Grafting submicron titania particles with gold nanoparticles using droplet microfluidics†
Josias B.
Wacker
,
Virendra K.
Parashar
and
Martin A.M.
Gijs
*
Laboratory of Microsystems, Ecole Polytechnique Fédérale de Lausanne (EPFL), CH-1015, Lausanne, Switzerland. E-mail: martin.gijs@epfl.ch; Fax: +41 (0) 21 693 59 50; Tel: +41 (0) 21 693 77 31
First published on 9th March 2012
Abstract
A microfluidic method to synthesize gold–titania nanocomposites is presented. Porous TiO2 particles are first loaded with a reducing agent and then suspended in water and mixed with chloroauric acid (HAuCl4) in a droplet-by-droplet manner on a microfluidic chip. The procedure allows strongly reducing the amount of reagents and easy control of the reaction.
An important topic in modern chemistry is eco-friendly synthesis and some of the key postulates of the “green chemistry” paradigm are the reduction of reaction by-products and waste, minimizing energy consumption throughout the reaction process, and an avoidance of environmentally hazardous solvents. In recent years, microfluidic reactors have shown their ability to cope with these requirements.1 Not only can the consumption of reagents be dramatically reduced, but the reaction efficiency is also higher in miniaturized systems, due to enhanced heat and mass exchange conditions.2 In parallel, the emergence of nanoscience and nanotechnology has promoted the synthesis of novel materials by enabling control at the molecular level. In this respect, composites made of metal oxides and metal nanoparticles play an exemplary role, since the physical and chemical properties of these nanomaterials are distinct from the characteristics of the bulk material. In particular, metals that are inert in the bulk can become catalytically active in nanoparticle forms.3
In terms of catalytic activity, one of the most promising nanocomposites are TiO2 particles that are grafted with Au nanoparticles. Notably, this compound has shown its potential in environmentally relevant tasks, like the degradation of organic pollutants in water4 or the oxidation of CO.5 Besides conventional synthesis and assembly routes (reviewed by Primo et al.6), like the adsorption of preformed Au nanoparticles onto TiO2 surfaces or the deposition–precipitation of nanocomposites from liquid solutions, better controlled and more ecological methods recently emerged, such as the impregnation of a solid substrate with a microemulsion containing a metal salt or providing the metal precursors in a supercritical fluid.7 However, many of these catalyst production methods require bulky, energy-intensive equipment that often consume large amounts of resources and produce much more catalyst than necessary in a typical research-scale experiment. This mismatch in scale between catalyst synthesis and catalyst utilization is most acute when tiny amounts of noble metal nanocatalysts are used in a microfluidic environment.8,9 In an effort to synthesize small amounts of catalyst and limit reagent consumption during catalyst production, the simple volumetric reduction of a conventional reaction vessel has as limitation that small quantities (some μg) of catalyst are difficult to handle in a conventional, beaker-based environment. Furthermore, adjusting the reaction parameters can be cumbersome, since the presented techniques are batch processes that can only be optimized via complex analytical feed-back loops.
The most evident approach to bridge the size-gap between the synthesis and microfluidic application of Au–TiO2 catalysts, and to control the on-line reaction, is to integrate the synthesis process in a microfluidic device.10 To precisely control the amount of reagents involved in the synthesis of the catalysts, an interesting option is to confine them within micro-droplets in which mixing is achieved during transport through the microfluidic channels.11
Here, we propose a droplet-based microfluidic method for the novel synthesis of Au–TiO2 nanocomposites. Porous TiO2 particles (see transmission electron microscopy (TEM) image in Fig. 1) were synthesized as described in the ESI.† The presence of pores was further confirmed via a BET analysis which yielded a total surface of 55.2 m2 g−1, ∼10× bigger than expected for nanoparticles without pores. After washing and drying at 60 °C overnight, the TiO2 particles were infiltrated with 4-aminophenylacetic acid (APA) in a high-pressure vessel at 80 °C. APA incorporated into the TiO2 particles via bulk synthesis has proven to be an efficient reducing agent for the conversion of HAuCl4 to elemental gold.12 As shown in Fig. 2, in a microfluidic chip, made in polydimethyl siloxane (PDMS) with standard soft lithography techniques,13 we generated aqueous droplets of HAuCl4 in oleic acid (OA) and transported them in a microfluidic channel, alternated with droplets containing porous APA-loaded TiO2 particles (∼250 nm in diameter). The device works like a double T-junction droplet generator.14 The wall in the middle of the central channel after merging of the three fluid streams (shown on the right of the photograph in Fig. 2) is designed to avoid, at low relative flow rates (qOA/qreagents), the head-to-head merging of the two streams containing the reagents (APA-infiltrated TiO2 colloid and HAuCl4), which would lead to deposition of the reaction product on the channel walls. As shown in the diagram in Fig. 3(a), we found droplet merging only at low relative flow rates, due to the larger size of and smaller spacing between droplets in this flow regime. Fig. 3(b) and Fig. 3(c) are time-lapse micrographs taken with a high-speed camera and illustrate the merging of the droplets. The dark droplets in these photographs are dyed with ink and the bright droplets are composed of uncolored water. Droplets mostly merged in a lateral manner, as shown in the image sequence in Fig. 3(b). Lateral droplet merging happens when two droplets merge before entering the zig-zag constriction (middle of photograph). The evolution of the color intensity along the short axis of a laterally merged droplet is shown in the ESI.† After 2.6 s, the difference in color intensity between the left and the right side of the droplet is reduced to ∼10%, and after 5.9 s, mixing is complete. This period is well below the total residence time of a droplet on the chip (∼9.4 s). To force coalescence between droplets that did not merge laterally, we introduced two channel constrictions, by which these droplets coalesce in an apical manner, see Fig. 3(c). The mechanism of droplet coalescence in a constriction is discussed in ref. 14 and 15. In addition to facilitating droplet merging, the constriction also assures mixing inside the droplets: as shown in the image sequence in Fig. 3(c), the content of the lagging droplet quickly reaches the leading meniscus of the newly formed (merged) droplet (after ∼33 ms) and is distributed on both sides of the long axis of this droplet. The two liquids are further mixed due to the vortices that build up in droplets being transported in microchannels, as described in ref. 16. In a typical experiment, the flow rates of the two droplet-forming solutions were 5 nL s−1, while OA was delivered at 5–10 nL s−1. To analyze the reaction products, we captured the droplets on silicon dies or copper grids and instantaneously stopped the reaction by evaporation of the reagents at 120 °C.
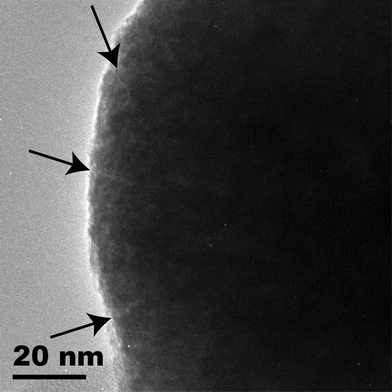 | ||
| Fig. 1 TEM micrograph of a TiO2 particle, with arrows indicating nanopores. | ||
 | ||
| Fig. 2 Experimental setup for the microfluidic, droplet-based synthesis of Au nanoparticle-grafted TiO2 particles. Aqueous HAuCl4 and TiO2 particles functionalized with APA are separately injected into a microfluidic chip, where the two streams form droplets in oleic acid, which are subsequently merged in a sequence of channel constrictions and expansions. The reaction product can be captured on a silicon die for subsequent analysis in a transmission electron microscope. | ||
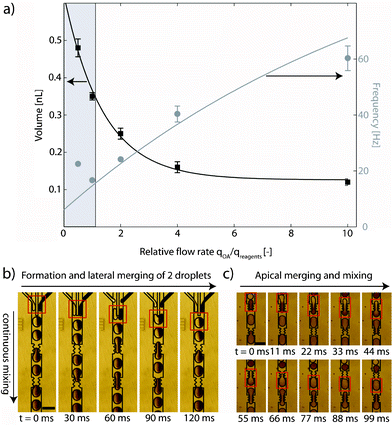 | ||
| Fig. 3 (a) Droplet volume and generation frequency. The grey area represents the window of operation of the microfluidic device, where one-to-one merging of HAuCl4 and TiO2 suspension droplets is assured. (b) Lateral merging of droplets. (c) Apical merging of droplets. The scale bars are 200 μm. | ||
In an attempt to reduce the consumption of the relatively toxic gold nanoparticle precursor HAuCl4, we followed two strategies: first, by using sub-nL sized droplets as reaction vessels, the overall consumption of HAuCl4 is drastically lowered compared to classical syntheses of Au–TiO2 nanocomposites prepared in stirring reactors, where typical synthesis volumes are in the range of mL to dL.17,18 Second, we optimized the use of HAuCl4 by a reduction reaction that is limited to the TiO2 particle surface, the only place where the APA is present. Fig. 4(a) shows TEM micrographs of TiO2 particles grafted with Au nanoparticles synthesized at different concentrations of APA and HAuCl4. The highest amount of Au nanoparticles was found for 0.1 M APA and 1 mM HAuCl4 (4–5% w/w, as estimated from the TEM images). We noted Au nanoparticle formation at concentrations of HAuCl4 as low as 10 μM. Furthermore, the TEM micrographs revealed that the Au nanoparticles are spherical in shape and well distributed over the whole surface of the TiO2 particles.
 | ||
| Fig. 4 (a) Micrographs and (b) corresponding size histograms of Au nanoparticles on TiO2 particles for different concentrations of HAuCl4 and APA used on-chip. | ||
As reported by others, the catalytic activity of Au nanoparticles is tightly linked to their small size and rapidly decreases for particles bigger than ∼10 nm.19,20 The size histograms of Fig. 4(b) show that our droplet-based synthesis method fulfils this prerequisite. We ascribe the small size of the Au nanoparticles, together with an acceptable size variation, to the localized reduction of HAuCl4 on the APA-infiltrated pores of TiO2 particles and to the short and well-controlled reaction time on chip.
In this article, we presented a microfluidic droplet-based method to graft TiO2 particles with Au nanoparticles, an important step in efficient production of nanocomposites. We showed that the use of microdroplets, together with a TiO2 surface located reducing agent, allowed a dramatic lowering of the quantities of hazardous reaction volumes compared to conventional batch-type syntheses, which makes our approach an attractive alternative for the synthesis of small quantities of catalysts, as used e.g. in microreactors. At the same time, our approach offers the possibility for upscaling nanocomposite synthesis via parallelization of the reaction using multiple microfluidic devices. We expect that the proposed synthesis scheme will be further extended to other types of supported metal nanoparticles.
References
- S. J. Haswell and P. Watts, Green Chem., 2003, 5, 240–249 RSC.
- A. J. deMello, Nature, 2006, 442, 394–402 CrossRef CAS.
- M. Haruta, N. Yamada, T. Kobayashi and S. Iijima, J. Catal., 1989, 115, 301–309 CrossRef CAS.
- A. Orlov, M. S. Chan, D. A. Jefferson, D. Zhou, R. J. Lynch and R. M. Lambert, Environ. Technol., 2006, 27, 747–752 CrossRef CAS.
- M. Haruta, S. Tsubota, T. Kobayashi, H. Kageyama, M. J. Genet and B. Delmon, J. Catal., 1993, 144, 175–192 CrossRef CAS.
- A. Primo, A. Corma and H. Garcia, Phys. Chem. Chem. Phys., 2011, 13, 886–910 RSC.
- J. M. Campelo, D. Luna, R. Luque, J. M. Marinas and A. A. Romero, ChemSusChem, 2009, 2, 18–45 CrossRef CAS.
- J. R. Adleman, D. A. Boyd, D. G. Goodwin and D. Psaltis, Nano Lett., 2009, 9, 4417–4423 CrossRef CAS.
- N. J. Divins, E. Lopez, M. Roig, T. Trifonov, A. Rodriguez, F. Gonzalez de Rivera, L. I. Rodriguez, M. Seco, O. Rossell and J. Llorca, Chem. Eng. J., 2011, 167, 597–602 CrossRef CAS.
- B. P. Mason, K. E. Price, J. L. Steinbacher, A. R. Bogdan and D. T. McQuade, Chem. Rev., 2007, 107, 2300–2318 CrossRef CAS.
- H. Song, D. L. Chen and R. F. Ismagilov, Angew. Chem., Int. Ed., 2006, 45, 7336–7356 CrossRef CAS.
- H. Zhang, Y. Xie, Z. Liu, R. Tao, Z. Sun, K. Ding and G. An, J. Colloid Interface Sci., 2009, 338, 468–473 CrossRef CAS.
- Y. Xia and G. M. Whitesides, Angew. Chem., Int. Ed., 1998, 37, 550–575 CrossRef CAS.
- B. Zheng, J. D. Tice and R. F. Ismagilov, Anal. Chem., 2004, 76, 4977–4982 CrossRef CAS.
- N. Bremond, A. R. Thiam and J. Bibette, Phys. Rev. Lett., 2008, 100, 024501 CrossRef.
- H. Kinoshita, S. Kaneda, T. Fujii and M. Oshima, Lab Chip, 2007, 7, 338–346 RSC.
- R. Zanella, S. Giorgio, C. R. Henry and C. Louis, J. Phys. Chem. B, 2002, 106, 7634–7642 CrossRef CAS.
- C. Subramaniam, R. T. Tom and T. Pradeep, J. Nanopart. Res., 2005, 7, 209–217 CrossRef CAS.
- A. Corma and H. Garcia, Chem. Soc. Rev., 2008, 37, 2096–2126 RSC.
- M. C. Daniel and D. Astruc, Chem. Rev., 2004, 104, 293–346 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: Detailed experimental procedure, mixing inside droplets, microscopic inspection, EDX and FTIR spectra. See DOI: 10.1039/c2ra01048h/ |
| This journal is © The Royal Society of Chemistry 2012 |
