High efficient PANI/Pt nanofiber counter electrode used in dye-sensitized solar cell†
Ziying
Tang
,
Jihuai
Wu
*,
Min
Zheng
,
Qunwei
Tang
,
Qin
Liu
,
Jianming
Lin
and
Jiangli
Wang
Institute of Materials Physical Chemistry, Huaqiao University, Quanzhou, 362021, P.R. China. E-mail: jhwu@hqu.edu.cn
First published on 6th March 2012
Abstract
A one-dimensional PANI nanofiber supported Pt nanoparticle film is prepared by a two-step electrochemical deposition method. The PANI/Pt film possesses high conductivity, surface area and catalytic activity. A dye-sensitized solar cell based on the PANI/Pt film achieves a high light-to-electric energy conversion efficiency of 7.69%.
Since the first prototype of a dye-sensitized solar cell (DSSC) was reported by O'Regan and Gratzel1 in 1991, it has aroused intensive interest over the past few decades due to its low cost, simple preparation procedure and high conversion efficiency over 12%.2 However, determining how to enhance its efficiency and decline costs is still a crucial issue. The counter electrode, as an important and expensive component in DSSCs, should have a low resistance and high electrocatalytic activity for an I−/I3− redox reaction to keep a low overvoltage and decelerated charge recombination.3 On the other hand, in order to reduce the cost of DSSCs, conductive polymers and carbon materials, such as PANIs (polyanilines),4 polypyrrole (PPy),5 carbon nanotubes (CTNs),6 graphene,7 poly(3,4-ethylenedioxythiophene) (PEDOT)8,9 have been widely attempted.
Among conductive polymers, PANI is one of the most attractive conducting polymers, due to its easy synthesis, high-conductivity, good environmental stability and interesting redox properties.10 The PANI nanofiber has attracted more interest because of its surface-to-volume ratios and potential applications in electrochemical devices.11 PANI nanofibers can be facilely synthesized by either chemical oxidation12 or electrochemical polymerization13 under mild conditions. Recently, several oxidation polymerization methods to fabricate polyaniline nanofibers without surfactants or templates have been developed, such as interfacial polymerizations,14 rapidly mixed reactions,12 dilute polymerizations,15 and two-step growths etc.16
Here, a polyaniline–platinum hybrid nanofiber (PANI/Pt) film is directly grown on a conductive glass substrate by a facile electrochemical deposition method on an electrochemical workstation. The resultant PANI/Pt film is used as the counter electrode for a DSSC, based on the PANI/Pt counter electrode, and the DSSC achieves a high conversion efficiency of 7.69%.
Scheme 1 shows the synthesis of the hybrid PANI/Pt film electrode, which is prepared by a two-step electrochemical deposition method (see ESI†). Firstly, a PANI nanofiber film is deposited onto a conductive glass substrate; secondly, the Pt nanoparticles are further deposited onto the surface of the PANI nanofibers, thus the hybrid PANI/Pt film on an indium tin oxide (ITO) glass substrate is formed.
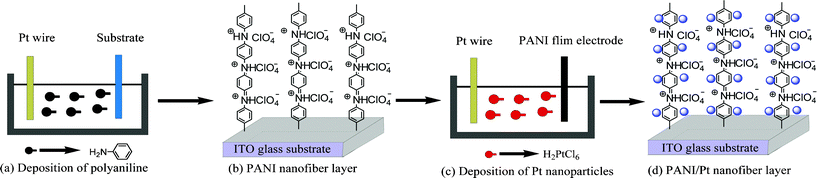 | ||
| Scheme 1 The two-step electrodeposition of the PANI/Pt hybrid electrode. | ||
Fig. 1 gives the morphologies of the Pt, PANI and PANI/Pt electrodes at different magnifications. Fig. 1a and b are the scanning electron microscope (SEM) images of the PANI electrode, it can be seen that the PANI nanofibers possess a large amount of pores and a one-dimensional structure, which provides a high effective surface area for the PANI film.17 The SEM images of the PANI/Pt are also presented in Fig. 1c and d, it can be observed that the PANI/Pt nanofiber’s one-dimensional structure still remains. Comparing the morphologies of the pristine PANI and the hybrid PANI/Pt, the PANI/Pt hybrid has a much rougher surface than the pristine PANI fiber does, which indicates that the Pt nanoparticles have been deposited onto the surface of the PANI nanofibers.11Fig. 1e and f are the SEM images of the Pt electrode, the Pt particles are ball-like and separately deposited on the substrate’s surface. From the magnified SEM image (Fig. 1f), the Pt particles are agglomerated and exist independently with a diameter of about 200 nm. The agglomerated structure of Pt is disadvantageous for its electrocatalytic performance.
 | ||
| Fig. 1 SEM images of the PANI electrode (a, b), PANI/Pt electrode (c, d) and Pt electrode (e, f) at different magnifications. | ||
The formation of the PANI/Pt hybrid nanofibers was characterized by energy dispersive X-ray spectroscopy (EDS). The EDS spectrum of the PANI sample (ESI,† Fig. S2a) shows the peaks corresponding to the C, N, Sn and O elements, while the PANI/Pt sample (ESI,† Fig. S2b) shows a new peak corresponding to Pt beside that of the C, N, Sn and O elements, which indicates the existence of Pt and confirms the successful synthesis of PANI/Pt hybrid nanofibers.
To further detect the structures of the PANI nanofibers and the PANI/Pt hybrid nanofibers, Fourier transform infrared spectroscopy (FTIR) spectra of the PANI and PANI/Pt nanofibers were measured and are shown in Fig. S3 (ESI†). For the PANI sample, all the PANI characteristic absorption peaks are observed in the spectra. Compared with the Pt sample, the PANI/Pt sample has no obvious absorption peak changes except that the peaks are slightly red or blue shifted. For example, the band at 1120 cm−1 in the PANI sample (corresponding to the C–H in-plane deformation, which has been used by Chiang and MacDiarmid18 as a measure of the extent of the electron delocalization in PANI) red shifts to 1140 cm−1 in the PANI/Pt sample; this implies that the PANI/Pt film has a higher degree of protonation and electrical conductivity than the PANI sample.19 The FTIR spectra further confirm the formation of the PANI/Pt nanofibers after the second electrodeposition process.
Using an I−/I3− redox as the supporting electrolyte, the cyclic voltammogram curves of the I−/I3− redox mediator for the PANI, Pt and PANI/Pt electrodes are shown in Fig. 2a. In DSSCs, electrons are injected into a photo-oxidized dye from I− ions in the electrolyte [eqn (1)], and the I3− ions produced are reduced on the counter electrode [eqn (2)].20
| 3I− − 2e− = I3− | (1) |
| I3− + 2e− = 3I− | (2) |
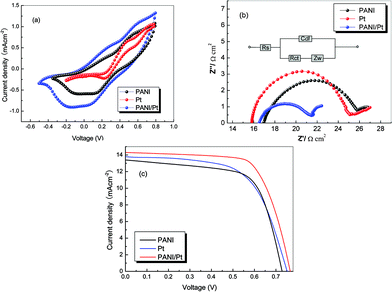 | ||
| Fig. 2 (a) Cyclic voltammograms (CVs) of the PANI, Pt and PANI/Pt electrodes using an acetonitrile solution containing 0.1 M LiClO4, 0.01 M LiI and 0.001 M I2 as the supporting electrolyte, scan rate = 10 mV s−1; (b) EIS spectra of the cells with two identical electrodes, the PANI, Pt and PANI/Pt were used as the working electrodes, respectively (Rs is serial resistance, Cdl is the constant phase element, Rct is the charge-transfer resistance and Zw is the diffusion impedance). (c) Photocurrent–voltage curves of the DSSCs with the PANI, Pt and PANI/Pt electrodes. | ||
The current peak of the positive potential (around 0.5 V) is assigned to the oxidation reaction (eqn (1)) and the current peak of the negative potential (about −0.2 V) is assigned to the reduction reaction (eqn (2)).21,22 In Fig. 2a, the PANI/Pt electrode shows a much larger current density for the I3− reduction and I− oxidation than both of the Pt and PANI electrodes, which means a faster redox reaction rate and a better electrocatalytic activity for the I−/I3− redox couple on the PANI/Pt electrode. This is ascribed to the unique 1-D nanofiber structure, the large active surface area and the increased catalytic active sites of the PANI/Pt electrode.23 According to the SEM observation, the PANI/Pt film is interconnected and microporous, thus, this structure is favorable for the electrolyte permeation and I3− reduction. Moreover, the Pt nanoparticles are uniformly dispersed on the surfaces of the 1-D PANI nanofibers, which provides more catalytic active sites and faster electron transportation channels, logically, leading to an enhanced electrochemical activity of the PANI/Pt hybrid film electrode.
Electrochemical impedance spectroscopy (EIS) measurements were carried out to compare the charge transfer and ion transport characteristics of the different electrodes. In Fig. 2b, the EIS results show well-defined single semicircles over the high frequency range, followed by short straight lines in the low-frequency region for the Pt electrodes. The PANI/Pt electrode has the lowest Rct of 2.51 Ω cm2, which is lower than both that of the PANI (4.53 Ω cm2) and Pt (4.73 Ω cm2) electrodes. The lower Rct for the PANI/Pt electrode implies that the reduction of I3− is more advantageous on the PANI/Pt electrode than that of the other two electrodes.8 In view of the excellent electrocatalytic activity, and lower charge transfer resistance, it is expected that the DSSC based on a PANI/Pt counter electrode can achieve an improved performance.
Fig. 2c shows the photocurrent–voltage curves of the DSSCs with the PANI, Pt and PANI/Pt electrodes under a simulated solar light illumination of 100 mW cm−2. The photovoltaic parameters of the DSSCs such as short current density (JSC), open voltage (VOC), fill factor (FF) and the light-to-electric energy conversion efficiency (η) are listed in Table 1.
| Counter electrode | Rct / Ω cm−2 | JSC / mA cm−2 | VOC / V | FF | η / % |
|---|---|---|---|---|---|
| PANI | 4.53 | 13.4 | 0.728 | 0.676 | 6.58 |
| Pt | 4.73 | 13.8 | 0.752 | 0.628 | 6.52 |
| PANI/Pt | 2.51 | 14.3 | 0.766 | 0.704 | 7.69 |
Among the three DSSCs, the DSSC with the PANI counter electrode shows the smallest JSC, which may be ascribed to the lower conductivity of the PANI film. While the DSSC with the Pt counter electrode has the smallest light-to-electric conversion efficiency and FF, which may be ascribed to the aggregation and discontinuous distribution of the Pt particles on the substrate. The DSSC with the PANI/Pt electrode shows the best photovoltaic performance and a light-to-electric conversion efficiency of 7.69%, which is a great improvement when compared with the DSSCs with the Pt and PANI counter electrodes. The higher light-to-electric efficiency for the DSSC with the PANI/Pt electrode is attributed to the following reasons: (i) according to the SEM images, a thin layer of Pt nanoparticles was evenly coated on the PANI nanofibers, which provides good conductivity and more catalytic active sites for the reduction of I3− compared with the PANI counter electrode; (ii) the microporous PANI/Pt nanofibers have higher accessible surface areas24 compared with the Pt counter electrode, which facilitates the electrolyte–electrode interfacial contact and contributes to the enhanced charge collection efficiency; (iii) from the CV and EIS measurements, the PANI/Pt hybrid counter electrode shows an enhanced electrochemical activity and lower Rct compared with the PANI and Pt electrodes. The above reasons are beneficial for the I3−/I− redox couple regeneration and the electron transportation, logically, the photovoltaic performance of the DSSC with a PANI/Pt electrode can be improved.
In summary, a one-dimensional PANI nanofiber supported Pt nanoparticle film was prepared by a two-step electrochemical deposition method. The PANI/Pt film possesses high conductivity, high surface area and high catalytic activity. Using the PANI/Pt film as a counter electrode, a dye-sensitized solar cell achieves a light-to-electric energy conversion efficiency of 7.69% under a simulated solar illumination with an intensity of 100 mW cm−2, which is higher than those with pure PANI or Pt counter electrodes.
Acknowledgements
This work was supported by the National High Technology Research and Development Program of China (No. 2009AA03Z217) and the National Natural Science Foundation of China (Nos. 90922028 and 51002053). Dr Bin Xu of the Institute of Urban Environment, Chinese Academy of Sciences, is also acknowledged for his assistance in the SEM measurements.References
- B. O'Regan and M. Gratzel, Nature, 1991, 353, 737 CrossRef CAS.
- A. Yella, H. Lee, H. Tsao, C. Yi, A. Chandiran, M. Nazeeruddin, E. Diau, C. Yeh, S. Zakeeruddin and M. Gratzel, Science, 2011, 334, 629 CrossRef CAS.
- A. Nattestad, A. Mozer, M. Fischer, Y. Cheng, A. Mishra, P. Bauerle and U. Bach, Nat. Mater., 2010, 9, 31 CrossRef CAS.
- C. Lin, K. Huang, J. Huang, C. Wu, C. Liu, H. Chen, C. Chu, J. Lin and K. Ho, J. Mater. Chem., 2011, 21, 10384 RSC.
- S. Jeon, C. Kim, J. Ko and S. Im, J. Mater. Chem., 2011, 21, 8146 RSC.
- Y. Xiao, J. Wu, G. Yue, J. Lin, M. Huang and Z. Lan, Electrochim. Acta, 2011, 56, 8545 CrossRef CAS.
- Y. Hu, H. Wang and B. Hu, ChemSusChem, 2010, 3, 782 CrossRef CAS.
- H. Tian, Z. Yu, A. Hagfeldt, L. Kloo and L. Sun, J. Am. Chem. Soc., 2011, 133, 9413 CrossRef CAS.
- S. Ahmad, J. Yum, X. Zhang, M. Grätzel, H. Butt and M. Nazeeruddin, J. Mater. Chem., 2010, 20, 1654 RSC.
- G. Torres-Gomez, E. M. Tejada-Rosales and P. Gomez-Romero, Chem. Mater., 2001, 13, 3693 CrossRef CAS.
- S. Guo, S. Dong and E. Wang, Small, 2009, 5, 1869 CrossRef CAS.
- J. Huang and R. Kaner, Angew. Chem., Int. Ed., 2004, 43, 5817 CrossRef CAS.
- R. David, M. William and J. Argersinger, J. Am. Chem. Soc., 1962, 84, 3618 CrossRef.
- J. Huang, S. Virji, B. Weiller and R. Kaner, J. Am. Chem. Soc., 2003, 125, 314 CrossRef CAS.
- N. Chiou and A. Epstein, Adv. Mater., 2005, 17, 1679 CrossRef CAS.
- Q. Tang, J. Wu, X. Sun, Q. Li, J. Lin and M. Huang, Chem. Commun., 2009, 2166 RSC.
- W. Huang, B. Humphrey and A. MacDiarmid, J. Chem. Soc., Faraday Trans., 1986, 82, 2385 RSC.
- J. Chiang and A. MacDiarmid, Synth. Met., 1986, 13, 193 CrossRef CAS.
- Q. Tang, J. Wu, X. Sun, Q. Li and J. Lin, Langmuir, 2009, 25, 5253 CrossRef CAS.
- D. Alexander and I. Popov, J. Am. Chem. Soc., 1958, 80, 5346 CrossRef.
- Y. Saito, W. Kubo, T. Kitamura, Y. Wada and S. Yanagida, J. Photoch. Photobio. A., 2004, 164, 153 CrossRef CAS.
- Q. Qin, J. Tao and Y. Yang, Synth. Met., 2010, 160, 1167 CrossRef CAS.
- S. Jeon, C. Kim, J. Ko and S. Im, J. Phys. Chem. C, 2011, 115, 22035 CAS.
- L. Liu, S. Yoo and S. Park, Chem. Mater., 2010, 22, 2681 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2ra20180a/ |
| This journal is © The Royal Society of Chemistry 2012 |
