N-heterocyclic carbenes with negative-charge tags: direct sampling from ionic liquid solutions†
Priscila M.
Lalli
a,
Thyago S.
Rodrigues
b,
Aline M.
Arouca
b,
Marcos N.
Eberlin
*a and
Brenno A. D.
Neto
*b
aThomson Mass Spectrometry Laboratory, Institute of Chemistry, University of Campinas, UNICAMP, Campinas, SP, Brazil, 13083-970. E-mail: eberlin@iqm.unicamp.br; Fax: +55 (19) 35213073
bUniversity of Brasilia, Chemistry Institute, IQ-UnB Campus Universitário Darcy Ribeiro, CEP, Brazil, 70904-970,, P.O. Box 4478, Brasília, DF, Brazil. E-mail: brenno.ipi@gmail.com; Fax: +55 (61) 32734149; Tel: + 55 (61) 31073867
First published on 2nd February 2012
Abstract
Herein we report on the sampling and characterization via electrospray ionization (tandem) mass spectrometry of free, long-lived N-heterocyclic carbenes (NHC) bearing negative-charge tags. To facilitate electrospray “ion fishing” via electrostatic ejection directly to the gas phase, negative-charge tagged NHC were formed in imidazolium-based ionic liquid solutions via double deprotonation of imidazolium cations bearing acid side groups, viz. CH2CO2H or (CH2)3SO3H. Via ESI-MS/MS experiments, the gaseous N-heterocyclic carbenes were found to display structurally diagnostic dissociations and bimolecular reactions. In perfect parallel to solution chemistry, the gaseous negative-charge tagged NHC were found to react promptly with CO2 by carboxylation to form negative-charge tagged imidazolium carboxylates. Neutral carbenes were inaccessible for mass spectrometry, but the charge tag strategy opens many new possibilities to explore the intrinsic chemistry of these key but elusive species.
Introduction
Carbenes1 are key but elusive chemical entities. Many reactions are known to involve carbenes as their pivotal intermediates but such highly reactive and fascinating hypervalent species were for a long time inaccessible to experimental observation. N-heterocyclic carbenes (NHC) such as those of the imidazolidene type 2 (Scheme 1) were the first carbenes to be isolated,2 and these “bottleable” carbenes have since found many applications.3 | ||
| Scheme 1 NHC (2) formation from imidazolium-based IL (1). | ||
Ionic liquids (IL)4 are of great academic, industrial and technological importance, and are used in many applications such as green solvents, catalysts and media for nanoparticle growth. Due to the relative acidity of the C2–H hydrogen (pKa in the range of 21–23),5 imidazolium (1)-based IL coexist with NHC (2);6 hence 2 have been shown to have a major influence on IL properties, such as stabilizing metal complex derivatives and metal nanoparticles and acting as catalysts for reactions performed in these “noninnocent” IL.7
Mass spectrometry (MS) is inherently blind to neutral species;8 hence neutral carbenes have been investigated via gas phase MS experiments mainly in their ionized forms9 or indirectly via neutralization–reionization MS (NRMS) experiments.10 Using the “charge tag” strategy,11 however, MS was made capable of handling “neutrals” and then, with the arrival of electrospray ionization (ESI),12 charge tagged species have been formed in solution and “fished”13 directly into the isolated gas phase environment for MS measurements and intrinsic reactivity investigations.14
Via ESI-MS of solutions of bromine salts of doubly, triply and quadruply charged imidazolium ions,15 ionic pairs of NHC bearing positive-charge tags (3) have been “fished” to the gas phase and dissociated to form free positive-charge tagged NHC (4). It also appeared that 4 was concurrently fished presumably due to the 3 ⇌ 4 solution equilibrium (Scheme 2).
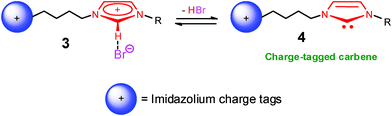 | ||
| Scheme 2 Positive-charge tagged NHC. | ||
Herein we report a negative-charge version of the “charge tag” approach which we apply to the direct and efficient fishing from ionic liquid solutions of free and long-lived NHC with negative-charge tags (Scheme 3).
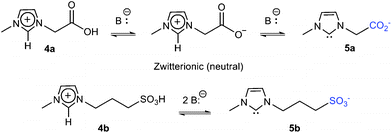 | ||
| Scheme 3 Negative-charge tagged NHC formation in IL solutions from deprotonation of imidazolium ions bearing acidic side groups. | ||
Results and discussion
To form NHC with negative-charge tags and to facilitate their ESI ejection in free, naked forms, we used imidazolium ions 4a,b with acid side groups attached to one of the ring nitrogens as precursors (Scheme 3).We postulated that double-deprotonation in solution of both acidic hydrogens of 4a,b by the action of a base should form the desired long-lived free charge-tagged NHC 5a,b in sufficient amounts for ESI fishing and MS detection and further manipulation in the gas phase. Fortunately, the mono-deprotonated zwitterionic species are neutral and therefore undetectable by ESI-MS, avoiding undesirable ion suppression effects from these intermediate species.
The chloride salts of 4a,b17 were therefore diluted in methanol to form 100 μM solutions and submitted to ESI-MS in the negative ion mode after addition of excess of KOtBu. Fortunately, the resulting ESI(−)-MS (Fig. 1) displayed predominant and abundant ions of m/z 139 (139.0529) and m/z 203 (203.0388) corresponding to the intact, free and long-lived negative-charge tagged NHC 5a,b. ESI16 is known to be a technique that does not form ions, but transfers those already present in solution directly to the gas phase; hence, 5a,b were, most likely, directly fished via electrostatic ion ejection18 from the IL solution.
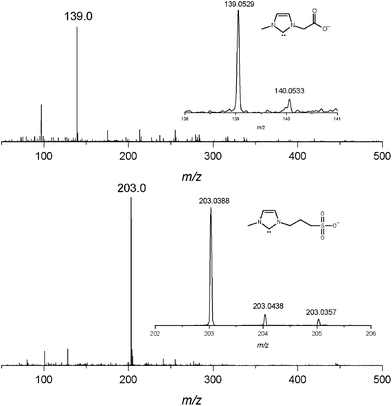 | ||
| Fig. 1 ESI(−)-MS of methanolic solutions of IL 4a and 4b after addition of KOtBu. Note the detection of the negative-charge tagged NHC 5a (m/z 139) and 5b (m/z 203). | ||
To confirm the formation and interception of the unprecedented 5a,b, these anions were isolated and subjected to dissociation and structurally diagnostic ion/molecule reactions17 with CO2. The resulting spectra after dissociation (Fig. 2) reveal structurally diagnostic chemistries that support the negative-charge tagged NHC structures 5a,b (Scheme 4).
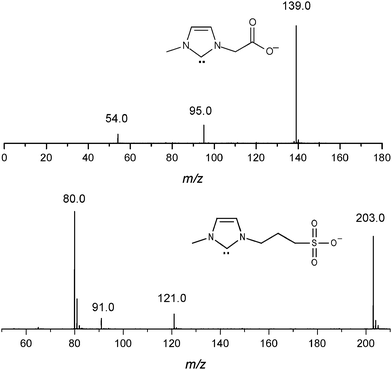 | ||
| Fig. 2 ESI(−)-MS/MS for collision-induced dissociation of the negative-charge tagged NHC 5a,b. | ||
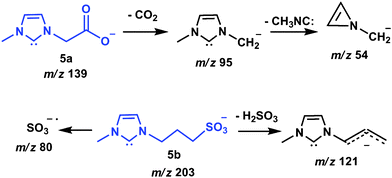 | ||
| Scheme 4 Proposed routes and structures for the collision induced dissociation of the gaseous negative-charge tagged NHC 5a,b. | ||
NHC are known to display rich reactivity,3 acting mostly as strong electrophiles or nucleophiles depending on the nature of the counter reactant.18 Recently, it was reported that NHC in IL solutions freely react with CO2 to form imidazolium carboxylates.19 The gaseous 5a,b were therefore reacted with CO2 (Fig. 3) and, in perfect parallel with the solution chemistry, found to react promptly with CO2 (Scheme 5) to form abundant negative-charge tagged imidazolium carboxylates 6a of m/z 183 (183.0432) and 6b of m/z 247 (247.0299).
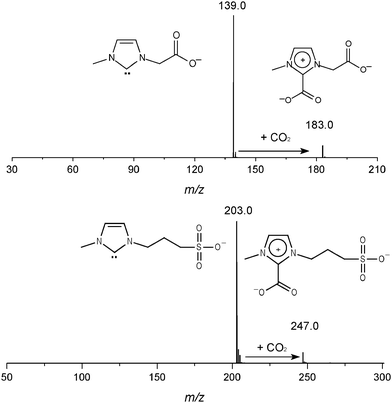 | ||
| Fig. 3 ESI(−)-MS/MS for ion/molecule reactions of the negative-charge tagged NHC 5a,b with CO2 generating 6a (m/z 183) and 6b (m/z 247). | ||
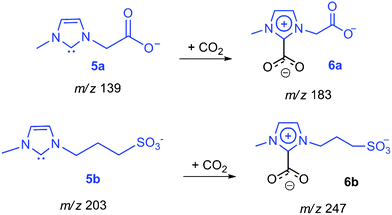 | ||
| Scheme 5 Gas-phase carboxylation (ion/molecule reaction) of 5a,b. | ||
Conclusions
Both the structurally diagnostic unimolecular dissociation chemistry upon collision with argon as well as the bimolecular ion/molecule reactivity towards CO2 provided evidence that, via ESI, long-lived free negative-charge tagged NHC can be efficiently fished from solutions of imidazolium ions bearing acid side groups directly to the isolated gas phase environment of a mass spectrometer. The gentle transfer of these “naked” NHC to the gas phase and the finding of the prompt addition of these ions to CO2, which perfectly parallels solution chemistry, opens unique opportunities to investigate, via MS experiments, the intrinsic physico-chemical properties and the solvent and counter-ion free reactivity of many types of reactants with gaseous NHC. These pivotal species can now be efficiently prepared in solution, gently ejected to the gas phase by ESI and properly manipulated inside mass spectrometers with the assistance of either positive15 or negative-charge tags.Acknowledgements
We thank the Brazilian Science Foundations FAPESP, CNPq, FAPDF, FINEP and MCT for financial assistance.References
- (a) W. Kirmse, Carbene Chemistry, Academic Press, New York, 1971 Search PubMed; (b) R. Breslow, J. Am. Chem. Soc., 1958, 80, 3719 CrossRef CAS; (c) H. W. Wanzlick, Angew. Chem., 1962, 74, 129 CrossRef CAS.
- A. J. Arduengo, R. L. Harlow and M. Kline, J. Am. Chem. Soc., 1991, 113, 361 CrossRef CAS.
- (a) N. Marion, S. Diez-Gonzalez and I. P. Nolan, Angew. Chem., Int. Ed., 2007, 46, 2988 CrossRef CAS; (b) N. Marion, S. Diez-Gonzalez and I. P. Nolan, Angew. Chem., Int. Ed., 2007, 46, 2988 CrossRef CAS; (c) W. A. Herrmann, Angew. Chem. Int. Edit., 2002, 41, 1291 Search PubMed; (d) O. Navarro, R. A. Kelly and S. P. Nolan, J. Am. Chem. Soc., 2003, 125, 16194 CrossRef CAS; (e) O. Back, M. A. Celik, G. Frenking, M. Melaimi, B. Donnadieu and G. Bertrand, J. Am. Chem. Soc., 2010, 132, 10262 CrossRef CAS; (f) O. Back, B. Donnadieu, P. Parameswaran, G. Frenking and G. Bertrand, Nat. Chem., 2010, 2, 369 CrossRef CAS; (g) V. Lavallo, A. El-Batta, G. Bertrand and R. H. Grubbs, Angew. Chem., Int. Ed., 2011, 50, 268 CrossRef CAS; (h) D. Enders and T. Balensiefer, Acc. Chem. Res., 2004, 37, 534 CrossRef CAS; (i) R. Singh, R. M. Kissling, M. A. Letellier and S. P. Nolan, J. Org. Chem., 2004, 69, 209 CrossRef CAS; (j) D. Enders and U. Kallfass, Angew. Chem., Int. Ed., 2002, 41, 1743 CrossRef CAS.
- (a) J. P. Hallett and T. Welton, Chem. Rev., 2011, 111, 3508–3576 CrossRef CAS; (b) N. V. Plechkova and K. R. Seddon, Chem. Soc. Rev., 2008, 37, 123–150 RSC; (c) J. Dupont and J. D. Scholten, Chem. Soc. Rev., 2010, 39, 1780–1804 RSC.
- T. L. Amyes, S. T. Diver, J. P. Richard, F. M. Rivas and K. Toth, J. Am. Chem. Soc., 2004, 126, 4366–4374 CrossRef CAS.
- (a) C. M. Jin, B. Twamley and J. M. Shreeve, Organometallics, 2005, 24, 3020–3023 CrossRef CAS; (b) L. S. Ott, M. L. Cline, M. Deetlefs, K. R. Seddon and R. G. Finke, J. Am. Chem. Soc., 2005, 127, 5758–5759 CrossRef CAS; (c) X. Mi, S. Luo and J.–P. Cheng, J. Org. Chem., 2005, 70, 2338–2341 CrossRef CAS; (d) J. D. Scholten and J. Dupont, Organometallics, 2008, 27, 4439–4442 CrossRef CAS; (e) J. Dupont and J. Spencer, Angew. Chem., Int. Ed., 2004, 43, 5296–5297 CrossRef CAS; (f) A. A. M. Lapis, L. F. de Oliveira, B. A. D. Neto and J. Dupont, ChemSusChem, 2008, 1, 759–762 CrossRef CAS.
- J. D. Scholten, G. Ebeling and J. Dupont, Dalton Trans., 2007, 5554–5560 RSC.
- F. Coelho and M. N. Eberlin, Angew. Chem., Int. Ed., 2011, 50, 5261–5263 CrossRef CAS.
- (a) R. R. Julian, J. A. May, B. M. Stoltz and J. L. Beauchamp, Int. J. Mass Spectrom., 2003, 228, 851 CrossRef CAS; (b) S. M. Villano, N. Eyet, W. C. Lineberger and V. M. Bierbaum, J. Am. Chem. Soc., 2008, 130 Search PubMed.
- (a) F. A. Wiedmann, J. N. Cai and C. Wesdemiotis, Rapid Commun. Mass Spectrom., 1994, 8, 804 CrossRef CAS; (b) F. Turecek, in Transient intermediates of chemical reactions by neutralization–reionization mass spectrometry, Topics in Current Chemistry, Springer-Verlab, Berlin, 1971, 225, p. 77 Search PubMed; (c) D. J. Lavorato, T. K. Dargel, W. Koch, G. A. McGibbon, H. Schwarz and J. K. Terlouw, Int. J. Mass Spectrom., 2001, 210, 43 CrossRef.
- K. G. Stirk and H. I. Kenttamaa, J. Am. Chem. Soc., 1991, 113, 5880 CrossRef CAS.
- J. B. Fenn, M. Mann, C. K. Meng, S. F. Wong and C. M. Whitehouse, Science, 1989, 246, 64 CAS.
- (a) C. Hinderling, C. Adlhart and P. Chen, Angew. Chem., Int. Ed., 1998, 37, 2685 CrossRef CAS; (b) C. Adlhart and P. Chen, Helv. Chim. Acta, 2000, 83, 2192 CrossRef CAS; (c) L. S. Santos, C. H. Pavam, W. P. Almeida, F. Coelho and M. N. Eberlin, Angew. Chem., Int. Ed., 2004, 43, 4330 CrossRef CAS; (d) A. Sabino, A. Machado, C. Correia and M. Eberlin, Angew. Chem., Int. Ed., 2004, 43, 2514 CrossRef CAS.
- (a) M. A. Schade, J. E. Feckenstem, P. Knochel and K. Koszinowski, J. Org. Chem., 2010, 75, 6848 CrossRef CAS; (b) P. Chen, Angew. Chem., Int. Ed., 2003, 42, 2832 CrossRef CAS.
- (a) E. Alcalde, N. Mesquida and M. Vilaseca, Rapid Commun. Mass Spectrom., 2000, 14, 1443–1147 CrossRef CAS; (b) E. Corilo, F. M. Nachtigall, P. V. Abdelnur, G. Ebeling, J. Dupont and M. N. Eberlin, RSC Adv., 2011, 1, 73–78 RSC.
- (a) J. B. Fenn, M. Mann, C. K. Meng, S. F. Wong and C. M. Whitehouse, Mass Spectrom. Rev., 1990, 9, 37–70 CrossRef CAS.
- (a) S. Gronert, Chem. Rev., 2001, 101, 329–360 CrossRef CAS; (b) J. S. Brodbelt, Mass Spectrom. Rev., 1997, 16, 91–110 CrossRef CAS; (c) R. G. Cooks, H. Chen, M. N. Eberlin, X. Zheng and W. A. Tao, Chem. Rev., 2006, 106, 188–211 CrossRef CAS; (d) M. N. Eberlin, J. Mass Spectrom., 2006, 41, 141–156 CrossRef CAS; (e) R. G. Cooks, H. Chen, M. N. Eberlin, X. Zheng and W. A. Tao, Chem. Rev., 2006, 106, 188–211 CrossRef CAS.
- (a) O. Illa, H. Gornitzka, V. Branchadell, A. Baceiredo, G. Bertrand and R. M. Ortuno, Eur. J. Org. Chem., 2003, 16, 3147–3152 CrossRef; (b) K. A. Williams and C. W. Bielawski, Chem. Commun., 2010, 46, 5166–5168 RSC.
- H. A. Duong, T. N. Tekavec, A. M. Arif and J. Louie, Chem. Commun., 2004, 112–113 RSC.
Footnote |
| † Experiments were performed using a QTOF (Waters Manchester, UK) mass spectrometer with a hybrid quadrupole/orthogonal acceleration time-of-flight (oa-TOF) geometry. Instrument ESI source conditions were as follows: capillary voltage 2.5 kV, sample cone 30 V, extraction cone 3 V, source temperature 100 °C, desolvation temperature 100 °C, and desolvation flow rate 300 mL min−1 of nitrogen. Ion/molecule reactions were performed by selecting the desired reactant at the quadrupole analyzer and by adding CO2 to the collision cell at a pressure of ca. 1 mbar. |
| This journal is © The Royal Society of Chemistry 2012 |
