Sterically congested pyrrole-fused tetrathiafulvalene decamers as highly conductive amorphous molecular materials†
Masayoshi
Takase
*,
Naofumi
Yoshida
,
Tomoyuki
Narita
,
Takashi
Fujio
,
Tohru
Nishinaga
and
Masahiko
Iyoda
*
Department of Chemistry, Graduate School of Science and Engineering, Tokyo Metropolitan University, Hachioji, Tokyo, 192-0397, Japan. E-mail: mtakase@tmu.ac.jp; iyoda@tmu.ac.jp
First published on 7th March 2012
Abstract
Sterically congested pyrrole-fused tetrathiafulvalene (TTF) decamer 4 was designed and synthesized via the SNAr reaction of decafluorobiphenyl with the corresponding pyrrolyl sodium salt, which formed amorphous spin-coated films showing good conductivity of up to 4.4 S cm−1 after iodine doping as the result of multi-dimensional π–π stacking between the TTF units.
The anisotropic nature of molecular conductors is a critical issue because the conjugated π-systems employed for electric conduction usually deviate greatly from spherical symmetry and the molecular orbitals contributing to the conduction are conjugated π-orbitals parallel to the π-planes. On the other hand, because low-dimensional charge conduction is intrinsically unfavorable and because the tuning of crystalline morphology brings different conductivities, conductive donor–acceptor complexes have disadvantages. Among the numerous molecular conductors, tetrathiafulvalene (TTF) and its analogs are the most important class of π-donors due to their thermodynamically stable 6π-electrons in 1,3-dithiolium cations formed by one-electron oxidation. Given the fascinating properties of TTF and the essential problems with molecular conductors, supramolecular chemistry has recently been applied to this research area to create conductive soft TTF materials.1–3 In this context, we recently reported the synthesis and the redox and conductive properties of a series of star-shaped TTF oligomers 1–3 (Fig. 1a).3d The less-conjugated but still rigid multiple pyrrole-benzene linkages that form propeller structures resulted in no intramolecular interactions between neutral, oxidized, and neutral/oxidized TTFs, although an increase in the charge carrier density resulting from the accumulation of radical cation moieties on the TTF oligomers did not greatly affect their conductivities.
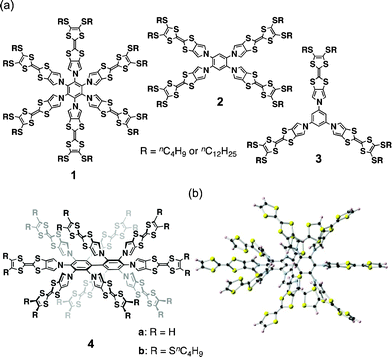 | ||
| Fig. 1 (a) Star-shaped TTF oligomers 1–4 and (b) a DFT-optimized structure of 4a (B3LYP/6-31G(d)). | ||
In addition to well-defined donor–acceptor segregated crystals and well-programmed supramolecular assemblies, amorphous molecular materials composed of small organic compounds have also been studied as a new class of functional organic materials.4 Although these materials are prepared by solution deposition, as are polymers, the films of small compounds are pure materials with well-defined molecular structures and definite molecular weights. Among the numerous compounds forming amorphous molecular materials, key compounds are multi-branched compounds such as triarylamines4a,d and spiro compounds.4c Based on these findings, we attempted to prepare the sterically congested TTF decamer 4 because spatially substituted TTF units are expected to form amorphous films with multi-dimensional conduction pathways. Herein, we present the synthesis of highly substituted TTF oligomers via the nucleophilic aromatic substitution (SNAr) of decafluorobiphenyl with a pyrrole-fused TTF. We also describe the redox behaviors and conductivities of the solution-processed films.
As shown in the model structure of D2 symmetric 4a, ten TTF units are introduced in a heavily congested manner, with the dihedral angles between the pyrroles and the central benzene rings in the range of 52–60° (Fig. 1b). Because the synthesis of desired decamer 4b was supposed to be difficult due to the multiple bulky substitutions, we first investigated the SNAr reaction with the parent pyrrole (Scheme 1a). Following the reported procedure,3d,5 decafluorobiphenyl was added to a DMF solution of 20 equiv. of pyrrolyl sodium salt at 0 °C, and then the reaction mixture was slowly allowed to warm to 80 °C to yield decapyrrolobiphenyl 5 quantitatively. X-ray single-crystal structure analysis clearly revealed the congested structure of 5 (Fig. 2).6 Two benzene rings of the biphenyl moiety are orthogonally-crossed with the torsion angle of 69.4°, and the peripheral pyrroles substituted on both benzene rings form propeller structures (torsion angles of 48–65°), resulting in a D2 symmetric conformation. The two pairs of adjacent pyrroles in the ortho-positions of the biphenyl moiety have slipped-stacking geometries with short Cα–Cα contact distances of 3.19 and 3.20 Å and N–N distances of 3.11 and 3.16 Å. The stacking structure of these two pairs is in contrast to the reported crystal structure of the phenyl analog, decaphenylbiphenyl,7 in which only one pair of phenyl rings is stacked due to the larger torsion angles through the central and peripheral phenyl rings of 51–79°. From this pseudo D2 symmetric configuration of 5 in the solid state, effective conduction pathways are expected through both the intra- and intermolecular π-stackings.
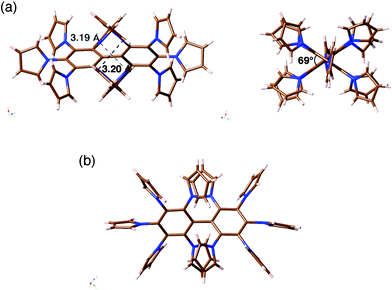 | ||
| Fig. 2 X-ray crystal structure of decapyrrolobiphenyl 5: (a) two side views and (b) top view. | ||
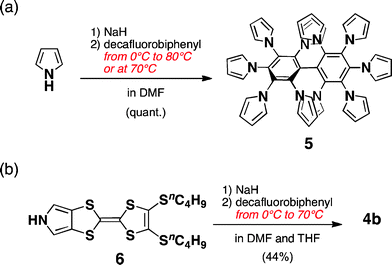 | ||
| Scheme 1 Synthesis of pyrrole-fused TTF decamer 4b and decapyrrolobiphenyl 5. | ||
With these results in hand, we then applied the SNAr reaction to the TTF oligomer 4b with pyrrole-fused TTF 63d,8 (Scheme 1b). Following a similar procedure to 5, 4b was isolated in 44% yield. The obtained structure was confirmed using 1H NMR, LDI-TOF MS, and elemental analysis. For example, in the 1H NMR spectra, α-protons of pyrroles were observed at δ 5.98, 5.86, and 5.65 ppm, reflecting the substitution positions on the biphenyl moiety. Interestingly, when the reaction was conducted at 70 °C without starting from lower temperature, only TTF oligomers up to nonamers were detected using LDI-TOF MS, in which the fluorine atoms remained on the biphenyl moiety, whereas 5 was quantitatively obtained under similar conditions.9
The electrochemical properties of 4b were investigated using cyclic voltammetry (CV) and differential pulse voltammetry (DPV) in benzonitrile (0.1 mM) (Fig. 3a, b). To our surprise, the voltammograms showed two reversible oxidation peaks at 0.11 and 0.44 V (vs. Fc/Fc+), a typical behavior for a TTF system that indicates the sequential formation of the TTF radical cation and the TTF dications,3d although the reference compound 5 possesses a slipped-stacking geometry of the pyrrole moieties in the crystal state. To investigate the possible intramolecular TTF interactions in the oxidized states chemical oxidation was performed with Fe(ClO4)3 in a mixture of CH2Cl2 and CH3CN (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) by monitoring the absorption spectra. Fig. 3c shows the absorption spectra of the neutral, radical cation (TTF•+) and dication (TTF2+) states of 4b. When the oxidant was added incrementally, the spectra exhibited isosbestic points, which strongly supports the sequential oxidation of TTF units from neutral to radical cation 4b10(•+) and then to dication 4b10(2+). Because similar spectral changes were observed for our previous star-shaped TTF oligomer 2,3d it should be noted again that pyrrole–benzene linkages in pyrrole-fused TTF 6 play an important role in stabilizing multiple radical cations and positive charges within a TTF oligomer, even those with a biphenyl π-system.10
1, v/v) by monitoring the absorption spectra. Fig. 3c shows the absorption spectra of the neutral, radical cation (TTF•+) and dication (TTF2+) states of 4b. When the oxidant was added incrementally, the spectra exhibited isosbestic points, which strongly supports the sequential oxidation of TTF units from neutral to radical cation 4b10(•+) and then to dication 4b10(2+). Because similar spectral changes were observed for our previous star-shaped TTF oligomer 2,3d it should be noted again that pyrrole–benzene linkages in pyrrole-fused TTF 6 play an important role in stabilizing multiple radical cations and positive charges within a TTF oligomer, even those with a biphenyl π-system.10
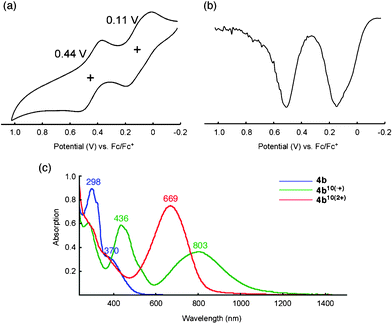 | ||
Fig. 3 (a) Cyclic and (b) differential pulse voltammograms of 4b (0.1 mM) in benzonitrile with 0.1 M nBu4NPF6 as the supporting electrolyte, Ag/AgNO3 as the reference electrode, Pt wire as the counter electrode, and a scan rate of 100 mV s−1. Values are half-wave potentials. (c) Absorption spectra of 4b (blue), 4b10(•+) (green), and 4b10(2+) (red) (0.02 mM) in the presence of stoichiometric amount of oxidant, Fe(ClO4)3·6H2O, in a mixture of CH2Cl2 and CH3CN (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) at room temperature. 1, v/v) at room temperature. | ||
To study the conductive properties, spin-coated films were prepared from a CH2Cl2 solution of 4b (1000 rpm). The flat and uniform morphology of the films was observed by atomic force microscopy (AFM) and scanning electron microscopy (SEM), and the thickness was controlled by changing the concentration and/or the number of drops of the solution in spin-coating, yielding films with thicknesses of ca. 140, 350, and 2000 nm. As shown in the absorption spectrum of the film (350 nm thick), a bathochromic shift was observed relative to the spectrum of a solution in a mixture of CH2Cl2 and CH3CN (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
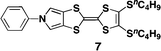
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) (Fig. 4a), indicating the presence of J-type interactions in the film state. However, the X-ray diffractogram as well as AFM and SEM of the films clearly revealed an amorphous nature, as expected (Fig. 4b). Thus, the interactions between TTF units, which caused the bathochromic shift in the absorption spectra, appear to extend radially. The electric conductivity of the 350 nm thick film was then measured after iodine doping with the standard four-terminal method; the obtained value was 4.4 S cm−1 at room temperature. In the case of a 2000 nm thick film, a lower conductivity of 0.24 S cm−1 was observed, whereas the 140 nm thick film showed a comparable value of 2.7 S cm−1 at room temperature. It should be noted that all values are two to three orders of magnitude greater than those for films of previously reported star-shaped TTF oligomers 1–3 (2.5–6.9 × 10−3 S cm−1).3d In stark contrast, a spin-coated film of mono-substituted TTF 7,11 which has an amorphous structure, showed a much lower conductivity of 3.1 × 10−2 S cm−1 under the same conditions. These results clearly support the hypothesis that the high conductivity in the film state is attributable to the sterically congested TTF arrangement, probably making the overall electrical conduction easier as a result of multi-dimensional π–π stacking between TTF units. Furthermore, because it is one of the most characteristic features of conductive molecular materials, the repeatability of the doping and undoping processes was also studied by monitoring the conductivity of a 140 nm thick film (Fig. 4c). The conductivities after doping were of moderate values in the range of 0.95–2.7 S cm−1 for up to six cycles, and the off conductivities after undoping were less than 4.2 × 10−3 S cm−1.
1, v/v) (Fig. 4a), indicating the presence of J-type interactions in the film state. However, the X-ray diffractogram as well as AFM and SEM of the films clearly revealed an amorphous nature, as expected (Fig. 4b). Thus, the interactions between TTF units, which caused the bathochromic shift in the absorption spectra, appear to extend radially. The electric conductivity of the 350 nm thick film was then measured after iodine doping with the standard four-terminal method; the obtained value was 4.4 S cm−1 at room temperature. In the case of a 2000 nm thick film, a lower conductivity of 0.24 S cm−1 was observed, whereas the 140 nm thick film showed a comparable value of 2.7 S cm−1 at room temperature. It should be noted that all values are two to three orders of magnitude greater than those for films of previously reported star-shaped TTF oligomers 1–3 (2.5–6.9 × 10−3 S cm−1).3d In stark contrast, a spin-coated film of mono-substituted TTF 7,11 which has an amorphous structure, showed a much lower conductivity of 3.1 × 10−2 S cm−1 under the same conditions. These results clearly support the hypothesis that the high conductivity in the film state is attributable to the sterically congested TTF arrangement, probably making the overall electrical conduction easier as a result of multi-dimensional π–π stacking between TTF units. Furthermore, because it is one of the most characteristic features of conductive molecular materials, the repeatability of the doping and undoping processes was also studied by monitoring the conductivity of a 140 nm thick film (Fig. 4c). The conductivities after doping were of moderate values in the range of 0.95–2.7 S cm−1 for up to six cycles, and the off conductivities after undoping were less than 4.2 × 10−3 S cm−1.
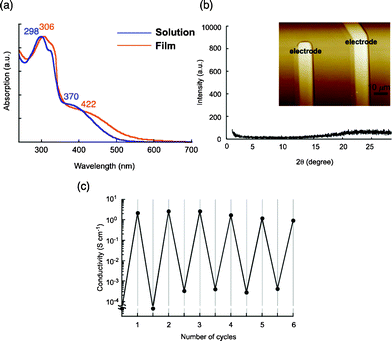 | ||
| Fig. 4 (a) Absorption spectra of the spin-coated amorphous film and the solution of 4b in a mixture of CH2Cl2 and CH3CN (2/1, v/v)), (b) XRD profile and AFM image of the 4b film on the electrode employed for the conductivity measurements, (c) repeated doping cycles of 4b film with monitoring electrical conductivities. | ||
In conclusion, we successfully synthesized sterically congested pyrrole-fused TTF decamer 4via ten SNAr reactions. The preliminary synthetic studies of 4 and reference decapyrrolobiphenyl 5 suggested the importance of the kinetic control to complete the introduction of pyrrole-fused TTF 6. In spite of the D2 symmetric crystal structure of 5 with slipped π–π stacking between the pyrrole moieties, no significant intramolecular interactions between neutral, oxidized, and neutral/oxidized TTFs were observed for 4b. Thus, spatial accumulation of redox-active TTF units was achieved in the decasubstituted TTF oligomer. The spin-coated film of 4b was amorphous, and the conductivity after iodine doping was, to the best of our knowledge, the highest among those of the previously reported TTF-containing amorphous films.12 The three-dimensionally well-defined structure of 4 would effectively involve the conductive mechanism. These results will provide new and valuable information on the design of molecular conductors to develop less-conjugated and easily processable highly conductive amorphous molecular materials.
This research was supported by Grants-in-Aid for Science and Research (No. 23750045) from MEXT, Japan, and research grants from the Noguchi Institute and the Asahi Glass Foundation.
References
- (a) For selected reviews: M. R. Bryce, J. Mater. Chem., 1995, 5, 1481 Search PubMed; (b) M. B. Nielsen, C. Lomholt and J. Becher, Chem. Soc. Rev., 2000, 29, 153 RSC; (c) J. L. Segura and N. Martín, Angew. Chem., Int. Ed., 2001, 40, 1372 CrossRef CAS; (d) D. Canevet, M. Sallé, G. Zhang, D. Zhang and D. Zhu, Chem. Commun., 2009, 2245 RSC; (e) M. Iyoda, T. Nishinaga and M. Takase, Top. Heterocycl. Chem., Springer, 2009, 18, 103 Search PubMed.
- (a) T. Kitamura, S. Nakaso, N. Mizishita, Y. Tochigi, T. Shimomura, M. Moriyama, K. Ito and T. Kato, J. Am. Chem. Soc., 2005, 127, 14769 CrossRef CAS; (b) T. Akutagawa, K. Kakiuchi, T. Hasegawa, S. Noro, T. Nakamura, H. Hasegawa, S. Mashiko and J. Becher, Angew. Chem., Int. Ed., 2005, 44, 7283 CrossRef CAS; (c) J. Puigmartí-Luis, Á. P. del Pino, E. Laukhina, J. Esquena, V. Laukhin, C. Rovira, J. Vidal-Gancedo, A. G. Kanaras, R. J. Nichols, M. Brust and D. B. Amabilino, Angew. Chem., Int. Ed., 2008, 47, 1861 CrossRef.
- (a) Y. Kobayashi, M. Hasegawa, H. Enozawa and M. Iyoda, Chem. Lett., 2007, 36, 720 CrossRef CAS; (b) M. Hasegawa, H. Enozawa, Y. Kawabata and M. Iyoda, J. Am. Chem. Soc., 2007, 129, 3072 CrossRef CAS; (c) S. Ahn, Y. Kim, S. Beak, S. Ishimoto, H. Enozawa, E. Isomura, M. Hasegawa, M. Iyoda and Y. Park, J. Mater. Chem., 2010, 20, 10817 RSC; (d) M. Takase, N. Yoshida, T. Nishinaga and M. Iyoda, Org. Lett., 2011, 13, 3896 CrossRef CAS.
- (a) Y. Shirota, J. Mater. Chem., 2000, 10, 1 RSC; (b) P. Strohriegl and J. V. Grazulevicius, Adv. Mater., 2002, 14, 1439 CrossRef CAS; (c) T. P. I. Saragi, T. Spehr, A. Siebert, T. Fuhrman-Lieker and J. Salbeck, Chem. Rev., 2007, 107, 1011 CrossRef CAS; (d) Y. Shirota and H. Kageyama, Chem. Rev., 2007, 107, 953 CrossRef CAS.
- (a) H. A. M. Biemans, C. Zhang, P. Smith, H. Kooijman, W. J. J. Smeets, A. L. Spek and E. W. Meijer, J. Org. Chem., 1996, 61, 9012 CrossRef CAS; (b) M. Takase, V. Enkelmann, D. Sebastiani, M. Baumgarten and K. Müllen, Angew. Chem., Int. Ed., 2007, 46, 5524 CrossRef CAS; (c) T. Dutta, K. B. Woody and M. D. Watson, J. Am. Chem. Soc., 2008, 130, 452 CrossRef CAS.
- Crystal data for 5 (from CH2Cl2/hexane): C52H40N10, Mw = 804.94, orthorhombic, space group Pbca (No. 61), a = 14.853(3), b = 22.747(3), c = 25.640(4), α = β = γ = 90.00°, V = 8662.76 Å3, Z = 8, Dc = 1.234 g cm−3, T = 293(2) K, λ(Mo Kα) = 0.71073 Å, R1 = 0.0431, wR2 = 0.1430, GOF = 0.998 (I > 2σ(I)). CCDC 850832†.
- L. Tong, H. Lau, D. M. Ho and R. A. Pascal Jr., J. Am. Chem. Soc., 1998, 120, 6000 CrossRef CAS.
- (a) J. O. Jeppesen, K. Takimiya, F. Jensen and J. Becher, Org. Lett., 1999, 8, 1291 CrossRef; (b) J. O. Jeppesen, K. Takimiya, F. Jensen, T. Brimert, K. Nielsen, N. Thorup and J. Becher, J. Org. Chem., 2000, 65, 5794 CrossRef.
- The different reactivities with respect to the SNAr reaction with the parent pyrrole and 6 would be the result of the bulkiness of the substituents. See ESI†.
- Although the crystal structure of 5 indicates the slipped-stacking geometries of the adjacent pyrroles, there is no possible stacking between TTF units and little interactions in their HOMOs.
- (a) B. Yin, Y. Yang, Z. Cong and K. Imafuku, Heterocycles, 2004, 63, 1577 CrossRef CAS; (b) H. Li and C. Lambert, Chem.–Eur. J., 2006, 12, 1144 CrossRef CAS; (c) J. Li, G. Zhang, D. Zhang, R. Zheng, Q. Shi and D. Zhu, J. Org. Chem., 2010, 75, 5330 CrossRef CAS.
- (a) For the examples of conductive non-crystalline materials based on TTF: S. Inagi, K. Naka and Y. Chujo, J. Mater. Chem., 2007, 17, 4122 Search PubMed , and references therein; (b) Y. Hou, X. Wan, M. Yang, Y. Ma, Y. Huang and Y. Chen, Macromol. Rapid Commun., 2008, 29, 689 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedures and the characterization data, DFT calculation results (RB3LYP/6-31G(d)) for 4a, and decafluorobiphenyl. CCDC reference number 850832. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c2ra00035k |
| This journal is © The Royal Society of Chemistry 2012 |