Clicking dendritic peptides onto single walled carbon nanotubes†
Eli
Moore
a,
Peng-Yuan
Wang
ab,
Andrew P.
Vogt
ac,
Christopher T.
Gibson
a,
Vattekat
Haridas
d and
Nicolas H.
Voelcker
*a
aFlinders University, School of Chemical and Physical Sciences, Sturt Road, Bedford Park, Adelaide, SA 5042, Australia. E-mail: nico.voelcker@flinders.edu.au; Tel: +61 882015338; Fax: +61 88201 2905
bDepartment of Chemical Engineering, National Taiwan University, No. 1, Roosevelt Road, Sec. 4, Taipei, 106, Taiwan
cPreparative Macromolecular Chemistry, Institut für Technische Chemie und Polymerchemie, Karlsruhe Institute of Technology (KIT), Engesserstr. 18, 76128, Karlsruhe, Germany
dDepartment of Chemistry, Indian Institute of Technology, Hauz-Khas, New Delhi 110016, India
First published on 19th December 2011
Abstract
Here, we demonstrate a straightforward click-chemistry-based approach for the functionalisation of single walled carbon nanotubes with oligo-lysine dendrons. Azide-functionalised nanotubes were reacted with alkyne-focal dendrons using the 1,3 dipolar Cu-catalysed azide alkyne cycloaddition reaction. Peptide dendron functionalised nanotubes showed significantly increased biocompatibility in rat mesenchymal stem cell culture.
Theoretical and practical applications for carbon nanotubes (CNTs) in the biomedical field have soared over the past few years. Targeted and slow release drug delivery and bioimaging are just a few applications under particular scrutiny.1 Unfortunately, due to the physical and chemical nature of CNTs, it is often difficult to form a suitable biointerface allowing for the safe introduction of CNTs into a biological environment. Two major hurdles to overcome in this regard are hydrophobicity and toxicity of the tubes.2 A variety of both covalent and noncovalent methods have been developed for the functionalisation of CNTs to help overcome the problem of solubility in water.3 In some cases, functionalisation of CNTs has also positively impacted on the biocompatibility of CNTs.4 However, the currently available methods have drawbacks. Noncovalent approaches suffer from poor stability whilst covalent functionalisation of CNTs often involves cumbersome synthetic procedures.
Click chemistry has been readily embraced from the macro- to the nanoscale as a means to functionalise materials due to the efficient, quantitative nature of the reactions resurrected by Sharpless et al. in 2001.5 In particular, CNTs have been functionalised with a range of chemical species via the 1,3-dipolar Cu-catalysed azide alkyne cycloaddition (CuAAC) click reaction.6CuAAC is characterised by mild reaction conditions, fast reaction times and high yields.
Here, we demonstrate a novel approach for functionalising CNTs with dendritic oligo-lysine (K3) (Scheme 1).7 Dendritic peptides combine the advantages of dendrimers in terms of displaying multiple peripheral functional groups giving rise to multivalencies and a tunable size depending on the dendritic generation with the inherent biocompatibility of peptides. In particular, we demonstrate that click-functionalisation with alkyne-focal dendritic peptide moieties significantly improves the biocompatibility of the CNTs in adult stem cell culture.
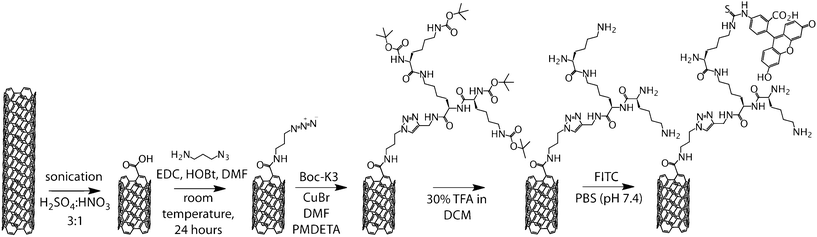 | ||
| Scheme 1 Synthesis of FITC labelled, K3 functionalised CNTs. | ||
Our synthetic approach first involved a new method of functionalising acid-cut CNTs with an azide-functional linker. The presence of covalently bound azides on the CNTs allows for further functionalisation with a plethora of alkyne-terminated species viaCuAAC.
As prepared single walled carbon nanotubes (AP-SWCNTs) were first carboxylated (COO−-CNTs) by cutting in concentrated acid under sonication, as previously reported.8 This yielded CNTs averaging 250–400 nm in length with 4 wt% carboxylic acid functionalisation (data not shown). 3-Azidopropylamine was reacted with the cut nanotubesviaactivation of the carboxylic acids with N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC), obtaining azide functionalised CNTs (N3-CNTs). At this point, solubility of the CNTs was still very low in aqueous and organic solvents and sonication was used to maintain the material in dispersion.
N3-CNTs were dispersed in N,N-dimethylformamide (DMF) along with excess N-tert-butoxycarbonyl (Boc) protected K3 (Boc-K3). A solution of copper bromide (CuBr) and N,N,N′,N′′,N′′-pentamethyldiethylenetriamine (PMDETA) in DMF was added to the CNT mixture. The reaction was allowed to proceed for 48 h to ensure complete reaction between the bulky constituents. After this time, the Boc-K3-CNT composite was isolated from the catalyst and unreacted Boc-K3. A solution of trifluoroacetic acid (TFA) in dichloromethane (DCM) was then used for deprotection to produce K3-CNTs.
Atomic force microscopy (AFM), Raman spectroscopy and infrared (IR) spectroscopy were used to characterise the Boc-K3-CNT composite. Confirmation of successful azide functionalisation of CNTs came from IR spectroscopy, where a new azide band at 2100 cm−1 appeared for the functionalised nanotubes as shown in Fig. 1a (red trace).9 Additionally, the IR analysis showed C–H stretching vibrations in the range of 2750–3000 cm−1 significantly increased in intensity that were not observed in cut SWCNTs (Fig. 1a).
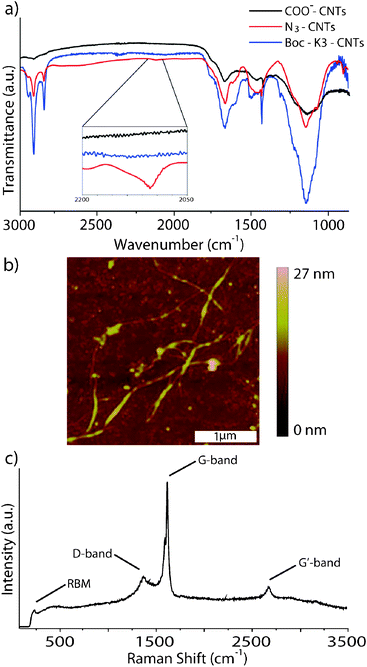 | ||
| Fig. 1 (a) IR spectra comparing COO−-CNTs, N3-CNTs and Boc-K3-CNTs; (b) AFM image of Boc-K3-CNTs; (c) Raman spectra of Boc-K3-CNTs. | ||
IR analysis of Boc-K3-CNTs showed an additional increase of intensity in the C–H bands, which is attributed to the increased number of aliphatic carbons from the Boc-K3. The disappearance of the azide band at 2100 cm−1 strongly suggests that the azide was consumed throughout the click reaction. A drastic change in solubility was seen in the K3-CNT composite, after removal of the Boc groups. The K3-CNTs were no longer soluble in DCM, even after periods of sonication. The composite floated on the surface of the DCM as rafts. However, the K3-CNTs were found to be very soluble in ethanol and water with a short period of sonication to help disperse the composite.
AFM height images of the Boc-K3-CNTs were acquired using a tapping mode in air. No discernible difference was seen between functionalised and non-functionalised nanotubes. However, due to the relatively low molecular weight of the peptide and the fact that the majority of the functionalisation occurs at the ends of the nanotubes, this is to be expected. Woo et al. used AFM to image CNTs after noncovalent functionalisation with dendrons.10 The authors did not observe any distinguishable change in the diameter of their nanotubes after functionalisation, which they presumed to be because of the binding nature leading to a minute change in height and the resolution limit of AFM imaging. Palacin et al. also imaged CNTs functionalised with zinc porphyrin dendrons. They noticed a slight increase in diameter of between 0.5 to 3.5 nm and attributed this change to dendron functionalisation.11
Raman analysis of the Boc-K3-CNTs showed the characteristic bands associated with SWCNTs (Fig. 1c). The G (graphitic) band at 1590 cm−1 and the radial breathing mode (RBM) at 186 cm−1 indicates that the SWCNT structure was retained throughout the functionalisation procedure, which is further supported by the low intensity D (disorder) band at 1350 cm−1 in comparison to the G band and RBM. Comparison of the D band between pristine CNTs and Boc-K3-CNTs showed an increase in the number of nanotube defects (Fig. S1†). This supports an increase in functionality over pristine CNTs.
Biocompatible CNTs have the potential to be used for drug delivery and as scaffolds for stem cell technologies.12 Here, we investigated the toxicity of K3-CNTs in comparison to COO−-CNTs of the same length in a rat mesenchymal stem cell (rMSC) culture. In order to visualise the CNTs, fluorescein isothiocyanate (FITC) was reacted with K3-CNTs to yield FITC-K3-CNTs (Scheme 1). Different doses of both FITC-K3-CNTs and COO−-CNTs in pH 7.4 phosphate buffered saline (PBS) were added to rMSCs cultured on microplates. After 48 h in culture, cell viability (Fig. 2a) and morphology (Fig. 2b) were assessed. Cytotoxicity of the CNTs was determined by means of a dye-uptake assay, which is based on the neutral red absorption of living cells. Cell viability was observed to gradually decrease with increasing concentration of CNTs over a 48 h period at concentrations higher than 20 μg mL−1. In this concentration range, FITC-K3-CNTs were significantly more biocompatible than unmodified COO−-CNTs (Fig. 2a).
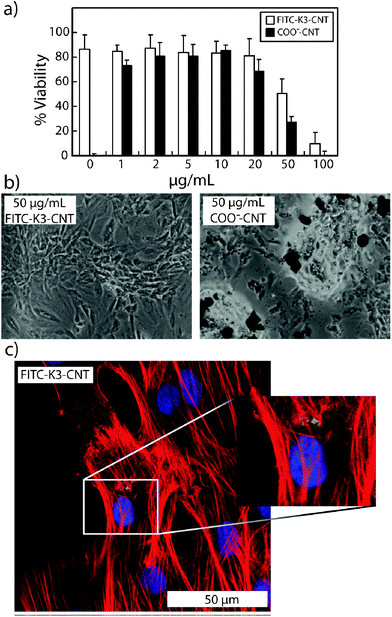 | ||
| Fig. 2 (a) rMSC viability assay after 48 h incubation with various concentrations of COO−-CNTs and FITC-K3-CNTs; (b) brightfield microscopy images of rMSCs after 48 h incubation with 50 μg mL−1 of FITC-K3-CNTs and COO−-CNTs; (c) confocal microscopy images of rMSCs incubated with FITC-K3-CNTs. | ||
The first observations related to reduced aggregation of CNTs in the cell culture medium after functionalisation with peptide. Also, rMSCs were observed to detach from the microplate surface or assumed a spherical morphology after 48 h when the CNT concentration exceeded 100 μg mL−1 and 50 μg mL−1 for FITC-K3-CNTs and COO−-CNTs, respectively (Fig. 2b). For concentrations of 10 μg mL−1 or lower, high cell densities were observed for both FITC-K3-CNTs and COO−-CNTs, suggesting that cells will continue to grow at these low CNT concentrations. However, considerably higher cell densities were observed in the samples exposed to FITC-K3-CNTs (11725 ± 3511 cells cm−2) over the unmodified COO−-CNTs (6221 ± 2353 cells cm−2). Hardly any difference was observed between the control cells not exposed to CNTs, compared to cells incubated with FITC-K3-CNTs (Fig. S2†). The FITC-labelled nanotubes could in some cases be clearly observed coexisting with the MSC while typical morphology was retained after 48 h of incubation (Fig. 2c).
In summary, we have demonstrated the attachment of dendritic oligo-lysine dendrons to azide-functionalised SWCNTs viaCuAAC. This composite nanostructure showed improved water solubility over plain acid cut CNTs and azide functionalised CNTs. Stem cell viability studies indicated increased biocompatibility of the nanocomposites. The development of straightforward covalent surface functionalisation techniques for CNTs leading to improved biocompatibility has the potential to significantly expand their biomedical applications by enabling selective cell targeting and control over cell uptake and biodistribution.
References
- (a) M. Bottini, N. Rosato and N. Bottini, Biomacromolecules, 2011, 12, 3381–3393 Search PubMed; (b) K. Balasubramanian and M. Burghard, Anal. Bioanal. Chem., 2006, 385, 452–468 CrossRef CAS; (c) P. Cherukuri, S. M. Bachilo, S. H. Litovsky and R. B. Weisman, J. Am. Chem. Soc., 2004, 126, 15638–15639 CrossRef CAS.
- Y. Wu, J. S. Hudson, Q. Lu, J. M. Moore, A. S. Mount, A. M. Rao, E. Alexov and P. C. Ke, J. Phys. Chem. B, 2006, 110, 2475–2478 CrossRef CAS.
- (a) B. Gebhardt, Z. Syrgiannis, C. Backes, R. Graupner, F. Hauke and A. Hirsch, J. Am. Chem. Soc., 2011, 133, 7985–7995 Search PubMed; (b) U. Hahn, S. Engmann, C. Oelsner, C. Ehli, D. M. Guldi and T. Torres, J. Am. Chem. Soc., 2010, 132, 6392–6401 CrossRef CAS; (c) D. Tasis, N. Tagmatarchis, A. Bianco and M. Prato, Chem. Rev., 2006, 106, 1105–1136 CrossRef CAS; (d) C. A. Dyke and J. M. Tour, Chem.–Eur. J., 2004, 10, 812–817 CrossRef CAS.
- (a) S. Murugesan, T.-J. Park, H. Yang, S. Mousa and R. J. Linhardt, Langmuir, 2006, 22, 3461–3463 CrossRef CAS; (b) C. M. Sayes, F. Liang, J. L. Hudson, J. Mendez, W. Guo, J. M. Beach, V. C. Moore, C. D. Doyle, J. L. West, W. E. Billups, K. D. Ausman and V. L. Colvin, Toxicol. Lett., 2006, 161, 135–142 CrossRef CAS; (c) A. Nimmagadda, K. Thurston, M. U. Nollert and P. S. McFetridge, J. Biomed. Mater. Res., Part A, 2006, 76A, 614–625 CrossRef CAS.
- H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004–2021 CrossRef CAS.
- (a) R.-S. Lee, W.-H. Chen and J.-H. Lin, Polymer, 2011, 52, 2180–2188 Search PubMed; (b) Y. Song, K. Qu, C. Xu, J. Ren and X. Qu, Chem. Commun., 2010, 46, 6572–6574 RSC; (c) S. Rana and J. W. Cho, Nanoscale, 2010, 2, 2550–2556 RSC; (d) Y. Zhang, H. He, C. Gao and J. Wu, Langmuir, 2009, 25, 5814–5824 CrossRef CAS; (e) S. Rana, I. Kumar, H. J. Yoo and J. W. Cho, J. Nanosci. Nanotechnol., 2009, 9, 3261–3263 CrossRef CAS; (f) I. Kumar, S. Rana, C. V. Rode and J. W. Cho, J. Nanosci. Nanotechnol., 2008, 8, 3351–3356 CrossRef CAS; (g) P. Wu, X. Chen, N. Hu, U. C. Tam, O. Blixt, A. Zettl and C. R. Bertozzi, Angew. Chem., Int. Ed., 2008, 47, 5022–5025 CrossRef CAS; (h) Z. Guo, L. Liang, J.-J. Liang, Y.-F. Ma, X.-Y. Yang, D.-M. Ren, Y.-S. Chen and J.-Y. Zheng, J. Nanopart. Res., 2007, 10, 1077–1083.
- (a) V. Haridas, Y. K. Sharma, R. Creasey, S. Sahu, C. T. Gibson and N. H. Voelcker, New J. Chem., 2011, 35, 303 RSC; (b) V. Haridas, Y. K. Sharma and S. Naik, Eur. J. Org. Chem., 2009, 1570–1577 CrossRef CAS.
- M. W. Marshall, S. Popa-Nita and J. G. Shapter, Carbon, 2006, 44, 1137–1141 CrossRef CAS.
- E. Lieber, C. N. R. Rao, T. S. Chao and C. W. W. Hoffman, Anal. Chem., 1957, 29, 916–918 CrossRef CAS.
- S. Woo, Y. Lee, V. Sunkara, R. K. Cheedarala, H. S. Shin, H. C. Choi and J. W. Park, Langmuir, 2007, 23, 11373–11376 CrossRef CAS.
- (a) T. Palacin, H. L. Khanh, B. Jousselme, P. Jegou, A. Filoramo, C. Ehli, D. M. Guldi and S. Campidelli, J. Am. Chem. Soc., 2009, 131, 15394–15402 CrossRef CAS; (b) I. R. Shapiro, S. D. Solares, M. J. Esplandiu, L. A. Wade, W. A. Goddard and C. P. Collier, J. Phys. Chem. B, 2004, 108, 13613–13618 Search PubMed.
- K. T. Constantopoulos, C. J. Shearer, A. V. Ellis, N. H. Voelcker and J. G. Shapter, Adv. Mater., 2010, 22, 557–571 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures. See DOI: 10.1039/c2ra00791f |
| This journal is © The Royal Society of Chemistry 2012 |
