Lipid storage compounds in raw activated sludge microorganisms for biofuels and oleochemicals production†
Emmanuel D.
Revellame
a,
Rafael
Hernandez
*a,
William
French
a,
William E.
Holmes
a,
Tracy J.
Benson
b,
Patrisha J.
Pham
a,
Allison
Forks
a and
Robert
Callahan II
a
aRenewable Fuels and Chemicals Laboratory, Dave C. Swalm School of Chemical Engineering, Mail Stop 9595, Mississippi State University, Mississippi State MS 39762, U.S.A. E-mail: rhernandez@che.msstate.edu; Fax: 662 325 2482; Tel: 662 325 2480
bCenter for Chemical Energy Engineering, Dan F. Smith Department of Chemical Engineering, P.O. Box 10053, Lamar University, Beaumont, TX 77710, U.S.A. E-mail: tracy.benson@lamar.edu; Fax: 409 880 2197; Tel: 409 880 7536
First published on 11th January 2012
Abstract
Activated sludge contains a microbial population responsible for the biological treatment of wastewater. This microbial population mostly consists of heterothrophic bacteria which utilize the organic content of the wastewater for growth, either as part of their cellular structures or as energy and carbon storage compounds. These compounds are mostly lipidic in nature and are or could be important raw materials for a multitude of applications in biofuel and oleochemical industries. In this study, a municipal activated sludge was analyzed for lipid storage compounds and other compound classes present in significant concentrations. Three extraction techniques, namely; Bligh & Dyer (applied on dried and partially dewatered samples) and accelerated solvent extractions, were initially investigated to identify the one resulting in the highest gravimetric and biodiesel yields. The highest yields were obtained using the Bligh & Dyer of partially dewatered sludge samples and thus, the extracts from this extraction technique were subjected to a series of analytical procedures such as precipitation, solid phase extraction, thin layer chromatography (TLC), gas chromatography with flame ionization detector (GC-FID) and gas chromatography-mass spectrometry (GC-MS) to characterize the major compound classes present. Results indicated that the major compounds in the samples were polyhydroxyalkanoates, wax esters, steryl esters, triacylglycerides, free fatty acids, free sterols and phospholipids. Hydrocarbons, diacylglycerides and monoacylglycerides were also detected. These compounds are either synthesized by microorganisms or from exogenous contributions. Regardless of the source of these compounds, their persistent presence in activated sludge offers another feedstock for a wide range of applications.
Introduction
The drive for renewable transportation fuels has lead to the utilization of lipid feedstocks as renewable fuel resources. Currently, researchers in academe and petroleum industries alike, have begun to develop processes to convert lipid feedstocks into fuel-like mixtures.1–5 These renewable fuels include biodiesel, renewable diesel, ethanol and biobutanol. For example, biodiesel is a mixture of fatty acid alkyl esters produced from the reaction of lipids and an alcohol in the presence of a catalyst (i.e. acid, base, enzyme).6,7 Depending on the nature of the lipid feedstock and the applied catalyst, the reaction is called either transesterification or esterification.8 In contrast, renewable diesel is comprised of organic compounds similar to those contained in petroleum diesel.9 Renewable diesel is produced by hydrotreatment processes, where oxygenated compounds, such as lipids, are deoxygenated by a series of cracking and hydrogenation reactions to form mostly linear aliphatic hydrocarbons within the range of petroleum-derived diesel fuel compounds. Hydrotreating/hydrocracking processes generally use mixed sulfides of CoMo, NiMo or NiW supported on γ–Al2O3 as catalysts.10,11Lipid feedstocks could come from soybeans, canola, corn, castor, jatropha and microbial sources.6 Presently, most of the lipid feedstocks that are being used for fuel production are also used in the edible oil industry. To provide an alternative feedstock that addresses the biofuel versus food issue, this study focuses on lipids from microbial sources specifically, activated sludge.
Activated sludge is a product of a biological treatment associated with most municipal wastewater treatment plants (MWWTPs).12 It contains a mixed microbial community, most of which are heterotrophic bacteria, which utilize the biochemical oxygen demand (BOD) content of the wastewater for growth.6 Some of the lipidic components of bacteria include fatty acids, phospholipids, glycerides and wax esters.13–15 Depending on the type of wastewater and treatment process configurations, activated sludge may also contain other organic compounds aside from lipids, such as alkanes, polycyclic aromatic hydrocarbons, polychlorinated biphenyl, linear alkyl benzenes, polyhydroxyalkanoates, steroids and pentacyclic triterpanes.16–18
Compounds such as triacylglycerides, wax esters and polyhydroxyalkanoates are known to be carbon or energy storage materials which are produced by bacteria in response to stressful conditions (e.g. nitrogen, phosphorus or oxygen limitation).19 These compounds are important raw materials or intermediates for a variety of applications. For example, wax esters and fatty alcohols with C12 and higher are important basic materials for the production of fragrances, detergents, toothpastes, shampoos and lubricants.15,20,21 Depending on the bacterial strain or mixture of bacterial strains comprising the activated sludge, the relative amount of compounds that may be obtained varies.
Aside from transportation fuels, the world is also very much dependent on other products (i.e. crayons, eyeglasses, tires, heart valves, etc.) derived from petroleum oil.22 Thus, in the search for an alternative to petroleum oil, the importance of these other products must also be considered. In this context, activated sludge, as a feedstock for fuels and the production of oleochemicals, might have an advantage compared to other conventional feedstocks because of the variety of compounds that activated sludge can potentially offer.
Previous studies on activated sludges showed relatively low yields (3–6% weight of dry solid) of biodiesel. Raw activated sludge, applied just for biodiesel production is not economically competitive at current petroleum prices.6,13,23,24 On the study conducted by Revellame et al.13 a gravimetric yield as high as 15% (weight) was obtained on the in situ transesterification of partially dewatered activated sludge because of the extraction of other compounds aside from biodiesel. These compounds could be any or all of the compound classes mentioned earlier. Their sensitivity analysis indicated that a yield of at least 10% (weight) biodiesel yield must be attained for activated sludge to be economically competitive at current petroleum prices. If the other unidentified compounds (the difference between 3–6% and 15%) can be converted to biofuel, or other useful chemical reaction precursors, the economics of this feedstock may improve dramatically. However, identification and quantitation of these compounds are necessary for their strategic separation and utilization. Once identified, reaction pathways to fuel or oleochemical conversion can then be established.
This work seeks to provide characterization of raw activated sludge extracts to support the evaluation of activated sludge as a feedstock for renewable fuels and oleochemical production. Different extraction techniques have been employed to maximize extraction yields. A series of analytical tools have been utilized to characterize the extractables from activated sludge. Furthermore, potential products that can be obtained from different compound classes present in the sludge were identified based on available literature.
Materials and methods
Sample collection and preparation
All activated sludge samples used in this study were collected from Hilliard Fletcher municipal wastewater treatment plant in Tuscaloosa, AL, USA.25 The solids were concentrated by gravity-settling overnight, followed by either centrifugation using IEC Centra GP6 centrifuge (Thermo Electron Corp., Milford, MA, USA) operated at 3000 rpm for 20 min or vacuum filtration using a P8-creped cellulose fiber filter (Fisher Scientific, Pittsburgh, PA, USA). A portion of the concentrated sludge was frozen in a ColdTech freezer (Jimex Corp., Hayward, CA, USA) at −18 °C and was freeze-dried for 5 days either in a Freezone 2.5 or Freezone 6 Bulk Tray freeze dry system (Lanconco, Kansas City, MO, USA). The solid content of the concentrated sludge and freeze-dried sludge was determined using Ohaus MB45 infrared heater (Ohaus, Pine Brook, NJ, USA). The centrifugation/filtration of the sludge gave a concentrated sludge containing 8–16% weight solids. Freeze-drying of the concentrated sludge resulted in sludge with an average solids content of 95.74% weight.Extractions
Quantitation of FAMEs was conducted using an Agilent 6890N gas chromatograph equipped with flame ionization detector (GC-FID) (Agilent, Santa Clara, CA, USA) following the procedure by Revellame et al.6 (see Table S2 for details). Instrument calibration was conducted using a 14-component FAMEs standard mixture containing saturated, mono-unsaturated and poly-unsaturated C8–C24 fatty acids (Sigma-Aldrich, St. Louis, MO, USA).
Analysis of storage compounds
The isolated PHAs dissolved in chloroform were de-polymerized/derivatized by addition of 1 mL of 2.0 N HCl in methanol (Sigma-Aldrich, St. Louis, MO, USA). The mixture was refluxed using an Instatherm® heating block system (Ace Glass Inc., Vineland, NJ, USA) for 16 h at 80 °C in a screw-capped (PTFE-lined) vial. After the reaction, the mixture was allowed to cool to room temperature after which, 2.5 mL of distilled water containing 5% NaCl and 2% NaHCO3 was added. After addition of 1 mL chloroform, the mixture was vortex-mixed and set aside for phase separation. The organic (chloroform) layer was withdrawn and extraction using 1 mL chloroform was repeated twice more. The organic layers were pooled and dried using a TurboVap LV as in BDE experiments. The solid residue was re-dissolved in chloroform and was analyzed using a Varian 3400 GC equipped with a Saturn 2000 ion-trap mass spectrometer (GC-MS) (Varian Inc., Palo Alto, CA, USA). Electron impact (EI) and chemical ionization (CI) using acetonitrile as CI gas were utilized for peak identification.
Quantitation was conducted using an Agilent 6890N GC-FID as described earlier (see Analysis of extraction yields). The GC-FID and GC-MS were running at the same condition and were equipped with the same column. The calibration of the GC-FID was conducted using methyl 3-hydroxybutyrate and methyl 3-hydroxyvalerate standards (Sigma-Aldrich, St. Louis, MO, USA).
 | ||
| Fig. 1 Sequential elution scheme for the separation of PHA-free activated sludge extract using 1000-mg Extra-clean™ silica solid phase extraction column. HC = Hydrocarbon, SE = Steryl Ester, WE = Wax Ester, TG = Triacylglyceride, FFA = Free Fatty Acid, FFOH = Free Fatty Alcohol, St = Sterol, DG = Diacylglyceride, MG = Monoacylglyceride, PL = Phospholipid. The composition and volumes of solvents A–E are presented in Table 1. | ||
| Solvent components | Composition (by volume) | Vol. (mL) | Compound class eluted | |
|---|---|---|---|---|
| a Elution volumes are highly dependent on sample load and lipid class concentration. | ||||
| A | n-Hexane/Diethyl ether | 94/6 | 3.70 | Hydrocarbons |
| B | n-Hexane/Diethyl ether | 94/6 | 4.50 | Steryl Esters and Wax Esters |
| C | n-Hexane/Diethyl ether | 94/6 | 10.00 | Triacylglycerides |
| D | n-Hexane/Diethyl ether/Acetic acid | 85/15/2 | 15.00 | Free Fatty Acids, Free Fatty Alcohols, Sterols, Diacylglycerides and Monoacylglycerides |
| E | Methanol | Pure | 5.00 | Phospholipids |
The fractions obtained from the SPE were dried using a TurboVap LV as described in BDE experiments. The dried fractions were re-constituted in 100 μL of chloroform and were subjected to TLC based on the method by Hwang et al..28 Fractions (5–10 μL) were spotted on 20 × 20 cm glass-backed Analtech Uniplates™ pre-coated with 250 μm silica gel-G (Sigma-Aldrich, St. Louis, MO, USA). Sample applications were conducted using Drummond microcaps® disposable pipettes (Fisher Scientific, Pittsburgh, PA, USA). Representative standards (20–30 μg) for each compound class were also spotted on the plates. Plates were developed either in 94/6 (v/v) n-hexane/diethyl ether or 85/15/2 (v/v/v) n-hexane/diethyl ether/acetic acid. Bands were visualized by spraying the plates with a solution of 10% (w/v) cupric sulfate in 8% phosphoric acid aqueous solution. The plates were then allowed to dry for 5 min and the developed bands were charred in an oven at 150 °C for visualization.
The re-constituted fractions (in chloroform) were also analyzed on a HT-GC using a Varian 3600 GC (Varian Inc., Palo Alto, CA, USA) equipped with a flame ionization detector (FID). The GC column was a Rtx®-Biodiesel TG with Rxi® guard column (Restek, Bellefonte, PA, USA). Samples were analyzed using cool-on-column injection with an initial injector temperature of 50 °C and a final temperature of 380 °C, at a ramp rate of 180 °C min−1. The GC oven temperature was programmed at an initial temperature of 50 °C, held for 1 min, ramped to 180 °C at 15 °C min−1, then to 230 °C at 7 °C min−1, and to 370 °C at 20 °C min−1, and finally held for 11.20 min (see Table S3, ESI†).
Fraction 1: hydrocarbons. The hydrocarbon fraction was analyzed on a Varian 3400 GC equipped with a Saturn 2000 ion-trap mass spectrometer. Both EI and CI were used for compound identification. Quantitation of the peaks was conducted using an Agilent 6890N GC-FID equipped with a Restek Stabilwax-DA capillary column. The GC oven was programmed at an initial temperature of 50 °C for 2 min, ramped to 250 °C at 2 °C min−1, and was held at 250 °C for 18 min (see Table S3, ESI†). The calibration of the GC-FID was done using n-octacosane (Sigma-Aldrich, St. Louis, MO, USA) and all responses were calculated based on this compound.
Fraction 2: wax esters and steryl esters. The wax and steryl esters fraction was subjected to methanolysis using a modified procedure by Bernasconi et al..29 The fraction from SPE was dried under N2 using the procedure described in BDE experiments. After addition of 1 mL 14% BF3-methanol solution, the mixture was vortex-mixed and the methanolysis was carried out at 60 °C for 30 min. The mixture was then allowed to cool to room temperature. Products from methanolysis were extracted using 3 × 2 mL of chloroform. The chloroform extracts were pooled and dried using a TurboVap LV as in BDE experiments. The dried extract was dissolved in chloroform and was subjected to TLC as described in SPE experiments to determine if the methanolysis reaction achieved completion. The FAMEs were then separated from sterols and fatty alcohols using another 1000-mg SPE silica column. The methanolysis products, dissolved in minimal volume of chloroform, was loaded into a pre-conditioned column (2 × 5 mL n-hexane). FAMEs were eluted using 17 mL of 94/6 (v/v) n-hexane/diethyl ether solvent mixture and the sterols and fatty alcohols were eluted using 85/15/2 (v/v/v) n-hexane/diethyl ether/acetic acid solvent mixture. These two fractions were subjected to TLC as in SPE experiments to verify the separation of the methanolysis products.
Quantitation of FAMEs was conducted using an Agilent 6890N GC-FID using the procedure described earlier (see Analysis of extraction yields) while the sterols and fatty alcohols were analyzed using an Agilent 6890N GC equipped with a 5975 inert Mass Selective Detector (Agilent, Santa Clara, CA, USA). The column was a Restek Rxi®-1MS. The GC oven was programmed at an initial temperature of 50 °C for 1.50 min, then ramped to 100 °C at 35 °C min−1, then to 310 °C at 20 °C min−1 and was held at 310 °C for 5 min. Calibration of the instrument was accomplished using standards of saturated and monounsaturated primary fatty alcohols (C14–C21), and sterols (coprostanol, cholesterol, campesterol, stigmastanol, stigmasterol and β -sitosterol) (Sigma-Aldrich, St. Louis, MO, USA).
Fractions 3 and 5: triacylglycerides and phospholipids. These two fractions were individually subjected to methanolysis as was done with Fraction 2: wax esters and steryl esters, and quantitation of FAMEs was conducted using an Agilent 6890N GC-FID using the procedure described previously (see Analysis of extraction yields).
Fraction 4: free fatty acids, free fatty alcohols, sterols, diacylglycerides and monoacylglycerides. The free fatty acids, diacylglycerides and monoacylglycerides were converted to FAMEs by methanolysis using 14% BF3-methanol solution followed by SPE to separate the FAMEs from free fatty alcohols and free sterols. The procedure described earlier was employed (see Fraction 2: wax esters and steryl esters).
Results and discussion
The availability of a wide range of compounds that can be obtained from activated sludge is advantageous for its potential use as an alternative to petroleum oil. These compounds are either intermediates or products of microbial degradation of organic and inorganic compounds present in the wastewater. Some microorganisms that are usually involve in the activated sludge process include Xanthomonas, Vibrio, Sphingomonas, Achromobacter, Aerobacter, Alcaligenes, Bacillus, Brevibacterium, Corynebacterium, Comamonas, Flavobacterium, Micrococcus, Pseudomonas, Spirillum, Zooglea, and E. coli.30–32 All activated sludge samples used in this study came from Hilliard Fletcher WWTP in Tuscaloosa, AL USA, which utilizes a conventional aerobic treatment configuration.25 Based on the study conducted by Mondala et al.,33 the activated sludge from this facility contains bacteria in phyla Proteobacteria [α-/β-/γ-/δ-/ε-Proteobacteria (i.e. Rhodobacterales and Xanthomonadales)], Verrucomicrobia (class Verrucomicrobiae), Bacteriodetes (class Flavobacteria and Sphingobacteria), Firmicutes (class Clostridia) and Actinobacteria .Studies indicated that roughly 90% of dry cell weight of cells in activated sludge is organic in nature. The inorganics (remaining 10%) are comprised primarily of metals such as iron, calcium, magnesium and sodium. The organic portion contains 50–55% carbon, 25–30% oxygen, 10–15% nitrogen, 6–10% hydrogen, 1–3% phosphorus and 0.5–1.5% sulfur.34 The ultimate analysis of activated sludge from this MWWTP is presented in Table 2. Results showed that the inorganic content of the activated sludge used in this study is higher than usual (21.32% weight as ash). Nevertheless, the composition of the organic portion is within the established ranges, giving an empirical formula of C6.4H10.1O2.6N. This is very close to the accepted empirical formula of bacterial cell (C5H7O2N).35,36
Extractions
All extraction experiments were conducted using a single batch of sludge. The main purpose of evaluating different extraction procedures is to maximize extract yield from activated sludge, and thus, obtain more complete characterization results. However, since the major products from petroleum oil are fuels, the extraction procedure that maximized the yield of FAMEs/biodiesel was considered more intensively. Focus was given on those extraction procedures, which are known for effectiveness. The BDE procedure was applied on both partially dewatered (centrifuged) and dried sludges. BDE procedure, which is the most well-known method for determination of total lipid content in biological samples, utilizes a mixture of chloroform, methanol and water in a specific ratio as extraction solvent.26,37 The study conducted by Dufreche et al.23 on the ex situ biodiesel production from activated sludge showed that the highest yield could be obtained using a 60/20/20 volume ratio of hexane/methanol/acetone as solvent for ASE. Thus, this extraction procedure was also employed in this study.The two extraction procedures tested might not be economically feasible on an industrial scale. However, as mentioned, the main objective of the study is to maximize extraction yields for a more complete characterization. After identification of compound classes present in the sludge, an extraction protocol that is industrially sound can then be strategized and will be reported elsewhere.38
The results of extraction experiments are shown in Fig. 2. On average, the gravimetric yield of the BDE using partially dewatered sludge was the highest among the three extraction techniques tested. However, it was not significantly different from the yields of the other two extraction procedures. This also applies to the biodiesel yields. Regardless of the extraction technique used, the fatty acid profiles of the biodiesel obtained are similar (Fig. 3). This profile is also similar to those obtained by previous researchers who worked on activated sludge from the same MWWTP.6,13,23,24 The dominant fatty acids present in the sludge ranges from C14–C18, which has been suggested to reflect bacterial contribution.39
 | ||
| Fig. 2 Yield comparison for the extraction of activated sludge. | ||
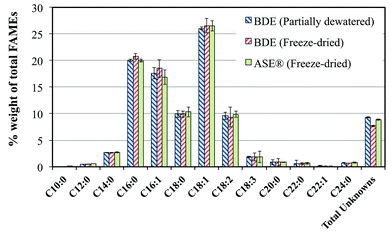 | ||
| Fig. 3 Fatty acid profiles of biodiesel from activated sludge using different extraction techniques. | ||
In selecting the suitable extraction procedure, the one that minimizes the changes or transformations of extractable compounds is preferable. Freezing has been known to trigger microbial cell damages resulting to a considerable breakdown of cellular organization. It can cause alterations in phospholipid composition of the cells due to either peroxidation or phospholipase activity.40 Furthermore, the accelerated solvent extraction was conducted at 100 °C using methanol as one of the extraction solvents. At this condition, significant production of FAMEs was observed (data not shown). Therefore, the extract from the BDE of partially dewatered sludge was used for the remainder of the study.
Three batches of activated sludge were collected from Hilliard Fletcher MWWTP in the months of April, June and October (coded A, J and O, respectively) during the plant's normal operation. Samples were extracted using the BDE procedure described above. The yield of extract ranges 6–16% (weight) based on the total solids. This wide range of extractables represents the inherent variability of activated sludge within a treatment plant. The range obtained corresponds to approximately 1.20–3.50% (weight) FAMEs yield based on dried activated sludge (see Table 3). This range is in agreement with the results obtained by Dufreche et al. on the ex situ biodiesel production from activated sludge obtained from the same MWWTP as this study. However, the range of biodiesel yield obtained is lower than the yields obtained by previous workers using in situ biodiesel production from activated sludge.6,13,23 This could be due to inherent variability of sludge sample with time or due to differences between the processes used. According to Dufreche et al., all saponifiable lipids are in contact with the reagents during the in situ process resulting to a higher biodiesel yield.23 The yields of FAMEs were calculated from the results of the SPE experiments. The FAMEs yield shown in Table 3 is the sum of FAMEs obtained from all SPE fractions.
| A | J | O | |
|---|---|---|---|
| Sludge Collection Date | 7-Apr-2010 | 30-Jun-2010 | 13-Oct-2009 |
| Aeration Basin T/°C | 24.80 | 20.10 | 26.00 |
| Bligh & Dyer extract yield, % weight of dry solid | 9.41 ± 0.21 | 5.88 ± 0.43 | 16.30 ± 1.28 |
| Total FAMEs yield, % weight of extract | 19.42 ± 0.33 | 20.68 ± 0.05 | 21.53 ± 0.32 |
| Total FAMEs yield, % weight of solid | 1.83 ± 0.05 | 1.22 ± 0.09 | 3.51 ± 0.28 |
| PHAs, % weight of extract | 1.95 ± 0.14 | 3.70 ± 0.72 | 2.88 ± 0.12 |
| FRACTION 1 | |||
| Hydrocarbons, ppm (based on weight extract) | 2.14 ± 0.18 | 1.39 ± 0.15 | 0.95 ± 0.20 |
| FRACTION 2 | |||
| Fatty Alcohol (from WEs), % weight of extract | 1.79 ± 0.22 | 5.24 ± 0.38 | 5.55 ± 0.36 |
| Sterols (from SEs), % weight of extract | 1.54 ± 0.09 | 2.83 ± 0.13 | 2.58 ± 0.27 |
| FAMEs yield, % weight of extract | 5.24 | 6.66 | 6.27 |
| FRACTION 3 | |||
| Triacylglycerides, % weight of extract | 2.82 ± 0.09 | 2.01 ± 0.00 | 2.08 ± 0.02 |
| FAMEs yield, % weight of extract | 2.84 | 2.02 | 2.09 |
| FRACTION 4 | |||
| Free Sterols, % weight of extract | 10.75 ± 0.01 | 18.42 ± 0.23 | 12.13 ± 3.55 |
| FAMEs yield, % weight of extract | 11.04 | 11.31 | 12.73 |
| FRACTION 5 | |||
| FAMEs yield, % weight of extract | 0.31 | 0.70 | 0.44 |
PHAs
PHAs are polyesters of hydroxyalkanoic acids and are well-known as biodegradable alternative to petroleum plastics.41,42 PHA is the main storage compound in most bacteria with poly(3-hydroxybutyrate) (PHB) as the most abundant one.43,44 Numerous microorganisms are known to accumulate PHAs. Some microorganisms such as Cupriavidus necator, Rhodopseudomonas palustris, Methylobacterium organophilum and Alcaligenes euthropus (also known as Ralstonia eutropha), require limitation of an essential nutrient (i.e. nitrogen, phosphorus, magnesium, manganese, iron, potassium, sodium and oxygen) with an excess of carbon source. On the other hand, some microorganisms are known to accumulate PHAs during the growth phase. Examples of these microorganisms include Alcaligenes latus and Azotobacter vinelandii. Efforts to increase the yield of PHAs by microbial fermentation include cloning and expression of genes involved in PHA biosynthesis in Escherichia coli. Recombinant E. coli can accumulate PHAs up to 80–90% of cell dry weight. Other microorganisms that can produce PHAs include Protomonas extorquens, Bacillus subtilis, Bacillus thuringiensis, Bacillus megaterium, Rhodococcus ruber, Rhodococcus opacus, Rhodococcus jostii RHAI, Syntrophomonas wolfei, Rhodospirillum rubrum, Rhizobium japonicum, Halobacterium mediterranei, Azotobacter beijerinckii, Zoogloea ramigera, Methylobacterium rhodesianum, Methylocystis pervus, Methylosinus trichosporium, Rhizobium meliloti, Thiocapsa pfennigii, Sphaerotilus natans, Streptomyces lividans, Protomonas aeruginosa, Protomonas mendocina, Pseudomonas flourescens, Pseudomonas testosterone, Pseudomonas denitrificans, Rickettsia prowazekii and Pseudomonas oleovorans.42,45–54 Nowadays, approximately 300 microbial species are known to produce PHAs.55 The properties of microbial PHAs such as molecular weight, polydispersity and hydroxyacid monomer length are highly dependent on the microorganism(s) involved, fermentation conditions and method of isolation.42,46 PHAs in microorganisms, particularly in bacteria, serve as carbon and energy reserve (storage) and/or as sink for redundant reducing power or electrons under stressful conditions.45,46Microorganisms in activated sludges are known to accumulate PHAs ranging from 0.30 to 22.70 mg polymer per gram of sludge.17 Reddy et al. in 2008 isolated PHA producing bacteria from activated sludge obtained from a MWWTP. Out of 480 bacterial isolates that they screened, 21.87% are PHA-accumulators. Futhermore, they identified seven Bacillus species, two Alcaligenes species, two Aeromonas species and one Chromobacterium species as PHA-accumulators.56 In a related study, Law et al.57 isolated Bacillus species from municipal activated sludge. They found that the species is closely similar to Brevibacillus laterosporus and Bacillus megaterium. The study conducted by Jiang et al.58 on PHA production from waste activated sludge showed that γ-Proteobacteria, β-Proteobacteria and α-Proteobacteria were the major PHA-producing microorganisms.
The PHAs content of the three batches analyzed is shown in Table 3 and a representative total ion chromatogram from GC-MS analysis is presented in Fig. 4. The activated sludge extracts contains about 2–4% PHAs based on the weight of extract, which corresponds to ∼1.83–4.69 mg PHAs per gram of dried solids. The isolated PHAs have purities of 80–90% by weight. Furthermore, only two hydroxyacid monomers were detected which are hydroxybutyric (HB) and hydroxyvaleric (HV) acids. These findings on the yields and monomers present are in agreement with the results obtained by other researchers on the isolation of PHAs from municipal activated sludges.17,55,59–61 On the average, the ratio of HB to HV of the isolated PHAs was 1.20 by weight. This result is lower than that obtained by Hesselmann et al., which was ∼2.91 by weight.60 The difference is potentially due to differences in influent wastewater characteristics, specifically the volatile fatty acids (VFA) content. According to Yan et al., aside from environmental stress (i.e. high C:N ratio), there is a direct correlation between VFA content of the wastewater and PHA production in activated sludge. The type of VFA (i.e. acetic, propiopic, butyric, valeric) and the presence of other carbon sources in the wastewater also affects the ratio of different PHA monomers in the activated sludge.59 For example, Alvarez et al. obtained PHAs consisting mostly of 3-hydroxyoctanoic acids from Pseudomonas species (Isolate 319) when either octanoate or octanol was used as carbon source.62 Takabatake et al. studied PHA production using 18 activated sludges from MWWTPs with excess acetate as carbon source. They concluded that PHA production is more affected by influent characteristics than activated sludge operating conditions.63 In a similar study, Takabatake et al. concluded that regulating the composition of VFA such as acetate and propionate in the wastewater influent could control the monomer units of PHAs from activated sludge.64
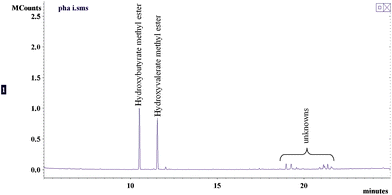 | ||
| Fig. 4 GC-MS analysis of PHAs isolated from activated sludge. | ||
Presently, there are several PHA products such as Biopol, Mirel and Nodax (USA), Biomer (Germany), Biocyle (Brazil), DegraPol (Italy) and Tianan PHBV and PHB (China) that are available commercially.45,65 Companies that manufacture microbial PHAs include ZENECA Bio-products (UK), Biotechnolgische Froschungs gessellschaft mbH (Austria), Petrochemia Danubai, Bio Ventures Alberta Inc. (Canada), Biocorp (USA), Metabolix (USA), Procter and Gamble (USA) and Asahi Chemicals and Institute of Physical and Chemical Research (Japan).50,66 The current production cost of microbial PHAs is about $4–6 per kilogram which is approximately 10 times higher than petroleum plastic.42,55 The cost of carbon source has caused the slow growth experienced by the PHA industry. For example, the cost of substrate or carbon source accounts for about 50% of the microbial PHA cost.57 Even with genetically engineered E. coli, the carbon source is still about 31% of PHAs’ production cost.67
Efforts to reduce the cost of microbial PHAs include searching for inexpensive carbon sources or substrates, advancement of fermentation, extraction and purification strategies and development of genetically engineered microorganisms.42,46,49,68 Carbon sources such as whey, wheat and rice brans, starch, molasses, waste vegetable and plant oils, CO2 and H2, methanol, industrial and biological wastes and wastewater are some of alternative substrates that have been considered to produce less expensive microbial PHAs.42,50,65,68–71 In the past several years, researchers all over the world have been looking at the production of PHAs concurrent with wastewater treatment, particularly by utilizing the biological or activated sludge treatment of wastewater. Aside from the fact that this configuration might not need additional infrastructure, this has the potential of reducing the amount of waste sludge to about 20% after PHA extraction.18,55–59,61,63,64,67,72–79
PHAs are attractive as packaging films and disposable commodity plastics (i.e. razor, utensils, diapers, cosmetic containers, bottles and cups, etc.) due to their biodegradability.50 In medicine, PHAs can be used as functionalized nano/micro beads for diagnostic and therapeutic applications, as devices for sutures and wound dressings, as conduits and carrier scaffolds for nerve repairs, as drug delivery systems, as drug eluting stents for cardiovascular applications, for soft and hard-tissue repair and regeneration and as heart valve in heart tissue engineering.48,66,80–84
PHAs from activated sludges might not be applicable for use as everyday commodities and medical devices. One possible application is to convert the PHAs to hydroxyacid alkyl esters by acid-/enzyme-/alkaline-catalyzed alcoholysis to produce biodiesel (see Table S2, ESI†). Zhang et al. studied the simultaneous H2SO4–catalyzed hydrolysis and methanolysis of PHAs. They used 200 mL of chloroform or acetyl butyrate to initially dissolve 15 grams of PHAs, after which 200 mL of 15% (v/v) acidic methanol was added. Reactions were conducted at 100 °C for 60 h, which gave recoveries of up to 65%. They also found that a 10% hydroxyacid methyl esters-ethanol blend enhances the combustion heat of ethanol from 27 to up to 35 KJ g−1. They estimated that the production cost of PHA-based biodiesel is about US$1,200 per ton.85 For both enzymatic and alkaline catalyzed biodiesel production from PHAs, a two-step process is required, hydrolysis of the polymer to its hydroxyacid monomers followed by acid-catalyzed alcoholysis. The enzymatic hydrolysis of PHAs can be accomplished by different extracellular lipases from a variety of microorganisms including Bacillus subtilis, Pseudomonas aeruginosa, Pseudomonas alcaligenes, Pseudomonas fluorescens, and Burkholderia cepacia (formerly Pseudomonas cepacia).86 PHA hydrolysis can also be accomplished using extracellular PHA depolymerases from Comamonas sp., Pseudomonas lemoignei, Pseudomonas fluorescens GK13 and Streptomyces sp. MG.86,87 These enzymes could be immobilized to a polypropylene matrix to improve their stability and reusability.88,89 Alkali-catalyzed hydrolysis of PHAs can be accomplished by mild alkaline treatment (0.5 N NaOH at 100 °C for 10 min).90–92 Although there are no available reports on 1-step alkali-catalyzed alcoholysis of PHAs, this seems to be an interesting process to consider.
Currently, biofuel production from PHAs is clearly not practical and economical. However, according to Thomson et al., all known chiral PHAs are purely composed of (R)-hydroxyacid monomers and thus, can be used as a good raw material for the production of enantiomerically pure drugs and speciality chemicals.41
SPE
The PHAs were removed prior to SPE to prevent their possible effect on the elution flow rate due to their high molecular weight. The results of TLC analysis of SPE fractions are shown in Fig. 5. To increase the resolution of the chromatograms, the TLC plates for Fractions 1 and 2 were developed in 94/6 hexane/diethyl ether solution while that of Fractions 3–5 were developed in 85/15/2 hexane/diethyl ether/acetic acid solution. Fraction 1 showed only one band corresponding to hydrocarbon (Fig. 5A-lanes A1, J1 and O1) while Fraction 2 showed several bands that correspond to steryl ester, wax ester and probably fatty acid alkyl esters (Fig. 5A-lanes A2, J2 and O2). As shown in Fig. 5B, Fraction 3 is composed mainly of triacylglycerides (lanes A3, J3 and O3), Fraction 4 predominantly contains free fatty acids, free fatty alcohol, sterols, diacylglycerides and monoacylglycerides (lanes A4, J4 and O4) and Fraction 5 is mainly phospholipids (lanes A5, J5 and O5). To verify these findings, the fractions (except Fraction 5) were injected to a HT-GC. The results are shown in Fig. 6 with the range of retention times for specific group of compounds. The retention time ranges were identified by analyzing standard mixtures of different compound groups (data not shown). As can be seen in Fig. 6A, the hydrocarbon fraction (Fraction 1) is characterized by the presence of an unresolved complex mixture (UCM). This will be discussed in the next section. | ||
| Fig. 5 Thin layer chromatography of fractions from solid phase extraction. (A) Fractions 1 and 2, developed in hexane/diethyl ether (94/6). (B) Fractions 3–5, developed in hexane/diethyl ether/acetic acid (85/15/2). S1 and S2 are standard mixtures: (1) n-octacosane, (2) behenyl oleate, (3) behenyl stearate, (4) palmityl palmitate, (5) lauryl palmitate, (6) triolein, (7) palmitoleic acid, (8) 1, 2-diolein, (9) monoolein, (10) cholesteryl myristate, (11) 1-hexadecanol, (12) cholesterol and (13) phospholipid standard mixture. | ||
 | ||
| Fig. 6 Representative high temperature gas chromatographs of fractions from solid phase extraction. A–D: Fractions 1–4. FAE: Fatty acid alkyl ester, WE: Wax ester, SE: Steryl ester, TG: Triacylglyceride, DG: Diacylglyceride, MG: Monoacylglyceride, FFA: Free fatty acid, FFOH: Free fatty alcohol, St: Sterol. | ||
As can be seen in Fig. 6B–6D, there were co-elutions of different compounds which makes quantitation of peaks or responses difficult. Thus, all the fractions were subjected to methanolysis. Although Fraction 1 appeared to contain only unsaponifiable hydrocarbons, it was also subjected to methanolysis for further verification. After methanolysis, the products were subjected to TLC and the results are shown in Fig. 7. The TLC result for Fraction 1 showed that it indeed contained unsaponifiable materials as indicated by the absence of methyl ester products (Fig. 7A-lanes A1, J1 and O1). Fraction 3 (Fig. 7B-lanes A3, J3 and O3) and Fraction 5 (Fig. 7B-lanes A5, J5 and O5) showed only one distinct band, which corresponded to methyl oleate. For Fraction 2 (Fig. 7A-lanes A2, J2 and O2) and Fraction 4 (Fig. 7B-lanes A4, J4 and O4), bands corresponding to fatty alcohol and sterol were also developed in addition to the methyl ester band. This result was expected since Fraction 2 contained wax esters and steryl esters while Fraction 4 contained free fatty alcohols and sterols as indicated by TLC and HT-GC of the original Fractions (Fig. 5A-lanes A2, J2 and O2, Fig. 5B-lanes A4, J4 and O4 and Fig. 6B and 6D).
 | ||
| Fig. 7 Thin layer chromatography of fractions after methanolysis. Developing solution: hexane/diethyl ether/acetic acid (85/15/2) (A) Fractions 1 and 2. (B) Fractions 3-5. S1 and S2 are standard mixtures: (1) n-octacosane, (2) behenyl oleate, (3) behenyl stearate, (4) palmityl palmitate, (5) lauryl palmitate, (6) triolein, (7) palmitoleic acid, (8) 1, 2-diolein, (9) monoolein, (10) cholesteryl myristate, (11) methyl oleate, (12) 1-hexadecanol, (13) cholesterol and (14) phospholipid standard mixture. | ||
The FAMEs analysis showed that Fractions 2–5 are dominated by saturated and unsaturated C16 and C18 fatty acids (Fig. 8). This result indicated microbial activity and is in agreement with the result obtained from extraction experiments and with other workers.6,13,23,24 The result of fatty alcohols and sterols analyses are shown in Fig. 9 and Table 3. As can be seen in Fig. 9, there were free sterols but no free fatty alcohols detected on the samples. Furthermore, results indicated that there were wax esters and steryl esters present in the samples (Fig. 9A). For more detailed discussions on these, see Wax esters and free fatty alcohols, and steryl esters and free sterols sections.
 | ||
| Fig. 8 Fatty acid profiles of different fractions from SPE. (A–D: Fractions 2–5). | ||
 | ||
| Fig. 9 Representative total ion chromatograms from GC-MS analysis of fatty alcohols and sterols from activated sludge. (A) Separated from Fraction 2. (B) Separated from Fraction 4. | ||
Hydrocarbons
The result of the hydrocarbon analysis is shown in Table 3. As mentioned earlier, the hydrocarbon fractions were characterized by the occurrence of UCM (Fig. 6A and Fig. 10). However, some major peaks were identified, quantified and presented as total hydrocarbons in Table 3. Identified peaks include hydrocarbons from C16–C20 and some linear alkyl benzene (LABs) particularly 1-pentyloctyl benzene and 1-butylnonyl benzene. According to Jardé et al., LABs are found as unsulphonated detergent residue and are characteristics of domestic sludges. Due to their resistance to microbial attack, LABS are recognized as molecular markers for domestic waste contribution. On the other hand, low molecular weight n-alkanes (from C15 to C22) are characteristics of petroleum products or fossil organic matter. This is supported by the presence of the UCM, which can be attributed to microbially degraded petroleum residue and are characteristics of petroleum-polluted sediments.16 | ||
| Fig. 10 A typical total ion chromatogram of the hydrocarbon fraction from SPE of activated sludge extract showing the presence of alkanes and unresolved complex mixture. | ||
Although the results of the hydrocarbon analysis showed contribution from petroleum products, it is still possible that some of these compounds were synthesized by activated sludge microorganisms. In a study by Moreda et al., aliphatic hydrocarbons on domestic sludges ranging from 230 to 1420 mg kg−1 of dry matter were detected.93 Some species of bacteria were known to produce small amounts of hydrocarbons (<1.0%). For example, 25% of the 5.9% cellular lipids of Desulfovibrio are straight chain hydrocarbons ranging from C15 to C31 while 17.4% of the 7.4% total lipids of Pseudomonas maltophilia are from C22 to C32 hydrocarbons. In addition to straight chain hydrocarbons, most bacteria produce trace amounts of isoprenoid hydrocarbons such as prispane, phytane and squalene.94 Other bacteria that produce hydrocarbons include Desulfovibrio desulfuricans, Pseudomonas flourecens, Clostridium pasteurianum, Clostridium tetanomorphum, Synechococcus elongatus, Anabaena variabilis, Micrococcus luteus, Micrococcus lysodeikiticus, Bacillus sp., E. coli, Mycobacterium sp. and Arthrobacter sp.95–100 Some microorganisms from the phyla Verucomicrobia, Planctomyces, Chloroflexi, Proteobacteria and Actinobacteria can also produce hydrocarbons.101 The hydrocarbons produced by these microorganisms can be either intracellular or extracellular. Furthermore, yeasts, fungal spores, fungal mycelia and algae were also reported to produce hydrocarbons.94,98,102–105 A broad list of microorganisms that produce intracellular and extracellular hydrocarbons is given by Ladygina et al.98
The formation of intracellular hydrocarbons in microorganisms is essential for the regulation of the cellular fatty acid pool.94 Furthermore, intracellular hydrocarbons might have protective functions (i.e. promote resistance to desiccation) and control some physicochemical properties of the cytoplasmic membrane.98 On the other hand, the extracellular hydrocarbons synthesized by microorganisms promote cell wall hydrophobicity, protecting them from extreme condition changes (i.e. high concentration of excreted acids). In most bacteria, extracellular hydrocarbons decrease glass adhesion of the cells and promote cell aggregations.95,98 Bagaeva and Zinurova obtained 3.7% and 6.9% (weight of biomass) intracellular and extracellular hydrocarbons from a culture of Clostridium pasteurianum grown in a 10% CO2–90% H2 atmosphere.95 In other microorganisms, hydrocarbons aid in cell development and interspecies interactions.98
Hydrocarbons are considered to be the most stable group of compounds and are the main component of petroleum-based fuels and thus, a very advantageous target for the biofuel industry. They can be used in existing engines, refineries and distribution systems without modifications.97,98 Since the role of hydrocarbons in microorganisms is not fully understood, genetic engineering seems to be the only way to increase microbial production of hydrocarbons.96,97,106 To date, there have been no reports of hydrocarbon production concurrent with wastewater treatment. However, in 2001, Park and co-workers isolated a halotolerant bacterial strain (close in characteristics to Vibrio furnissii) from sewage, which can accumulate large amount of extracellular lipids and hydrocarbons (120% of cell dry weight). The accumulated hydrocarbons included C15, C18, C21, C22 and C24 alkanes totalling to 50% of cell dry weight.107 In terms of industrial production, Robertson et al. claimed to develop a genetically engineered cyanobacteria capable of producing alkanes and ethanol on a commercial scale.108
Wax esters and free fatty alcohols
Wax esters (WEs) or waxes are another class of storage compounds that microorganisms can synthesize under stressful environment. In particular, some prokaryotes can accumulate large amount of WEs under nitrogen-limited conditions when there is an excess of carbon.109–112 WEs contain fatty acids which are ester-linked to long chain alcohols or fatty alcohols that can have chain length up to C64.15,39 Accumulation of WEs have been reported involving microorganisms in the genus Acinetobacter, Moraxella, Micrococcus, Fundibacter, Neisseria, Pseudomonas, Marinobacter, Corynebacterium, Nocardia, Mycobacterium and Rhodococcus.110,113 Microbial species that are known to be WE-producers include Aeromonas hydrophila, Fundibacter jadensis, Micrococcus cryophilus ATCC15174, Acinetobacter calcoaceticus, Rhodococcus opacus PD630, Rhodococcus jostii RHAI, Marinobacter hydrocarbonoclasticus [ATCC 49840], Marinobacter aquaeolei VT8, Pseudomonas nautica [IP85/617], Mycobacterium tuberculosis, Mycobacterium leprae, Mycobacterium bovis, Mycobacterium smegmatis, Streptomyces coelicolor, Alcanivorax borkumensis and Euglena gracilis [ATCC 12716].15,39,54,109,110,113–120 For a more complete listing of microbial species, see Kalscheuer.121 The list includes gram-negative bacteria (α-, β-, γ-, δ-Proteobacteria) and gram-positive bacteria (Actinobacteria, Bacteriodetes/Chlorobi). In the environment, waxes can be produced not only by microorganisms, but also by marine and terrestrial plants, marine animals, insects and birds.39Fatty alcohols (a.k.a. alkanols) normally exist in the environment as wax esters.39 In microbial cultivation, fatty alcohols serve as intermediates during aerobic catabolism of long chain n-alkanes for WE biosynthesis.121,122 However, in microbial catabolism of detergent fatty alcohols as polyethoxylates, free fatty alcohols can potentially be found as one of the cultivation products.39
WEs in microorganisms serve mainly as energy and carbon storage reserves during starvation. In addition, WEs also act as metabolic water reserves, buoyancy generators, thermal insulators and as sinks for toxic or useless fatty acids during growth on recalcitrant hydrocarbons.39,109,110,113 In some microorganisms such as Fundibacter jadensis and some strains of Acinetobacter sp., production of extracellular WEs has also been reported but their functions in living microbial cells are yet to be determined.113,123 Microorganisms can produce WEs from a variety of carbon sources including hydrocarbons, alkanols, fatty acids, triacylglycerides and phytol.109,110,115,122,124,125
Analyses showed that WEs are present in the samples (Fig. 5 and 7, Table 3). The complexity of the samples, however, made it impossible to analyze the WEs without derivatization. The methanolysis of WEs separated the fatty acid and fatty alcohol components of the molecule and independent analyses of the components were made without much interference. The fatty alcohol associated with WEs in the samples ranges from about 1.80–5.55% (weight) of extract which correspond to ∼0.17–0.90% (weight) based on dried sludge. According to Mudge et al.,39 due to the synthetic pathway for fatty alcohols, fatty acids should act as indicator of the likely fatty alcohols that can be found in bacteria. The fatty acids present in the samples have C12–C24, peaking at C14–C18 (Fig. 3 and 8). By looking at the fatty acid profile of the samples, it was expected that fatty alcohols from C14–C18 should be present in the samples. And as shown in Fig. 11, this was indeed the case. Saturated C14–C18 and monounsaturated C16 and C18 fatty alcohols were found in all the samples. Furthermore, odd numbered fatty alcohols were detected (C15 and C17), which are mainly produced by bacteria.126 Although, this is a good indication of bacterial activity, exogenous contributions cannot be neglected since fatty alcohols may come from other sources. For example, fatty alcohols from Ascophyllum nodosum and Fucus spiralis (brown algae) contain C12–C28 range peaking at C14–C18.39 The fatty alcohols of the samples may also have anthropogenic contribution. The sludge samples were obtained from a MWWTP and C12–C18 fatty alcohols are usually used in detergent applications.20,39 Fatty alcohols are not completely degraded in wastewater treatment facilities, with degradation fraction ranging from 0.993 for C6 to 0.159 for C22. The remaining fraction goes to air (0.004 for C6 to 0.000094 for C22), water (0.001 for C6 to 0.045 for C22) and sludge (0.470 for C12 to 0.729 for C18).39 Thus, the total fatty alcohols detected in the samples might be a sum of contributions from all these sources.
 | ||
| Fig. 11 Fatty alcohol profile of wax esters isolated from activated sludge extract. | ||
Although, as mentioned earlier, in the presence of detergent fatty alcohols, it is possible to find free fatty alcohols in microbial extracts, they were not detected on any of the samples (see Fig. 9B). Nagao et al.115 studied the conversion of vegetable oils to rare fatty acids and fatty alcohols using Aeromonas hydrophila. They detected wax esters but not free fatty alcohols in their samples. In their case, the microorganisms most likely synthesized the free fatty alcohols as precursors for WEs biosynthesis since they used vegetable oil as carbon source. As for the case of activated sludge, the same might be true. Detergent fatty alcohols especially in the form of polyethoxylates are considered to be bioavailable.39 Thus, the activated sludge microorganism might have used them as intermediates for WEs production, which is the main function of free fatty alcohols in living microbial cells.
WEs and fatty alcohols are important raw materials for a variety of surfactant, polymer, leather, solid coating, lubricants, toiletry, cosmetic, food and pharmaceutical products.21,109,122,123,127–129 The major sources of natural WEs are jojoba and carnauba oils. However, due to high price of jojoba oil (∼7,000 USD per ton), most commercial WEs available nowadays are of synthetic origin, which are mainly consumed by cosmetic and pharmaceutical industries.130,131 In the past several years, researchers have been considering other sources of WEs such as microbial (including genetically engineered microbes), and crambe and rice bran oils.15,129,132,133 Free fatty alcohols that are commercially available (i.e. Lurgi manufacturing company) are usually produced by catalytic hydrogenation of fatty acids or FAMEs at high temperature (523–573 K) and under high hydrogen pressure (25–35 MPa).134 They are also produced from ethylene via the Ziegler Alfol process and by hydroformylation of olefins.135
There are no reports regarding the production of WEs concurrent with wastewater treatment. Aside from possible applications of activated sludge WEs in different industries mentioned earlier, they could also be used as feedstocks for the renewable fuel industry. The fatty acid component of the WE can be converted to biodiesel (by methanolysis) or green fuel (via catalytic cracking).136 The fatty alcohol component can be converted to its alkyl acetate derivative (by transesterification/transacetylation), which has been recently considered as a new class of biofuel, or to green fuel (via catalytic cracking) (see Table S2, ESI†).137–139
Steryl esters and free sterols
Steryl esters and sterols are usually associated with lipids found in animals, plants, yeasts and fungi.113,140–142 Only a few species of bacteria are known to produce sterols. These include Flovobacterium dehydrogenes, Methylcoccus capsulatus, Methylosphaera hansonii, Nannocystis exedens, Rhodococcus rhodochrous, Bacillus sp., Cellulomonas dehydrogenans and Mycobacterium smegmitis.140,142–147 Most bacteria belonging to the genus Mycoplasma (i.e. M. salivarium PG-20, M. fermentans PG-18, and M. canis PG-14), are known to require sterol for growth.148 Some bacteria such as Streptobacillus moniliformis, Proteus mirabilis, Streptococcus pyogenes, and Staphylococcus aureus can incorporate cholesterol into their cell membranes.149 Moreover, some bacteria such as Staphylococcus epidermis, Propionibacterium acnes and Propionibacterium granulosum can esterify cholesterol if it is present in the growth medium.146 In Mycoplasma species and other sterol-requiring species (i.e. Borrelia afzelii and Helicobacter pyroli), the presence of steryl glycosides (sterol with an attached sugar moiety) has also been reported.150Other than structural functions in sterol-requiring microorganisms, the role of sterols and steryl esters in living bacterial cells is not clearly understood. It has been suggested that the steryl esters might be involved in the transport of sterols to different parts of plants.151 Sterols can also eliminate the thermotrophic transition (lamellar gel phase to liquid-crystalline phase) of phosphoglycerolipid bilayers. This will result in constant membrane properties such as membrane fluidity for wide temperature ranges.150 Aside from membrane fluidity, free sterols were also suggested to have important functions in the sensitivity of yeasts to the action of polyene antibiotics.152 In a study conducted by Grunwald151 on the effects of sterols, steryl esters and steryl glycoside on membrane permeability of barley roots, they found that free sterols particularly cholesterol and campesterol can greatly stimulate or inhibit (depending on the concentration) the permeability of the phospholipid layer of the barley root membrane. However, cholesteryl palmitate and cholesteryl glucoside did not have any effect on the membrane permeability. This study, as indicated by Grille et al., suggested that free sterols might have similar effect on permeability of most biomembranes.150 These functions of free sterols and steryl esters on plants, yeasts and biomembranes might be also true for bacteria.
There were four major sterols (as steryl esters and free sterols) present in the activated sludge samples (Fig. 12). In both cases, coprostanol and cholesterol are the dominant ones. Cholesterol has a variety of possible sources including animals and microalgae in addition to sewage while coprostanol is considered to be the principal indicator of mammalian sewage.126,140 Coprostanol is produced in the digestive track by anaerobic microbial hydrogenation of cholesterol and can comprise 24–89% of total sterols in human feces.16,153 Moreover, coprostanol might be a product of reduction of cholesterol during the activated sludge treatment process.16,154 Stigmasterol together with β-sitosterol is known to be a higher plant sterol and usually associated with herbivore fecal contamination.16,140 As was for the case of coprostanol, the stigmastanol detected in the samples could have been produced by hydrogenation of stigmasterol during the treatment process.154
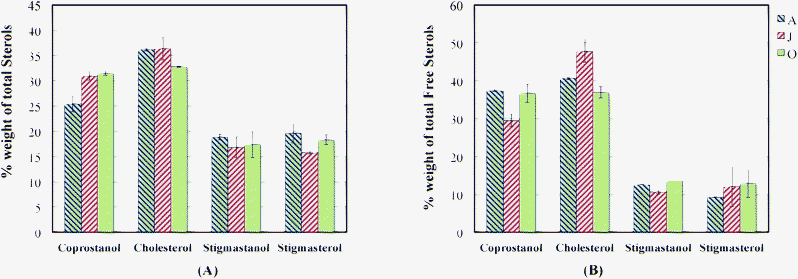 | ||
| Fig. 12 Profiles of sterols isolated from activated sludge extract. (A) Associated with Steryl Esters. (B) Free Sterols. | ||
Fecal sterols are known to be excreted in esterified form.153,155 Thus, the steryl esters in the samples could have been from the influent wastewater or due to microbial activity. On the average, the percentages of esterified sterols in the samples were 12.53%, 13.31% and 17.54% (weight of total sterols) for batch A, J and O, respectively. Based on these results, the steryl esters and free sterols in the samples could be the sum of contributions from two main sources, which are human and animal feces and treatment due to microbial activity.
Possible industrial application of steryl esters and free sterols from microorganisms could be the same as plant sterols. They can be used as starting material for steroids synthesis and steroid-based drug production, as bioactive pharmaceutical compounds, as food and nutraceutical additives and as surfactants.140 In addition to these possible applications, steryl esters and free sterols from activated sludge can also be used as feedstock for renewable fuel production. This might be possible via either catalytic cracking (hydroprocessing) or pyrolysis (thermal cracking) as indicated by several studies (see Table S2, ESI†).154,156–160
Glycerides and Free fatty acids
Triacylglycerides (TGs) are triesters of glycerol with fatty acids. They are commonly present in most eukaryotic organisms such as animals, plants, yeasts and fungi.161,162 In prokaryotic microorganisms, accumulation of TGs have been reported in some bacteria belonging to the genera Mycobacterium, Rhodococcus, Micromonospora, Dietzia, Gordonia, Nocardia (Streptomyces) and Acinetobacter.62,113,161,162 Species that are known to accumulate TGs include Rhodococcus opacus PD630, Rhodococcus opacus DSM1069, Rhodococcus jostii RHAI, Rhodococcus aetherivorans IAR1, Rhodococcus fascins, Rhodococcus erythropolis, Aeromonas hydrophila, Pseudomonas aeruginosa, Nocardia corallina, Nocardia globerula 432, Streptomyces coelicolor, Streptomyces lividans, Mycobacterium smegmatis, Rhodococcus ruber NCIMB 40126, Alcanivorax borkumensis, Mycobacterium tuberculosis, Dietzia maris, Gordonia amarae, Acinetobacter lwoffi and Acinetobacter calcoaceticus [see work by Alvarez and Steinbüchel for more complete listings].44,51,54,113,115–118,161–168 These microorganisms can use a wide range of carbon sources such as wastewater, sugars, vegetable oils, food wastes, hydrocarbons and halogenated aliphatics and aromatics.32,164,165,168,169The accumulation of TGs is usually triggered when a carbon source is available in excess in a nitrogen-limited environment. However, it has been also suggested that TG-accumulations in bacteria can be accomplished under limited aeration conditions.161 Similar to PHAs and WEs, the main function of TG is as a carbon and energy reserve compound. In addition to this, TG serves as a sink for reducing equivalents, as a reservoir of metabolic water, as a means of adjusting membrane fluidity by regulating the fatty acid pool of the membrane lipids, as a raw material for phospholipids biosynthesis, as an acceptor for toxic or unwanted fatty acids, as a means of balancing cell metabolism depending on environmental conditions by reducing pyridine nucleotides in the cells and as a precursor for antibiotics and mycolic acids biosynthesis.161,162
Diacylglycerides (DGs) and monoacylglycerides (MGs) serve mainly as intermediates for the synthesis of TGs and phospholipids.152,161 They are usually present in minute but detectable concentrations.14 In a study conducted by Wältermann et al.117 on lipid accumulation of Rhodococcus opacus PD630, they detected DGs and MGs along with TGs and free fatty acids. However, the concentrations of DGs, MGs and free fatty acids were almost negligible relative to the TGs. According to Alvarez, free fatty acids are biologically toxic and hence they do not occur in living cells in high quantity.161 DGs and MGs being intermediate compounds and free fatty acids being toxic are probably the reason why they occur in very small quantities in living cells. Furthermore, DGs, MGs and free fatty acids could be products of TGs and phospholipids degradations possibly during sample extraction, preparation and storage.
The result of the analysis of extract from activated sludge showed the presence of free fatty acids, MGs, DGs and TGs (Fig. 5B and 6C–D). As mentioned, the presence of DGs and MGs might have been due to sample extraction, preparation and storage. The same might be true about the presence of high proportion of free fatty acids in the samples. The extract from activated sludge contains 2–3% (weight of extract) TGs (Fraction 3) which yielded FAMEs in the same range. On the other hand, the FAMEs obtained from Fraction 4 (free fatty acids, DGs and MGs) were 11–13% (weight of extract), which constitute to more than 50% of total FAMEs obtained (Table 3). The BDE uses chloroform, methanol and water as solvents. In the presence of water and methanol, TGs, DGs and MGs (even WEs, SEs and phospholipids) can undergo hydrolysis and methanolysis, respectively. Aside from the occurrence of high proportion of free fatty acids, this is also evident by the presence of fatty acid alkyl esters in the samples [Fig. 5A (lanes A2, J2 and O2), Fig. 5B (lanes A4, J4 and O4) and Fig. 6B].
Glycerides are probably the most important basic oleochemicals including free fatty acids, fatty acid alkyl esters, fatty alcohols and fatty amines.135 Generally, possible applications of bacterial glycerides and fatty acids may be the same as those of vegetable sources, which include soaps, detergents, plastics, personal care products, resins and lubricants. Among these possible applications, glycerides and fatty acids from activated sludge might be well suited as renewable fuel feedstock either via alcoholysis or catalytic cracking.
Phospholipids
Like most biological membranes, bacterial membranes consist of a lipid bilayer. For gram-negative bacteria, in general, their outer membrane contains 25% phospholipids with 75% phosphatidylethanolamine, 20% phosphatidylglycerols and 5% cardiolipin.170 These phospholipids are also present in gram-positive bacteria but in different proportions. For example, phospholipids from Bacillus megaterium contains 16% phophatidylethanolamine, 40% phosphatidylglycerols, 40% cardiolipin and 4% other.171 However, the compositions and even the amount of phospholipids present in microorganisms are dictated by environmental conditions such as nutrient deficiency (C, N, Na and Mn) and temperature. Mn deficiency has been reported to reduce phospholipid content of Brevibacterium ammoniagenes. N–/C–/Na–limitation affects the composition of phospholipids in Rhodotorula glutinis and Staphylococcus aureus. As for temperature, unsaturation of phospholipid fatty acids increases with decreasing temperature as has been reported for Neurospora crassa and Paecilomyces persicinus.172In most cells, phospholipids play a vital role in cellular structure and functions. They also have a function in the transport of important cellular material such as protein and they regulate materials coming in and out of the cell.173,174 The yields of FAMEs from the phospholipid fraction of the sludge extract ranges 0.30–0.70% (weight extract) (Table 3). This range corresponds to 0.03–0.07% (weight dry sludge). These results are within the range obtained by Forney et al.175 on their study about fatty acids associated with activated sludge phospholipids obtained from different wastewater treatment facilities in the United States. They obtained a range of 0.40–15.3 nmol fatty acids mg−1 dry biomass which is equivalent to (as stearic acid) 0.01–0.44% (weight dry biomass).
Phospholipids can be used as a source of oleochemical fatty acids, which can be utilized for the production of fatty alcohols, biofuels and other useful products. They can be utilized for production of polymerizable phospholipids, which can be used in biomedical and microelectronic applications.176 Individually, phospholipids are nutritious, biodegradable, biocompatible and a good source of organic phosphate and choline. As a group, phospholipids can form supramolecular structures that self-assemble. Furthermore, phospholipids can spontaneously self-associate into bilayer membranes that can separate compartments of the same aqueous phase from each other geographically. The resulting structures from this self-association have predictable properties. Due to these properties of phospholipids, they are widely used in different industrial applications (i.e. paints, magnetic recording media, controlled microparticle crystallization), molecular biology (as genetic material carrier) and food technology (i.e. accelerated cheese ripening, reduction of bacterial spoilage and encapsulation of antioxidants).177
Concluding remarks
The Bligh & Dyer extracts of activated sludge obtained from a MWWTP in Tuscaloosa, AL, USA were analyzed for major bacterial storage compounds. Due to the diversity of the microbial community present in the sludge, all types of storage compounds were detected including PHAs, WEs, SEs and TGs. The input of PHAs in the activated sludge process is highly likely to be negligible and thus all the PHAs present in the sludge are due to microbial activity. Although there is a very high possibility that the WEs and TGs present were produced by activated sludge microorganisms, the probability of exogenous contributions may not be neglected. As for steryl esters, their occurrence in the sludge can be accounted mainly from anthropogenic contributions. Regardless of the source of these compounds, their persistent presence in the sludge offers a wide range of applications in the renewable fuel and oleochemical industries. The results also explain the high gravimetric yield (∼15% weight) obtained by previous researchers on the in situ transesterification of activated sludge. Other compounds, particularly fatty alcohols and sterols, were also extracted during the in situ transesterification resulting in a high gravimetric yield.The utilization of bacterial lipids as a feedstock for different applications can be made economically feasible by addressing three main conditions: (1) sustainable utilization of inexpensive bio-based carbon source, (2) high lipid yield, and (3) reproducibility, sustainability and quality of lipids produced.164 Activated sludges generated by wastewater treatment operations have the potential of meeting these three conditions. Strategies to increase the lipid yield and produce consistent quality lipids are part of a current study and will be published elsewhere.178
Acknowledgements
This work was funded by the United States Department of Energy, Office of Energy Efficiency and Renewable Energy (Grant No.: DE-FG36-06GO86025).References
- S. P. R. Katikaneni, J. D. Adjaye, R. O. Idem and N. N. Bakhshi, Ind. Eng. Chem. Res., 1996, 35, 3332–3346 CrossRef CAS.
- J. D. Adjaye, S. P. R. Katikaneni and N. N. Bakhshi, Fuel Process. Technol., 1996, 48, 115–143 CrossRef CAS.
- R. O. Idem, S. P. R. Katikaneni and N. N. Bakhshi, Fuel Process. Technol., 1997, 51, 101–125 CrossRef CAS.
- A. Corma, G. W. Huber, L. Sauvanaud and P. O'Connor, J. Catal., 2007, 247, 307–327 CrossRef CAS.
- J. Holmgren, C. Gosling, R. Marinangeli, T. Marker, G. Faracii and C. Perego, Hydrocarb. Process., 2007, 86, 67–72 CAS.
- E. Revellame, R. Hernandez, W. French, W. Holmes and E. Alley, J. Chem. Technol. Biotechnol., 2010, 85, 614–620 CrossRef CAS.
- D. de Oliveira, M. Di Luccio, C. Faccio, C. Dalla Rosa, J. P. Bender, N. Lipke, C. Amroginski, C. Dariva and J. V. de Oliveira, Appl. Biochem. Biotechnol., 2005, 121-124, 553–560 CrossRef CAS.
- M. J. Goff, N. S. Bauer, S. Lopes, W. R. Sutterlin and G. J. Suppes, J. Am. Oil Chem. Soc., 2004, 81, 415–420 CrossRef CAS.
- M. Brady, T. Ellis, K. Kimura, J. K. Lyons, H. D. Sinks and J. R. Stephens, unpublished work. Search PubMed.
- T. J. Benson, R. Hernandez, W. T. French, E. G. Alley and W. E. Holmes, Journal of Molecular Catalysis a-Chemical, 2009, 303, 117–123 CrossRef CAS.
- L. Coulier, J. W. Niemantsverdriet and J. A. R. van Veen, Eindhoven University of Technology, 2001 Search PubMed.
- B. Günder, in The membrane-coupled activated sludge process in municipal wastewater treatment, Technomic Publishing Company, Inc., Lancaster, PA, 2001, ch. 1pp. 1–32 Search PubMed.
- E. Revellame, R. Hernandez, W. French, W. Holmes, E. Alley and R. Callahan II, J. Chem. Technol. Biotechnol., 2011, 86, 61–68 CrossRef CAS.
- F. D. Gunstone and J. L. Harwood, in The lipid handbook with CD-ROM, ed. F. D. Gunstone, J. L. Harwood and A. J. Dijkstra, CRC Press/Taylor & Francis, Boca Raton, FL, 3rd edn, 2007, 37–141 Search PubMed.
- B. D. Wahlen, W. S. Oswald, L. C. Seefeldt and B. M. Barney, Appl. Environ. Microbiol., 2009, 75, 2758–2764 CrossRef CAS.
- E. Jardé, L. Mansuy and P. Faure, Water Res., 2005, 39, 1215–1232 CrossRef.
- D. Baetens, A.-M. Aurola, A. Foglia, D. Dionisi and M. C. M. van Loosdrecht, Water Sci. Technol., 2002, 46, 357–361 CAS.
- A. S. M. Chua, H. Takabatake, H. Satoh and T. Mino, Water Res., 2003, 37, 3602–3611 CrossRef CAS.
- M. Bassas Galià, in Handbook of Hydrocarbon and Lipid Microbiology, ed. K. N. Timmis, SpringerBerlin Heidelberg, 2010, 3725–3741 Search PubMed.
- U. R. Kreutzer, J. Am. Oil Chem. Soc., 1984, 61, 343–348 CrossRef CAS.
- T. Mimura, Shokubai, 2006, 48, 532–537 CAS.
- United States Department Of Energy. Energy Information Administration, What Fuels Are Made from Crude Oil?, http://www.eia.doe.gov/energyexplained/index.cfm?page=oil_refining, Accessed March 20, 2011.
- S. Dufreche, R. Hernandez, T. French, D. Sparks, M. Zappi and E. Alley, J. Am. Oil Chem. Soc., 2007, 84, 181–187 CrossRef CAS.
- A. Mondala, K. Liang, H. Toghiani, R. Hernandez and T. French, Bioresour. Technol., 2009, 100, 1203–1210 CrossRef CAS.
- U. S. A. City of Tuscaloosa AL, Hilliard Fletcher Wastewater Treatment Plant, http://www.ci.tuscaloosa.al.us/index.aspx?NID=645, Accessed March 10, 2010..
- E. G. Bligh and W. J. Dyer, Can. J. Biochem. Physiol., 1959, 37, 911–917 CrossRef CAS.
- Y. Kathiraser, M. K. Aroua, K. B. Ramachandran and I. K. P. Tan, J. Chem. Technol. Biotechnol., 2007, 82, 847–855 CrossRef CAS.
- K. T. Hwang, S. L. Cuppett, C. L. Weller, M. A. Hanna and R. K. Shoemaker, J. Am. Oil Chem. Soc., 2002, 79, 529–533 CrossRef CAS.
- R. Bernasconi, E. Bolzacchini, G. Galliani, F. Gugliersi, B. Rindone, M. Rindone, M. T. Tacconi and A. Terraneo, LWT-Food Sci. Technol., 2007, 40, 569–573 CrossRef CAS.
- F. F. Dias and J. V. Bhat, Appl. Environ. Microbiol., 1964, 12, 412–417 CAS.
- F. F. Dias and J. V. Bhat, Appl. Environ. Microbiol., 1965, 13, 257–261 CAS.
- K. P. Y. Fong and H. M. Tan, World Journal of Microbiology and Biotechnology, 2000, 16, 441–443 CrossRef.
- A. H. Mondala, R. Hernandez, T. French, L. McFarland, J. W. Santo Domingo, M. Meckes, H. Ryu and B. Iker, AIChE J., 2011 DOI:10.1002/aic.12655.
- D. Orhon and N. Artan,in Modelling of activated sludge systems, Technomic Publishing Co., Inc., Lancaster, PA, 1994, ch. 2pp. 39–110 Search PubMed.
- Y. Comeau, in Biological wastewater treatment : principles, modelling and design, ed. M. Henze, M. van Loosdrecht, G. Ekama and D. Brdjanovic, IWA Publishing, London, 2008, ch. 2, pp. 9–32 Search PubMed.
- L. K. Wang, Z. Wu and N. K. Shammas, in Biological Treatment Processes, ed. L. K. Wang, N. C. Pereira and Y.-T. Hung, Humana Press, New York, 2009, vol. 8, ch. 9, pp. 371–434 Search PubMed.
- F. Smedes and T. K. Askland, Mar. Pollut. Bull., 1999, 38, 193–201 CrossRef CAS.
- E. Revellame, R. Hernandez and W. French, unpublished work Search PubMed.
- S. M. Mudge, S. E. Belanger and A. M. Nielsen, Fatty alcohols: anthropogenic and natural occurrence in the environment, Royal Society of Chemistry, Cambridge, UK, 2008 Search PubMed.
- A. Clarke, G. Coulson and G. J. Morris, Plant Physiol., 1982, 70, 97–103 CrossRef CAS.
- N. Thomson, I. Roy, D. Summers and E. Sivaniah, J. Chem. Technol. Biotechnol., 2010, 85, 760–767 CrossRef CAS.
- E. Akaraonye, T. Keshavarz and I. Roy, J. Chem. Technol. Biotechnol., 2010, 85, 732–743 CrossRef CAS.
- A. Steinbüchel and T. Lütke-Eversloh, Biochem. Eng. J., 2003, 16, 81–96 CrossRef.
- A. Arabolaza, E. Rodriguez, S. Altabe, H. Alvarez and H. Gramajo, Appl. Environ. Microbiol., 2008, 74, 2573–2582 CrossRef CAS.
- S. Y. Lee, Biotechnol. Bioeng., 1996, 49, 1–14 CrossRef CAS.
- A. J. Anderson and E. A. Dawes, Microbiol. Rev., 1990, 54, 450–472 CAS.
- W. Punrattanas in PhD Dissertation (PhD), Virginia Polytechnic Institute and State University, 2001 Search PubMed.
- M. Zinn, B. Witholt and T. Egli, Adv. Drug Delivery. Rev., 2001, 53, 5–21 CrossRef CAS.
- R. J. Van Wegen, Y. Ling and A. P. J. Middelberg, Chem. Eng. Res. Des., 1998, 76, 417–426 CrossRef CAS.
- C. S. K. Reddy, R. Ghai, Rashmi and V. C. Kalia, Bioresour. Technol., 2003, 87, 137–146 CrossRef CAS.
- H. M. Alvarez, R. Kalscheuer and A. Steinbüchel, Appl. Microbiol. Biotechnol., 2000, 54, 218–223 CrossRef CAS.
- H. Brandl, R. A. Gross, R. W. Lenz and R. C. Fuller, Appl. Environ. Microbiol., 1988, 54, 1977–1982 CAS.
- R. Kalscheuer, M. Wältermann, H. M. Alvarez and A. Steinbüchel, Arch. Microbiol., 2001, 177, 20–28 CrossRef CAS.
- M. A. Hernandez, W. W. Mohn, E. Martinez, E. Rost, A. F. Alvarez and H. M. Alvarez, BMC Genomics, 2008, 9, 600 CrossRef.
- H. Chua, P. H. F. Yu and C. K. Ma, Appl. Biochem. Biotechnol., 1999, 77-79, 389–399 CrossRef CAS.
- S. V. Reddy, M. Thirumala, T. V. K. Reddy and S. K. Mahmood, World J. Microbiol. Biotechnol., 2008, 24, 2949–2955 CrossRef CAS.
- K. H. Law, Y. C. Leung, H. Lawford, H. Chua, L. Wai-Hung and P. H. Yu, Appl. Biochem. Biotechnol., 2001, 91-93, 515–524 CrossRef CAS.
- Y. M. Jiang, Y. G. Chen and X. Zheng, Environ. Sci. Technol., 2009, 43, 7734–7741 CrossRef CAS.
- S. Yan, R. D. Tyagi and R. Y. Surampalli, Water Sci. Technol., 2006, 53, 175–180 CAS.
- R. P. Hesselmann, T. Fleischmann, R. Hany and A. J. Zehnder, J. Microbiol. Methods, 1999, 35, 111–119 CrossRef CAS.
- E. R. Coats, K. E. Vandevoort, J. L. Darby and F. J. Loge, Journal of Environmental Engineering, 2011, 137, 46–54 CrossRef CAS.
- H. M. Alvarez, O. H. Pucci and A. Steinbuechel, Appl. Microbiol. Biotechnol., 1997, 47, 132–139 CrossRef CAS.
- H. Takabatake, H. Satoh, T. Mino and T. Matsuo, Water Sci. Technol., 2002, 45, 119–126 CAS.
- H. Takabatake, H. Satoh, T. Mino and T. Matsuo, Water Sci. Technol., 2000, 42, 351–356 CAS.
- P. Y. Tian, L. A. Shang, H. Ren, Y. Mi, D. D. Fan and M. Jiang, Afr. J. Biotechnol., 2009, 8, 709–714 CAS.
- S. Philip, T. Keshavarz and I. Roy, J. Chem. Technol. Biotechnol., 2007, 82, 233–247 CrossRef CAS.
- C. K. Ma, H. Chua, P. H. Yu and K. Hong, Appl. Biochem. Biotechnol., 2000, 84-86, 981–989 CrossRef CAS.
- T. Yamane, Biotechnol. Bioeng., 1993, 41, 165–170 CrossRef CAS.
- M. G. E. Albuquerque, M. Eiroa, C. Torres, B. R. Nunes and M. A. M. Reis, J. Biotechnol., 2007, 130, 411–421 CrossRef CAS.
- S. A. Ataei, E. Vasheghani-Farahani, S. A. Shojaosadati and H. Abdul Tehrani, Macromol. Symp., 2008, 269, 11–16 CrossRef CAS.
- S. Bengtsson, A. Werker, M. Christensson and T. Welander, Bioresour. Technol., 2008, 99, 509–516 CrossRef CAS.
- E. R. Coats, F. J. Loge, M. P. Wolcott, K. Englund and A. G. McDonald, Water Environ. Res., 2007, 79, 2396–2403 CrossRef CAS.
- Y. Huang, Shengwu Jishu Tongbao, 2009, 59-61, 74 Search PubMed.
- H. Sato and T. Mino, Kagaku Sochi, 2002, 44, 55–61 CAS.
- H. Satoh, M. Onuki and T. Mino, Eco Industry, 2002, 7, 5–11 CAS.
- S. Chinwetkitvanich, C. W. Randall and T. Panswad, Water Sci. Technol., 2004, 50, 135–143 CAS.
- H. Chen, H. B. Li and Y. F. Xia, Enzyme. Microb. Technol., 2010, 46, 594–597 CrossRef.
- D. He, B. B. Zhang, Y. F. Tsang and H. Chua, WIT Transactions on Ecology and the Environment, 2008, 109, 837–844 CrossRef CAS.
- W.-T. Liu, T. Mino, T. Matsuo and K. Nakamura, J. Biosci. Bioeng., 2000, 90, 494–500 CAS.
- S. K. Misra, S. P. Valappil, I. Roy and A. R. Boccaccini, Biomacromolecules, 2006, 7, 2249–2258 CrossRef CAS.
- S. P. Valappil, S. K. Misra, A. R. Boccaccini and I. Roy, Expert Review of Medical Devices, 2006, 3, 853–868 CrossRef CAS.
- G.-Q. Chen and Q. Wu, Biomaterials, 2005, 26, 6565–6578 CrossRef CAS.
- S. F. Williams, D. P. Martin, D. M. Horowitz and O. P. Peoples, Int. J. Biol. Macromol., 1999, 25, 111–121 CrossRef CAS.
- Q. Wu, Y. Wang and G.-Q. Chen, Artificial Cells, Blood Substitutes, and Biotechnology, 2009, 37, 1–12 CrossRef CAS.
- X. J. Zhang, R. C. Luo, Z. Wang, Y. Deng and G. Q. Chen, Biomacromolecules, 2009, 10, 707–711 CrossRef CAS.
- K. E. Jaeger, A. Steinbuchel and D. Jendrossek, Appl. Environ. Microbiol., 1995, 61, 3113–3118 CAS.
- B. Calabia and Y. Tokiwa, Biotechnol. Lett., 2006, 28, 383–388 CrossRef CAS.
- J. Gangoiti, M. Santos, M. J. Llama and J. L. Serra, Appl. Environ. Microbiol., 2010, 76, 3554–3560 CrossRef CAS.
- W. Tischer and F. Wedekind, Top. Curr. Chem., 1999, 200, 95–126 CrossRef CAS.
- G.-E. Yu and R. H. Marchessault, Polymer, 1999, 41, 1087–1098 CrossRef.
- G.-Q. Chen and Q. Wu, Appl. Microbiol. Biotechnol., 2005, 67, 592–599 CrossRef CAS.
- F. P. Delafield, M. Doudoroff, N. J. Palleroni, C. J. Lusty and R. Contopoulos, J. Bacteriol., 1965, 90, 1455–1466 CAS.
- J. M. Moreda, A. Arranz, S. Fdez De Betoño, A. Cid and J. F. Arranz, Sci. Total Environ., 1998, 220, 33–43 CrossRef CAS.
- T. G. Tornabene, Cell. Mol. Life Sci., 1982, 38, 43–46 CrossRef CAS.
- T. V. Bagaeva and E. E. Zinurova, Biochemistry (Mosc.), 2004, 69, 427–428 CrossRef CAS.
- A. Schirmer, M. A. Rude, X. Li, E. Popova and S. B. del Cardayre, Science, 2010, 329, 559–562 CrossRef CAS.
- H. R. Beller, E.-B. Goh and J. D. Keasling, Appl. Environ. Microbiol., 2010, 76, 1212–1223 CrossRef CAS.
- N. Ladygina, E. G. Dedyukhina and M. B. Vainshtein, Process Biochem., 2006, 41, 1001–1014 CrossRef CAS.
- J. A. Frias, J. E. Richman and L. P. Wackett, Appl. Environ. Microbiol., 2009, 75, 1774–1777 CrossRef CAS.
- J. Han and M. Calvin, Proceedings of the National Academy of Sciences, 1969, 64, 436–443 CrossRef CAS.
- D. J. Sukovich, J. L. Seffernick, J. E. Richman, J. A. Gralnick and L. P. Wackett, Appl. Environ. Microbiol., 2010, 76, 3850–3862 CrossRef CAS.
- R. Bachofen, Cell. Mol. Life Sci., 1982, 38, 47–49 CrossRef CAS.
- P. Metzger and C. Largeau, Appl. Microbiol. Biotechnol., 2005, 66, 486–496 CrossRef CAS.
- H. C. Pinkart, R. Devereux and P. J. Chapman, J. Microbiol. Methods, 1998, 34, 9–15 CrossRef CAS.
- L. P. Wackett, Microbial Biotechnology, 2008, 1, 211–225 CrossRef CAS.
- D. C. Ducat, J. C. Way and P. A. Silver, Trends Biotechnol., 2011, 29, 95–103 CrossRef CAS.
- M. O. Park, M. Tanabe, K. Hirata and K. Miyamoto, Appl. Microbiol. Biotechnol., 2001, 56, 448–452 CrossRef CAS.
- D. Robertson, S. Jacobson, F. Morgan, D. Berry, G. Church and N. Afeyan, Photosynth. Res., 2011, 107, 269–277 CrossRef CAS.
- T. Nagao and Y. Shimada, Lipid Technology, 2010, 22, 250–252 CrossRef CAS.
- J.-F. Rontani, in Handbook of hydrocarbon and lipid microbiology, ed. K. Timmis, Springer, New York, 2009, 1, 459–470 Search PubMed.
- L. M. Fixter, M. N. Nagi, J. G. McCormack and C. A. Fewson, J. Gen. Microbiol., 1986, 132, 3147–3157 CAS.
- D. B. Finkelstein, S. C. Brassell and L. M. Pratt, Geology, 2010, 38, 247–250 CrossRef CAS.
- M. Wältermann and A. Steinbüchel, J. Bacteriol., 2005, 187, 3607–3619 CrossRef.
- J.-F. Rontani, P. C. Bonin and J. K. Volkman, Appl. Environ. Microbiol., 1999, 65, 221–230 CAS.
- T. Nagao, Y. Watanabe, K. Hiraoka, N. Kishimoto, T. Fujita and Y. Shimada, J. Am. Oil Chem. Soc., 2009, 86, 1189–1197 CrossRef CAS.
- M. Wältermann, A. Hinz, H. Robenek, D. Troyer, R. Reichelt, U. Malkus, H.-J. Galla, R. Kalscheuer, T. Stöveken, P. von Landenberg and A. Steinbüchel, Mol. Microbiol., 2005, 55, 750–763 CrossRef.
- M. Wältermann, H. Luftmann, D. Baumeister, R. Kalscheuer and A. Steinbüchel, Microbiology-SGM, 2000, 146, 1143–1149 Search PubMed.
- R. Kalscheuer, T. Stöveken, U. Malkus, R. Reichelt, P. N. Golyshin, J. S. Sabirova, M. Ferrer, K. N. Timmis and A. Steinbüchel, J. Bacteriol., 2007, 189, 918–928 CrossRef CAS.
- S. Koritala, J. Am. Oil Chem. Soc., 1989, 66, 133–134 CrossRef CAS.
- N. J. Russell and J. K. Volkman, J. Gen. Microbiol., 1980, 118, 131–141 CAS.
- R. Kalscheuer, in Handbook of hydrocarbon and lipid microbiology, ed. K. Timmis, Springer, New York, 2009, 1, 527–535 Search PubMed.
- T. Ishige, A. Tani, Y. R. Sakai and N. Kato, Curr. Opin. Microbiol., 2003, 6, 244–250 CrossRef CAS.
- R. Bredemeier, R. Hulsch, J. O. Metzger and L. Berthe-Corti, Mar. Biotechnol., 2003, 5, 579–583 CrossRef CAS.
- T. Ishige, A. Tani, K. Takabe, K. Kawasaki, Y. Sakai and N. Kato, Appl. Environ. Microbiol., 2002, 68, 1192–1195 CrossRef CAS.
- S. Dewitt, J. L. Ervin, D. Howes-Orchison, D. Dalietos, S. L. Neidleman and J. Geigert, JAOCS, J. Am. Oil Chem. Soc., 1982, 59, 69–74 CrossRef CAS.
- S. M. Mudge and C. E. Duce, Environ. Pollut., 2005, 136, 209–220 CrossRef CAS.
- T. Kaneshiro, L. K. Nakamura, J. J. Nicholson and M. O. Bagby, Curr. Microbiol., 1996, 32, 336–342 CrossRef CAS.
- P. S. Keng, M. Basri, M. R. S. Zakaria, M. B. A. Rahman, A. B. Ariff, R. N. Z. A. Rahman and A. B. Salleh, Industrial Crops and Products, 2009, 29, 37–44 CrossRef CAS.
- S. R. Vali, Y. H. Ju, T. N. B. Kaimal and Y. T. Chern, J. Am. Oil Chem. Soc., 2005, 82, 57–64 CrossRef CAS.
- A. S. Carlsson, Production of wax esters in crambe: outputs from the EPOBIO project, CPL Press, Newbury, 2006 Search PubMed.
- S. M. Radzi, M. Basri, A. B. Salleh, A. Ariff, R. Mohammad, M. B. A. Rahman and R. Rahman, J. Chem. Technol. Biotechnol., 2006, 81, 374–380 CrossRef CAS.
- S. Gunawan, S. R. Vali and Y. H. Ju, J. Am. Oil Chem. Soc., 2006, 83, 449–456 CrossRef CAS.
- A. Steinbüchel, Industrial Bioprocessing, 2006, 28, 9 Search PubMed.
- L. Giraldo, G. Camargo, J. Tirano and J. C. Moreno-Pirajan, E-Journal of Chemistry, 2010, 7, 1138–1147 CAS.
- J. O. Metzger and U. Bornscheuer, Appl. Microbiol. Biotechnol., 2006, 71, 13–22 CrossRef CAS.
- T. J. Benson, R. Hernandez, M. G. White, W. T. French, E. E. Alley, W. E. Holmes and B. Thompson, Clean-Soil Air Water, 2008, 36, 652–656 CrossRef CAS.
- P. Kaewkool and K. Krisnangkura, Chem. Phys. Lipids., 2010, 163, 685–688 CrossRef CAS.
- S. N. Shah, B. K. Sharma and B. R. Moser, Energy Fuels, 2010, 24, 3189–3194 CrossRef CAS.
- V. I. Komarewsky, C. H. Riesz and G. Thodos, J. Am. Chem. Soc., 1939, 61, 2525–2527 CrossRef CAS.
- J. K. Volkman, Appl. Microbiol. Biotechnol., 2003, 60, 495–506 CAS.
- T. Czabany, K. Athenstaedt and G. n. Daum, Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids, 2007, 1771, 299–309 CrossRef CAS.
- D. C. Lamb, C. J. Jackson, A. G. S. Warrilow, N. J. Manning, D. E. Kelly and S. L. Kelly, Mol. Biol. Evol., 2007, 24, 1714–1721 CrossRef CAS.
- N. A. Sorkhoh, M. A. Ghannoum, A. S. Ibrahim, R. J. Stretton and S. S. Radwan, Journal of Applied Bacteriology, 1990, 69, 856–863 CrossRef CAS.
- N. Carballeira, A. Guzmn, J. Nechev, K. Lahtchev, A. Ivanova and K. Stefanov, Lipids, 2000, 35, 1371–1376 CrossRef CAS.
- D. C. Lamb, D. E. Kelly, N. J. Manning and S. L. Kelly, FEBS Lett., 1998, 437, 142–144 CrossRef CAS.
- S. M. Puhvel, The Journal of investigative dermatology, 1975, 64, 397–400 CAS.
- C. J. Jackson, D. C. Lamb, A. G. S. Warrilow, J. E. Parker, N. R. de Melo, D. E. Kelly and S. L. Kelly, Acta Chim. Slov., 2008, 55, 58–62 CAS.
- S. Razin and J. G. Tully, J. Bacteriol., 1970, 102, 306–310 CAS.
- S. Razin and Z. Shafer, J. Gen. Microbiol., 1969, 58, 327–339 CAS.
- S. Grille, A. Zaslawski, S. Thiele, J. Plat and D. Warnecke, Prog. Lipid Res., 2010, 49, 262–288 CrossRef CAS.
- C. Grunwald, Plant Physiol., 1971, 48, 653–655 CrossRef CAS.
- J. B. M. Rattray, in Microbial lipids, ed. C. Ratledge and S. G. Wilkinson, Academic Press, London; San Diego 19881555–698 Search PubMed.
- K. O. Isobe, M. Tarao, M. P. Zakaria, N. H. Chiem, L. Y. Minh and H. Takada, Environ. Sci. Technol., 2002, 36, 4497–4507 CrossRef CAS.
- S. J. Gaskell and G. Eglinton, Nature (Lond.), 1975, 254, 209–211 CrossRef CAS.
- D. V. McCalley, M. Cooke and G. Nickless, Water Res., 1981, 15, 1019–1025 CrossRef CAS.
- H. L. Falk, S. Goldfein and P. E. Steiner, Cancer Res., 1949, 9, 438–447 CAS.
- A. I. Rushdi, G. Ritter, J. O. Grimalt and B. R. T. Simoneit, Org. Geochem., 2003, 34, 799–812 CrossRef CAS.
- K. Hoffelner, H. Libert and L. Schmid, Zeitschrift fur Ernahrungswissenschaft, 1964, 25, 16–21 CrossRef CAS.
- W. Meredith, C.-G. Sun, C. E. Snape, M. A. Sephton and G. D. Love, Org. Geochem., 2006, 37, 1705–1714 CrossRef CAS.
- L. Schmid, Mitteilungen aus dem Gebiete der Lebensmitteluntersuchung und Hygiene, 1962, 53, 507–510 CAS.
- H. M. Alvarez, in Triglycerides and cholesterol research, ed. L. T. Welson, Nova Science Publishers, Inc., New York, 2006ch. VI, pp. 159–176 Search PubMed.
- H. M. Alvarez and A. Steinbüchel, Appl. Microbiol. Biotechnol., 2002, 60, 367–376 CrossRef CAS.
- R. Kalscheuer, H. Luftmann and A. Steinbuchel, Appl. Environ. Microbiol., 2004, 70, 7119–7125 CrossRef CAS.
- M. Kosa and A. J. Ragauskas, Trends Biotechnol., 2011, 29, 53–61 CrossRef CAS.
- K. Hori, M. Abe and H. Unno, J. Biosci. Bioeng., 2009, 108, 319–324 CrossRef CAS.
- N. J. Garton, H. Christensen, D. E. Minnikin, R. A. Adegbola and M. R. Barer, Microbiology-Sgm, 2002, 148, 2951–2958 CAS.
- H. M. Alvarez, F. Mayer, D. Fabritius and A. Steinbuechel, Arch. Microbiol., 1996, 165, 377–386 CrossRef CAS.
- H. M. Alvarez, M. F. Souto, A. Viale and O. H. Pucci, FEMS Microbiol. Lett., 2001, 200, 195–200 CrossRef CAS.
- J. Hall, M. Hetrick, T. French, R. Hernandez, J. Donaldson, A. Mondala and W. Holmes, J. Chem. Technol. Biotechnol., 2011, 86, 54–60 CrossRef CAS.
- G. Seltmann and O. Holst, in The bacterial cell wall, Springer, Berlin; New York, 2002ch. 2pp. 9–99 Search PubMed.
- M. H. Filgueiras and J. A. F. O. den Kamp, Biochimica et Biophysica Acta (BBA) - Lipids and Lipid Metabolism, 1980, 620, 332–337 CrossRef CAS.
- S. L. Neidleman, in Phospholipids handbook, ed. G. Cevc, Marcel Dekker, Inc., New York, 1993ch. 2pp. 23–38 Search PubMed.
- M. P. Lechevalier, in Practical handbook of microbiology, ed. W. M. O'Leary, CRC Press LLC, Boca Raton, FL, 1989, 455–562 Search PubMed.
- C. van den Does, N. Nouwen and A. J. M. Driessen,in Protein secretion pathways in bacteria, ed. B. Oudega, Kluwer Academic, Dordrecht; Boston, 2003, ch. 2pp. 23–50 Search PubMed.
- L. J. Forney, W. T. Liu, J. B. Guckert, Y. Kumagai, E. Namkung, T. Nishihara and R. J. Larson, Ecotoxicol. Environ. Saf., 2001, 49, 40–53 CrossRef CAS.
- A. Singh and J. M. Schnur, in Phospholipids handbook, ed. G. Cevc, Marcel Dekker, Inc., New York, 1993, ch. 7pp. 233–291 Search PubMed.
- R. R. C. New, in Phospholipids handbook, ed. G. Cevc, Marcel Dekker, Inc., New York, 1993, ch. 26pp. 855–878 Search PubMed.
- E. Revellame, R. Hernandez, W. French, W. Holmes, A. Forks and R. Callahan II, unpublished work. Search PubMed.
Footnote |
| † Electronic Supplementary Information (ESI) available: Bligh & Dyer extraction details, chemical structures of different compounds present in municipal activated sludge, analytical equipment and conditions. See DOI: 10.1039/c2ra01078j/ |
| This journal is © The Royal Society of Chemistry 2012 |
