related nanocomposites CoFC@Silica-Py(- - - gray) and CoFC@PG-Py(— gray); (b) SAXS diffractograms of the pristine mesoporous silica (black) and of CoFC@Silica-Py (gray). Inset: magnification of (b). | ||
SAXS measurements were performed on samples of silica and CoFC@Silica-Py. As shown Fig. 2b, the initial functionalized silica has a hexagonal porous structure with the characteristic peaks for an SBA-15 type material: the peaks at 2θ = 0.9°, 1.5° and 1.8° correspond respectively to the 100, 110 and 200 planes. After pore filling by the nanoparticles, an identical hexagonal type structure with the characteristics of an SBA-15 porous texture was obtained (Table 2). Combining SAXS and the adsorption experiment gives an easy and accurate method for the determination of the pore diameter (Φ(nm)) (Table 2) thanks to the following equation:28
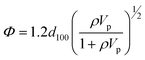 | (3) |
Note that the pristine hybrid glass PG and its respective nanocomposite CoFC@PG-Py are amorphous and cannot be characterized by SAXS.
The results of the nitrogen physisorption and SAXS were substantiated by TEM. The TEM images of the pristine mesoporous silica clearly show the hexagonal ordering of the pores (Fig. 3a). The TEM measurements performed for the related nanocomposite CoFC@Silica-Py indicate that the hexagonal structure of the host materials is still retained (Fig. S1a, ESI†). No visible particles separated out of the surface of pores were observed. As expected, no aggregates can be observed into the pores which is indicative of a homogeneous dispersion of the CoFC cyano-bridged polymer network in the silica matrix. The CoFC nanoparticles can be seen after removal of silica from the nanocomposite material using an extractive replica technique (see experimental section for details) (Fig. 3b). The sample presents a relatively narrow size distribution of nanoparticles centered at 5.8 ± 1.4 nm (Table 2). This value is slightly smaller than the pore channel mean diameter of the respective host silica of 10 nm obtained from BET and SAXS measurements (Table 2).
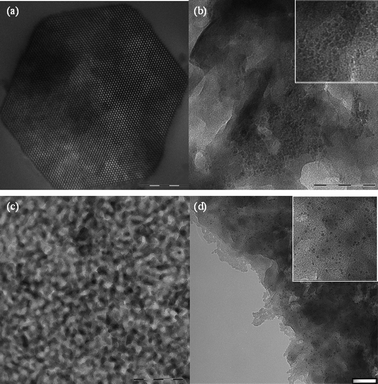 | ||
| Fig. 3 TEM pictures for (a) silica (scale bar = 200 nm); (b) the nanoparticles of CoFC after removal of the silica from the nanocomposite CoFC@Silica-Py (scale bar = 100 nm); (c) PG glass pearls (scale bar = 200 nm) and (d) for the nanoparticles of CoFC after removal of the glass from the nanocomposite CoFC@PG-Py (scale bar = 50 nm). Insets show magnifications of 3b and 3d. | ||
The same conclusions may be obtained from TEM observations of PG glass (Fig. 3c), the nanocomposite CoFC@PG-Py (Fig. S1b, ESI†), and after removal of the glass from the nanocomposite material using an extractive replica (Fig. 3d). Nanoparticles with a size distribution centered at 2.7 ± 0.8 nm are obtained (Table 2).
Structural characterisation of the nanocomposites
IR spectroscopy was performed on Silica-Py and PG-Py matrices before and after nanoparticles formation especially in the spectral window 2000–2300 cm−1, i.e. in the vicinity of the CN stretching mode, which is a fingerprint of structural and electronic changes occurring in Prussian blues analogous. The CN stretching frequency of a free CN− ion is 2080 cm−1, whereas upon coordination to a metal ion, it shifts to higher frequencies.17The IR spectra of both obtained nanocomposites (Table 1, Fig. S2, ESI†) show two characteristic absorption bands in the CN stretching region at 2159 cm−1 and 2117 cm−1 for CoFC@Silica-Py and at 2159 cm−1 and 2121 cm−1 for CoFC@PG-Py. The high frequency bands can be attributed to the stretching of the CN ligand bridged between Co2+ and Fe3+ in a Co2+–CN–Fe3+ mode and the low frequency band can be attributed to the linkage isomer with a Co2+–NC–Fe3+ coordination mode, as it was reported for the bulk cyano-bridged coordination polymer29,30 (Table 2). As expected, the IR spectra of the nanocomposites also present SiO2 vibration bands at 1080, 948, 798 and 459 cm−1.
XRD powder patterns of the nanocomposites materials containing cyano-bridged coordination polymer nanoparticles (silica and glass) in the range 10–60° (2Θ) were compared with the XRD powder pattern of the bulk material (Fig. 4). Both nanocomposites materials show characteristic peaks very similar to those of bulk CoFC, meaning the presence of a significant amount of the crystalline cyano-bridged coordination polymer in these materials.
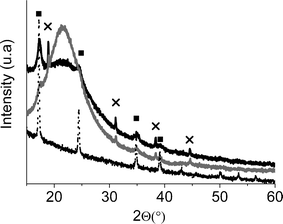 | ||
| Fig. 4 XRD powder pattern for the nanocomposite materials CoFC@Silica-Py (black) and CoFC@Glass-Py (gray) compared of the XRD powder pattern of bulk CoFC (dotted line, and ■), peaks (X) attributed to K2SiF6 present as an impurity. | ||
Cesium sorption experiments
The sorption of an element in solution on a solid support depends on the characteristics of this latter such as ionic exchange capacity, porosity, composition, as well as on the solution parameters such as ionic concentration, pH, stirring conditions. Two types of adsorption may be operational: (i) a physisorption in which the ions in solution are adsorbed on the solid by electrostatic forces, and (ii) chemisorption for which the ions form a chemical bond with the solid. The Cs+ sorption mechanism on the bulk metalhexacyanoferrates depends on their chemical composition. In the case of anionic metal hexacyanoferrate complexes, KM[Fe(CN)6], it is assumed in the literature that there is a true exchange between potassium and cesium ions3,31 without modification of the crystal structure.32It is also shown that not all the potassium ions can be exchanged by cesium and the exchange degree depends on the transition metal used.32,33 On the contrary, in the case of neutral metal hexacyanoferrates, M3[Fe(CN)6]2, the Cs+ sorption mechanism is far from being well described and understood. Some authors assume an ionic exchange between the M2+ ion and the cesium3,33–35 especially at the surface layer of the crystallites,31,36 while others accept that Cs+ ions may be incorporated into the cage structure of the metal hexacyannoferrate as an ion pair with nitrate.27 In addition, the Cs+ sorption efficiency and selectivity depend on the experimental conditions that induces some difficulties when comparing various materials based on neutral metal hexacyanoferrates.33
After each sorption experiment, iron concentration in solution was measured lower than 0.001 mmol L-1, meaning that no dissolution of the nanoparticles occurs during the immersion of these nanocomposites in solution.
Adsorption kinetics
Investigation of the sorption kinetics permits the determination of the time required to reach an equilibrium as well as the empirical order of the reaction and the experimental exchange capacity, thanks to a kinetic reaction model. Moreover, a diffusive model helps to determine the sorption kinetics limiting steps.37 The effect of the contact time on the amount of adsorbed cesium performed for both nanocomposite material, CoFC@Silica-Py and CoFC@Glass-Py, as well as for the bulk CoFC was analyzed by using these two models, the kinetic reaction model and the diffusive model (Fig. 5). The obtained results demonstrate that the kinetics of adsorption of both nanocomposites are more rapid than the one of the bulk CoFC: the process is quite rapid and the equilibrium is reached after 1 h in the case of the nanocomposites and after 10 h in the case of the bulk solid. On the other hand, the adsorption capacity at equilibrium is four or twenty times smaller in the case of nanocomposites compared to the bulk materials. However, this result is not surprising taking into account that the adsorption capacities have been calculated as mmol of adsorbed Cs+ per gram of whole materials and that the amount of CoFC nanoparticles into the matrices is 10 and 3 wt% for CoFC@Silica-Py and CoFC@Glass-Py, respectively. This point will be discussed in the adsorption isotherm section.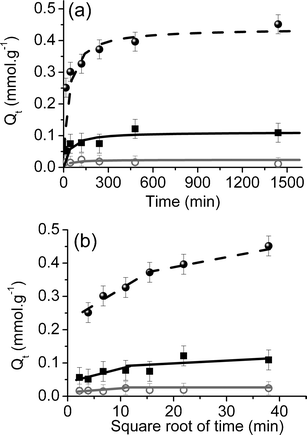 | ||
| Fig. 5 Effect of the contact time on the amount of the adsorbed cesium on the nanocomposites, CoFC@Silica-Py (■) and CoFC@Glass-Py (○), and on the bulk CoFC (•) (a) kinetic reaction model (solid lines correspond to the fit of experimental data with the kinetic reaction model) and (b) diffusive model presented as a quantity of adsorbed cesium, Qt, (mmol g−1) vs. square root of time (lines are a guide for the eye). | ||
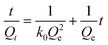 | (4) |
Except for the third one, all other steps are diffusion limited. The determination of the limiting step of the adsorption process may be achieved by plotting the amount of adsorbed ions as a function of the square root of time. The diffusion in the liquid phase (i.e. the first diffusion step) is neglected due to the stirring of the solutions. Fig. 5b shows the plot of the quantity of adsorbed cesium, Qt (mmol g−1), vs. the square root of time for both nanocomposite materials as well as for the bulk CoFC. The curve performed for the bulk CoFC shows two linear zones but the saturation and thus equilibrium stage are not totally reached.34 According to the literature, the presence of these two linear region on the curve may be explained by the presence of two sorption processes: an external mass transfer such as the boundary layer diffusion for the first linear region and an intra-particle diffusion for the second.37,38 Note also that the absence of saturation has also been noted for bulk copper hexacyanoferrates, and has been attributed to a small reorganization of the solid accompanied by a copper release in the solution.34
The curves plotted for the nanocomposite samples, CoFC@Silica-Py and CoFC@Glass-Py don't show clear linear regions (Fig. 5b). In these cases, any diffusion process appears as a rate-controlled step. This difference could be attributed to the presence of nano-sized CoFC particles inside the porous silica or glass support presenting a large surface for cesium sorption without rate limiting steps, whereas in the bulk sample, the diffusion limited process would be due to the micron-size of the sorbent. This observation can also be linked with a sorption mechanism involving mainly the surface layer of the crystallites.31,36
Adsorption isotherm
The sorption isotherms' measurements give the quantity of the adsorbed cesium at equilibrium (Qe, mmol g−1) vs. concentration of cesium in solution (Ce, mmol L−1).37Qe is defined as: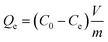 | (5) |
Fig. 6 presents the adsorption isotherms for the three investigated sorbents. The three curves are concave to the concentration axis which reflects the efficiency of these materials for the sorption of cesium ions in a wide range of concentration. The curves present a similar shape with a high slope for low Cs+ concentrations indicating that all these materials have a good affinity to cesium ions. The presence of a plateau for the high Cs+ concentrations specifies that the saturation of the sorbent is achieved when all the available sites for cesium uptake were used.
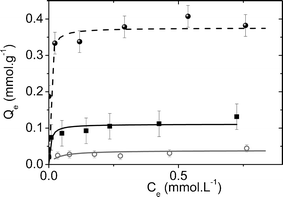 | ||
| Fig. 6 Cs+ adsorption isotherm from pure water for (•) CoFC; (■) CoFC@Silica-Py and (○) CoFC@Glass-Py. Solid lines represent the fits with the Langmuir isotherm model. | ||
Two main parameters should be taken into account in order to evaluate the sorbent efficiency: (i) the maximum adsorption capacity (Qmax) which indicates the efficiency of the materials to remove Cs+ at (or near) saturation. It can be estimated from the isotherm's plateau and more precisely obtained by using the Langmuir model (see above); (ii) distribution coefficient, Kd (mL g−1), evaluates the selectivity of the sorbent to extract the cesium ions at very low concentrations in the presence of other competitive ions such as Na+, K+, Ca2+ at high concentrations.42 In other words, high Kd values represent high selectivity for Cs+. Note that the affinity of the bulk Prussian blue analogues for alkaline ions follows as Na ≪ K < Cs.3,43,44
Given that these two parameters are independent and the highest Kd value does not necessarily offer the highest sorption capacity for the sorbent and vice versa, the evaluation of a sorbent in the decontamination process requires first a high distribution coefficient and second a high sorption capacity.
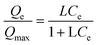 | (6) |
| Sorbent | Q max/mmol g−1 of composite | Q max a/mmol g−1 of CoFC | K d/mL g−1 (sea water, Cs: 10 ppm) | K d/mL g−1 (radioactive sea water, Cs: 29 kBq L−1) |
|---|---|---|---|---|
| a The Cs equilibrium concentration in sea water here is out of the detection limit (lower than 0.5 ppm). | ||||
| CoFC | 0.38 | 0.4 | >104 | 6 × 105 |
| CoFC@ Silica-Py | 0.13 | 1.3 | >104 | 8 × 105 |
| CoFC@ Glass-Py | 0.04 | 1.3 | 103 | 3 × 105 |
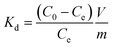 | (7) |
First off, the distribution coefficient of the bulk CoFC and the nanocomposite materials CoFC@Silica-Py, CoFC@Glass-Py were determined in sea water containing 10 ppm of natural Cs+ with a concentration of 0.5 g L−1 of the sorbent (V m−1 = 2 L g−1) (Table 3). Note that the used composition of the inactive sea water is closed to the one of the Fukushima site. In the case of the sea water, even if the sodium concentration is very high (9.6 g L−1 of Na+), the nanocomposite materials uptake very low concentrations of Cs+ with Kd higher than 104 and 103 for CoFC@Silica-Py and CoFC@Glass-Py, respectively. Therefore, these inorganic sorbents are the materials of choice for decontamination of 137Cs enriched water. The difference at Cs+ concentration of 10 ppm (sea water) between the values obtained for silica and glass supports can be attributed to the amount of CoFC inserted into the pores of these materials.
Secondly, the sorbents have been tested with simulated radioactive sea water from Fukushima site with 29 kBq L−1 of 137Cs. The obtained distribution coefficients of 8 × 105 and 3 × 105 for CoFC@Silica-Py and CoFC@Glass-Py, respectively (Table 3), are very high and demonstrate that these materials extract Cs+ in very low concentrations (in trace) from the radioactively enriched sea water. Note also that these obtained distribution coefficients are comparable to the one obtained for bulk CoFC. Therefore our composites are promising materials for the decontamination of sea water enriched with 137Cs like the Fukushima site.
Furthermore, the same nanocomposites may be obtained by using functionalized glass pearls required as an excellent support for decontamination following a continuous process in a column. After Cs+ absorption, the porosity closing by using a soft method like a treatment at low temperature or a treatment in basic conditions may be performed that allow a Cs+ confinement for further storage. These studies are currently under way.
Conclusion
In summary, the concept of using nanocomposites containing Prussian blue type nanoparticles covalently linked to a matrix appears to be a promising route to the decontamination of cesium ions. In this article, we prepared two types of nanocomposites based on functionalized silica or glass matrices containing the cyano-bridged coordination polymer Co2+/[Fe(CN)6]3−. These nanocomposites were obtained by successive coordination of cobalt ions and hexacyanoferrate precursors at the specific amino sites of the matrix. This approach allows one to obtain uniformly-sized spherical NPs of 5.8 and 2.7 nm, respectively in the silica and glass matrix. The as-obtained nanocomposites have been used for selective Cs+ extraction from different effluents: pure water, sea water and simulated radioactive sea water from the Fukushima site.The first point to note is that porous glass has been used as the matrix for the growth of the Prussian blue nanoparticles for the first time. As in the case of mesostructured silica, the presence of the pyridine bonding sites avoids both aggregation and extrusion of the formed coordination polymer nanoparticles and allows control of their dispersion providing nanocomposite materials in which the nanoparticles are homogeneously distributed into the matrix pores.
The second point is that the kinetics of cesium sorption is ten times faster and the sorption capacities per gram of metal hexacyanoferrate are more than 3 times higher in the case of the nanocomposites compared to the bulk Prussian blue analogues. This fact may be attributed to the high surface area of the Prussian blue nanoparticles in the case of nanocomposites.
The third point is that the nanocomposite materials also present a high selectivity to Cs+ comparable to the bulk materials. Even in the presence of high concentration of sodium in sea water, these nanocomposites exhibit very high distribution coefficients and extract the Cs+ ion in trace amounts. Therefore, these inorganic sorbents present both, high selectivity and high capacity and are the materials of choice for decontamination of 137Cs enriched water.
Fourthly, the experiments done with the sea water enriched with the radioactive 137Cs ion simulating Fukushima's contaminated effluents demonstrate a high potential of these nanocomposite materials for an efficient decontamination column process. Moreover, the closing of the glass-based nanocomposites pores after the decontamination should permit an efficient confinement and further storage of radioactive Cs. This study sheds light on the importance of the use of sorbents at the nanoscale for the decontamination of cesium supported on inorganic matrices. The presented concept of using nanocomposites containing Prussian blue nanoparticles covalently linked to a glass or silica matrix may now be regarded by the scientific community to be extended to other sorbents for the immobilization of various contaminants.
Acknowledgements
This work was supported by the ANR (Memfis, ANR-2010-RMNP-003-02), the University Montpellier Sud de France, the Matinex and Paris National Research Groups, the CEA and the CNRS. We acknowledge M. Nemec for first trials on glass pearls and Guillaume Serve, and Célia Lepeytre for their help on radioactive experiments.References
- H. Sepehrian, R. Yavari, S. Waqif-Husain and M. Ghannadi-Maragheh, Sep. Sci. Technol., 2008, 43, 3269–3285 CrossRef CAS.
- A. Merceille, E. Weinzaepfel, Y. Barré and A. Grandjean, Adsorption, 2011, 17, 967–975 CrossRef CAS.
- P. A. Haas, Sep. Sci. Technol., 1993, 28, 2479–2506 CrossRef CAS.
- J. Lehto and L. Szirtes, Radiat. Phys. Chem., 1994, 43, 261–264 CrossRef CAS.
- H. Mimura, J. Lehto and R. Harjula, J. Nucl. Sci. Technol., 1997, 34, 607–609 CAS.
- R. Harjula, J. Lehto, A. Paajanen, L. Brodkin and E. Tusa, Nucl. Sci. Eng., 2001, 137, 206–214 CAS.
- R. Harjula, J. Lehto, A. Paajanen, E. Tusa and P. Yarnell, React. Funct. Polym., 2004, 60, 85–95 CrossRef CAS.
- H. Mimura, M. Kimura, K. Akiba and Y. Onodera, Solvent Extr. Ion Exch., 1999, 17, 403–417 CrossRef CAS.
- A. Mardan and R. Ajaz, J. Radioanal. Nucl. Chem., 2002, 251, 359–361 CrossRef CAS.
- R. D. Ambashta, P. K. Wattal, S. Singh and D. Bahadur, J. Magn. Magn. Mater., 2003, 267, 335–340 CrossRef CAS.
- C. Y. Chang, L. K. Chau, W. P. Hu, C. Y. Wang and J. H. Liao, Microporous Mesoporous Mater., 2008, 109, 505–512 CrossRef CAS.
- T. Sangvanich, V. Sukwarotwat, R. J. Wiacek, R. M. Grudzien, G. E. Fryxell, R. S. Addleman, C. Timchalk and W. Yantasee, J. Hazard. Mater., 2010, 182, 225–231 CrossRef CAS.
- H. Mimura, M. Kimura, K. Akiba and Y. Onodera, Sep. Sci. Technol., 1999, 34, 17–28 CrossRef CAS.
- C. Loos-Neskovic, C. Vidal-Madjar, B. Jimenez, A. Pantazaki, V. Federici, A. Tamburini, M. Fedoroff and E. Persidou, Radiochimica Acta, 1999, 85, 143–148 CAS.
- S. Milonji, I. Bispo, M. Fedoroff, C. Loos-Neskovic and C. Vidal-Madjar, J. Radioanal. Nucl. Chem., 2002, 252, 497–501 CrossRef CAS.
- L. Sharygin, A. Muromskiy, M. Kalyagina and S. Borovkov, J. Nucl. Sci. Technol., 2007, 44, 767–773 CrossRef CAS.
- B. Folch, Y. Guari, J. Larionova, C. Luna, C. Sangregorio, C. Innocenti, A. Caneschi and C. Guerin, New J. Chem., 2008, 32, 2299–2299 RSC.
- A. Grandjean, Y. Barré, Y. Guari, J. Larionova and C. Guerin, France, FR2945756 A1 20101126 and WO 2010 133689 A2 20101125 Search PubMed.
- A. S. Tkachev, T. V. Antropova, V. P. Veiko and I. A. Drozdova, Glass Physics and Chemistry, 2004, 30, 173–179 CrossRef CAS.
- D. Y. Zhao, J. L. Feng, Q. S. Huo, N. Melosh, G. H. Fredrickson, B. F. Chmelka and G. D. Stucky, Science, 1998, 279, 548–552 CrossRef CAS.
- D. Y. Zhao, Q. S. Huo, J. L. Feng, B. F. Chmelka and G. D. Stucky, J. Am. Chem. Soc., 1998, 120, 6024–6036 CrossRef CAS.
- L. Mercier and T. J. Pinnavaia, Adv. Mater., 1997, 9, 500 CrossRef CAS.
- A. Cauvel, G. Renard and D. Brunel, J. Org. Chem., 1997, 62, 749–751 CrossRef CAS.
- R. J. P. Corriu, E. Lancelle-Beltran, A. Mehdi, C. Reye, S. Brandes and R. Guilard, J. Mater. Chem., 2002, 12, 1355–1362 RSC.
- C. Delchet, Unpublished work.
- J. Lehto, A. Paajanen and R. Harjula, J. Radioanal. Nucl. Chem., 1992, 164, 39–46 CrossRef CAS.
- D. V. Ca and J. A. Cox, Microchim. Acta, 2004, 147, 31–37 CAS.
- M. Kruk, M. Jaroniec and A. Sayari, J. Phys. Chem. B, 1997, 101, 583–589 CrossRef CAS.
- E. Reguera, J. F. Bertran, C. Diaz, J. Blanco and S. Rondon, Hyperfine Interact., 1990, 53, 391–395 CrossRef CAS.
- D. H. M. Buchold and C. Feldmann, Chem. Mater., 2007, 19, 3376–3380 CrossRef CAS.
- J. Lehto, R. Harjula and J. Wallace, J. Radioanal. Nucl. Chem., 1987, 111, 297–304 CrossRef CAS.
- C. Loos-Neskovic, S. Ayrault, V. Badillo, B. Jimenez, E. Garnier, M. Fedoroff, D. J. Jones and B. Merinov, J. Solid State Chem., 2004, 177, 1817–1828 CrossRef CAS.
- V. Avramenko, S. Bratskaya, V. Zheleznov, I. Sheveleva, O. Voitenko and V. Sergienko, J. Hazard. Mater., 2011, 186, 1343–1350 CrossRef CAS.
- S. Ayrault, B. Jimenez, E. Garnier, M. Fedoroff, D. J. Jones and C. Loos-Neskovic, J. Solid State Chem., 1998, 141, 475–485 CrossRef CAS.
- T. P. Valsala, A. Joseph, J. G. Shah, K. Raj and V. Venugopal, J. Nucl. Mater., 2009, 384, 146–152 CrossRef CAS.
- M. Ramaswamy, Solvent Extr. Ion Exch., 1999, 17, 1603–1618 CrossRef CAS.
- G. Crini, H. N. Peindy, F. Gimbert and C. Robert, Sep. Purif. Technol., 2007, 53, 97–110 CrossRef CAS.
- E. Metwally, R. O. A. Rahman and R. R. Ayoub, Radiochim. Acta, 2007, 95, 409–416 CrossRef CAS.
- A. Mardan, R. Ajaz, A. Mehmood, S. M. Raza and A. Ghaffar, Sep. Purif. Technol., 1999, 16, 147–158 CrossRef CAS.
- H. D. Liu, F. Z. Li, X. Zhao and G. C. Yun, Nucl. Technol., 2009, 165, 200–208 CAS.
- M. Ramaswamy, Solvent Extr. Ion Exch., 1997, 15, 1119–1131 CrossRef CAS.
- Y. H. Lin, G. E. Fryxell, H. Wu and M. Engelhard, Environ. Sci. Technol., 2001, 35, 3962–3966 CrossRef CAS.
- S. Sinha, B. D. Humphrey and A. B. Bocarsly, Inorg. Chem., 1984, 23, 203–212 CrossRef CAS.
- L. F. Schneemeyer, S. E. Spengler and D. W. Murphy, Inorg. Chem., 1985, 24, 3044–3046 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: TEM images of the nanocomposites (Fig. S1) and IR spectra of the nanocomposite (Fig. S2) and fit of the kinetics data with the pseudo second order model. See DOI: 10.1039/c2ra00012a |
| This journal is © The Royal Society of Chemistry 2012 |
