The ohmic resistance effect for characterisation of carbon nanotube paste electrodes (CNTPEs)†
Tomáš
Mikysek
a,
Matěj
Stočes
a,
Ivan
Švancara
a and
Jiří
Ludvík
*b
aUniversity of Pardubice, Faculty of Chemical Technology, Department of Analytical Chemistry, Studentská 573, CZ-53210, Pardubice, Czech Republic
bJ. Heyrovský Institute of Physical Chemistry ASCR, v.v.i., Dolejškova, 3, CZ-182 23, Prague 8, Czech Republic. E-mail: jiri.ludvik@jh-inst.cas.cz; Tel: +420-266 053 217
First published on 6th February 2012
Abstract
In this study, carbon nanotube paste electrodes (CNTPEs) have been characterised using their ohmic resistance, RCNTPE, when examining a set of six different electrodes prepared from three types of CNTs and two altered pasting liquids (binders); the latter represented by traditional mineral or silicone oils. It is shown that simple measurements of the ohmic resistance can alternate to the standard electrochemical characterisation by cyclic voltammetry with model systems. During the proper experimentation, particular attention has been paid to the changes of the consistency and of the resultant RCNTPE in dependence of both main constituents used and upon the carbon-to-pasting liquid ratio chosen. The results obtained were compared to those of “classical” carbon paste electrodes (CPEs) prepared from common graphite powder. The most evident and surprising difference ascertained is associated with the optimal carbon-to-pasting liquid: whereas the ordinary CPEs could contain up to 20% (w/w) oily phase, the CNTPEs reached the optimum at about 60% (w/w) binder, while retaining still satisfactory physical as well as electrochemical properties. The explanation of this finding is given and the problems connected with homogeneity and stability of various carbon pastes are discussed.
1. Introduction
Carbon paste electrodes (CPEs)1 have attracted the attention of electrochemists and electroanalysts for more than a half a century2–4 as apparently the most popular laboratory-made and easy-to-renew carbon-based electrodes. Whereas the first four decades had seen CPEs usually containing traditional mixtures from mineral (or silicone) oils and spectral graphite, the last decade was mainly in the sign of new, “special carbon pastes”.5In these configurations, either one or even both main constituents are purposely replaced by alternative moieties, giving rise to qualitatively new carbon(-like) paste(-like) mixtures. Among a wide spectrum of such composite materials,4,5 one can highlight the “glassy carbon paste electrode” (GCPE, described initially in ref. 6 and actually applied some years later)7 as one of the first prototypes of new carbon paste electrodes. Mainly, however, there are “carbon nanotube paste electrodes” (CNTPEs; mentioned firstly in 19968 and intentionally introduced in 2003)9,10 and the so-called “carbon ionic liquid electrodes” (CILEs; presented a few years later),11,12 both having already established two separate sub-classes amongst the above-postulated special CPEs. To date, it can be estimated that CNTPEs and CILEs have been the key topic in ca. 200 scientific reports,4,5 covering equally both categories, including their mutual combination (CNT-ILE, described also in the late-2000s).13 From a retrospective point of view,3 the rapidly growing popularity of carbon nanotubes (CNTs14) and room-temperature ionic liquids (RTILs15) for the preparation of CNTPEs and CILEs is a logical consequence of the well-known flexibility of CPEs; in this case, their adaptability to the outputs of new progressive technologies that are still more reflected in modern electrochemistry and electroanalysis.4,5
Apart from whether traditional or special (new) types of CPEs are to be chosen, the experimental characterisation of the freshly made CP-mixtures is of principal importance for their later successful use in practical measurements. In contrast to common electrodes from compact and homogenous materials (like glassy carbon, platinum, gold, or mercury), each carbon paste represents more or less an individual whose specific properties are best obtainable using experimental—and, usually, empirical—characterisation prior to the proper employment.2,5,16,17
In the past, there had been numerous proposals to perform and even unify the corresponding procedures (see e.g.ref. 17–30) which would aid the definition of the relation(s) between the quality of both major constituents, the carbon-to pasting liquid ratio, and the resultant performance of a CPE.
Mostly, it could be accomplished via so-called testing measurements, involving physicochemical and electrochemical experimentation with suitable model systems. Often, both traditional and new types of carbon pastes are characterised with the aid of cyclic voltammetry and the respective current/potential characteristics, such as anodic and cathodic limits, background currents, or peak-potential parameters, EP(i), EP(j), and ΔE,31 for the electrode processes studied. Furthermore, there are also some advanced assays involving special studies on reaction kinetics,21,23,25,26 adsorption/extraction capabilities,27–30 the carbon paste surface treatments,25,32,33 the effect of dissolved oxygen,34,35 or possible paste ageing.2,35,40 Finally, CPEs intended for electroanalytical application are being subjected to additional examination in association with their electroanalytical performance in quantitative analysis,17 including special tests for the proper “adjustment” of chemical/biological modifiers at the CP-surface or in the CP-bulk).5,36–38 Due to the fact that many of the above-described procedures are inevitably time-consuming, the recommended characterisations are often omitted and the users of CPEs rely wholly on optimisation measurements during the development of selected method(s) and its/their practical verification. According to the authors' experience (see ref. 5 and references therein), however, such simplification may lead to less effective results should the final procedure be elaborated with improperly prepared or poorly operating carbon paste mixture(s).
Recently, we have shown (ref. 39 and mainly 40) that rather unattractive electrochemical characterisation can be replaced by considerably simpler experiments based on measurements of the ohmic resistance, RCP, of the CPE(s) under testing. Although the effect of ohmic resistance upon some electrochemical properties of CPEs had been anticipated already in the early era of CPEs and, since then, of interest in numerous studies,11,22,23,33,41–46 none of them concluded on a direct association between RCP and proportion of both main components, mentioning the rather undesirable effect of the increased amount of the binder,23,33 the way of carbon paste filling,22,46 or a very low resistance of common carbon pastes as such,2,5,47 being incomparably lower than that of related screen-printed (carbon paste) electrodes.48 As experimentally proved previously,40 involving also comparisons with the results of CV for model redox pairs, there are fairly defined relationships between the carbon paste composition, the quality of each major constituent, the CP-consistency, the actual RCP, and the resultant electrochemical behaviour of the corresponding CP-mixture(s). Moreover, newly described—and formerly unreported—characteristic dependences of RCP on the carbon–binder ratio (having the shape of an ice-hockey stick) have allowed us to explain the principle of this effect according to a Model of the Closest Arrangement of Solid Spheres (see ref. 40 and references therein).
One of the often discussed themes connected to metallic impurities of carbon nanotubes have to be also mentioned. It has been shown that residual metallic catalyst impurities in CNTs are, in some cases, responsible for the ‘exceptional electrocatalysis’ of CNTs. Therefore purification of such carbonaceous material has to be considered. Several procedures have been already employed (usually washing with concentrated nitric acid).9,49,50 On the other hand, the required purity of each carbonaceous material depends strictly on the application.
In this paper, a study subsequent to the previous one40 is reported, dealing in detail with the relationship between RCP, composition and physical as well as electrochemical properties of carbon nanotube paste electrodes (CNTPEs) using a set of six different mixtures. The results were compared with standard CPEs of the mineral/silicone oil type and significant differences were ascertained, reflecting specific properties of nanomaterials.
2. Experimental
2.1 Chemicals and reagents
Potassium hexacyanoferrate(III), K3[Fe(CN)6] (p.a. grade), and potassium chloride, KCl (Suprapur®), were used as received (both from Merck). Throughout the experimental work, all solutions were prepared from doubly deionised water obtained by passing through a laboratory purification system (Milli-Q, Millipore, USA).2.2 Apparatus
A modular electrochemical system AUTOLAB equipped with PGSTAT-30 (Metrohm Autolab B.V., Utrecht, The Netherlands) was used in combination with the three-electrode cell and controlled by a GPES 4.9 software (from the same manufacturer). All the measurements were carried out in the cyclic voltammetric mode (CV).The ohmic resistance of the individual carbon pastes was measured with a Voltcraft multimeter (model “VC 404”, Conrad Electronics, Germany). The Raman spectra were excited by 1.96 eV using He-Ne laser being recorded with a Labram HR spectrometer (Horiba Jobin Yvon) interfaced to a optical microscope (model “Olympus BH2”) at a 50-fold magnification. The laser power impinging the sample was below 0.5 mW and the spectrometer was calibrated in the F1g mode of Si at 520.2 cm−1.
2.3 Electrodes
For the carbon nanotube paste electrodes (CNTPE), three types of nanotubes were used (single-walled, SWCNTs, diameter < 2 nm; multi-walled, type MWCNTs 1030, diameter 10–30 nm and the type MWCNTs 4060, diameter 40–60 nm; all Shenzhen Nanotech Port Co., China) together with two types of binder: (i) paraffin oil (PO; from Merck), and (ii) silicone oil (SO; LUKOIL MV 8000; Lučební závody, Kolín, Czech Republic). Hence, six different carbon nanotube paste mixtures (SW-CNT/SO, SW-CNT/PO, MW-CNT1030/SO, MW-CNT1030/PO, MW-CNT4060/SO and MW-CNT4060/PO) were prepared, each with different ratio of carbon and the binder, by thoroughly hand-mixing using a pestle and mortar.Freshly made carbon nanotube pastes were packed into identical electrode holders designed in our laboratories.51,52 The electrode surface was renewed by smoothing on dry filter paper or by cutting off with a sharp edge before starting a new series of experiments.
A traditional carbon paste electrode (CPE) was represented by two classical variants—(i) mineral oil-based (the “C/PO” type) and viscous (ii) silicone oil containing analogue (“C/SO”); both being made from graphite powder (“CR-5”, originally a gear lubricant; Maziva, Týn nad Vltavou, Czech Republic) according to the above described procedure. A Ag/AgCl electrode (containing 3 M KCl as the inner electrolyte) as the reference and a Pt-sheet as the auxiliary electrode completed the cell.
2.4 Procedures
3. Results and discussion
3.1 Characterisation of the nanotubes by Raman spectra
Fig. 1 shows the Raman spectra of studied samples excited by 1.96 eV laser excitation energy. Obviously, there are significant differences in the obtained spectra. The content of SWCNTs can be qualitatively deduced from the presence of radial breathing mode (RBM). The frequency at about 205 cm−1 gives a diameter slightly larger than 1 nm (ref. 53).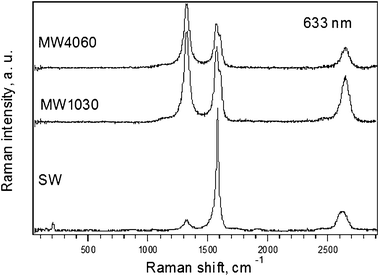 | ||
| Fig. 1 Raman spectra of MW4060, MW1030 and SW samples (from top to bottom) excited by 1.96 eV laser excitation energy. The spectra are offset for clarity but the intensity scale is the same for all spectra. | ||
According to the Kataura plot the laser excitation energy of 1.96 eV is in resonance with semiconducting carbon nanotubes in the range of diameters around 1 nm.54 This is also confirmed by a narrow tangential mode. Nevertheless, relatively low overall intensity of the spectra indicates a high content of amorphous carbon.
The analysis of the MWCNT is generally difficult. The MWCNTs do not exhibit the RBM mode; hence, it difficult to evaluate their diameter. Only rough estimation of the diameters can be made with the help of the double resonant G' mode, since it is also weakly dependent on the diameter of the tubes and its frequency tends to increase for the tubes with larger diameter.55 This is in agreement with our experimental observation since the MW samples should contain larger diameter tubes than SW and also the frequencies of the G' mode of the MW samples are slightly upshifted with respect to the G' frequency found for the SW sample.
To evaluate the number of defects, the D mode is often taken as a measure in the sp2 carbons. In other words this Raman feature gives the information about the quality of carbon nanotubes. Since it is difficult to compare absolute intensities of Raman bands for different samples, the ratio between the D and the G' mode intensities is usually taken. The calculated ratios D/G' (from the Lorentzian fits of the peak areas) for MW4060, MW1030 and SW samples were 1.4, 1.2, and 0.2, respectively. Hence, it can be concluded that the amount of defects in the studied samples has increased in the order SW < MW1030 < MW4060. Also, it can be quoted that, the D/G' ratio, without deeper analysis, gives only a rough qualitative estimation of defects, since this ratio is also a function of doping—because even natural doping is important56—and the laser excitation energy.57
3.2 Resistivity measurements
As mentioned above, the present study links up to our previous contribution40 dealing with traditional (and bare) carbon paste electrodes. The first part of the experiments was focused on ohmic resistance measurements of various CNTPEs, which were dependent on the paste composition. Similarly, like previously, the resistivity of electrodes with an increasing amount of binder in the carbon paste mixture did not dramatically change until the “break-point” was reached when the resistivity started to increase very strongly (see Fig. 2). Surprisingly, this effect was observed in carbon nanotube paste electrodes (CNTPE) with much higher content of the liquid binder compared to classical CPEs. Whereas the break point in CPE is around 20% w/w (30% v/v) of the binder, in CNTPE it is at about 60% (w/w). In addition to this, similarly unexpected was also the overall consistency of the pastes: whereas CPEs containing more than 30% oil had started to leak and the paste becomes notably fluid, CNTPEs with 60% of the binder were still compact and thus utilizable.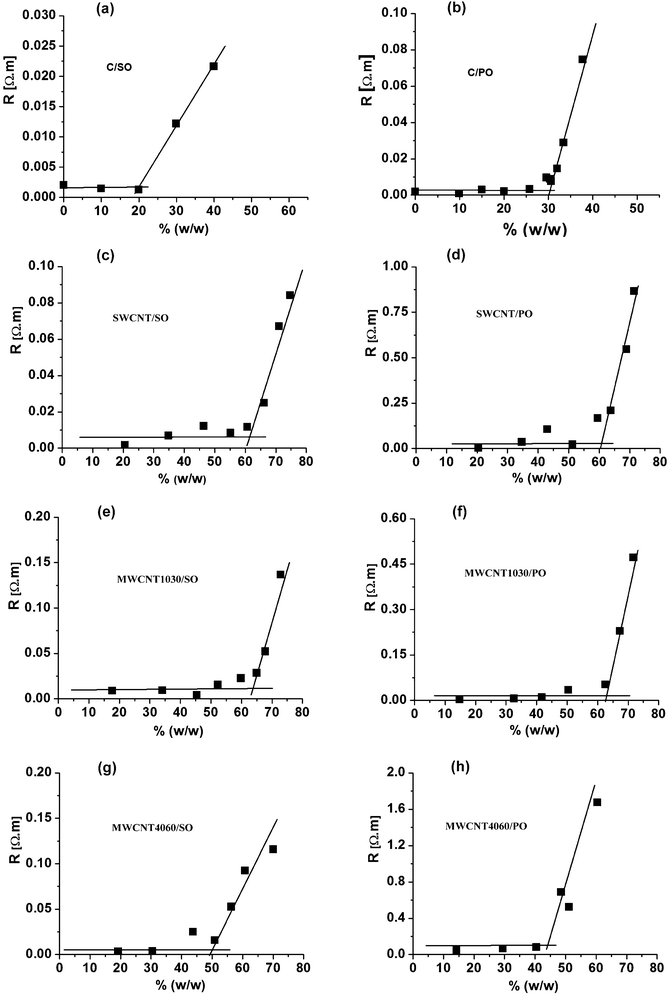 | ||
| Fig. 2 Dependence of the resistivity on content of binder in % (w/w) for eight different carbon paste mixtures. (a) “C/SO” type; (b) “C/PO” type; (c) “SWCNT/SO” type; (d) “SWCNT/PO” type; (e) “MWCNT1030/SO” type; (f) “MWCNT1030/PO” type; (g) “MWCNT4060/SO” type; (h) “MWCNT4060/PO” type; in all carbon paste mixtures, the content of the binder varied from 0–60% (v/v). | ||
The explanation of such a substantially different behaviour of CNTPEs is evidently connected with specific properties of this nanomaterial. In the case of a carbon paste mixture made from glassy carbon powder (GCPE type6,7) or pulverised graphite lubricant (common CPE), the particles have a shape that could be approximated by small spheres; therefore, the mentioned “close-packing” model can be applied: during addition of the first 20–30% of the oil, the binding liquid fills the external space between the particles being in the permanent contact. Since the penetration of oil into the structure of the carbon particles is not substantial, the additional amount of the oily phase (above those 20–30%) would cause the particles to start to float in the oil and the multiple contacts between the particles is progressively interrupted, resulting in the abrupt increase of the resistance (Fig. 2) accompanied by a decrease of the overall compactness.
In the case of CNTPEs, the remarkable shift of the breakpoint on the resistivity graph (Fig. 2) and simultaneously suitable density of the paste are most likely caused by two effects: (i) due to their fibrous microstructure and a bundle character, the nanotubes behave like a felt providing better electric contact among the carbon units with a higher proportion of the binder, as well as better mechanical properties of the electrode preventing the leakage of the oil and causing higher compactness; (ii) due to the relatively high proportion of the surface with respect to the volume hidden in the bulk of the material, which is a typical feature of all “nano” particles, carbon nanotubes are able to bind a relatively large amount of molecules of pasting liquid by their adhesion (adsorption) on the lipophilic surface of nanotubes. The eventual penetration of the oil into the SW-CNT is less probable because the estimated diameter of carbon nanotubes (see the above Raman characterisation) is approximately the same as the size of paraffin or polydimethylsiloxane molecules (about 1 nm58). The penetration of the oil into the MW-CNTs is not expected.
A shift was observed when comparing the resistivity dependence of pastes made from single-walled nanotubes (SW-CNT/SO and SW-CNT/PO) and multi-walled nanotubes (MW-CNT1030/SO and MW-CNT1030/PO), where the “breakpoint” is always at about 60% (w/w), with the mixtures based on larger multi-walled nanotubes (MW-CNT4060/SO and MWCNT4060/PO) where the “break-point” is reached “earlier”, already at about 50% (w/w).
This difference can be explained by a much lower total specific surface of the MW-CNT4060 material limiting the amount of adhered oil molecules, due to the fact that the carbon nanotubes of large diameter are filled with smaller nanotubes, the surface of which is inaccessible for the molecules of any binder.
3.3 Electrochemical characterisation
For the electrochemical characterisation of the carbon paste electrodes, data of the cyclic voltammetry experiments have been employed using a well-known system of [Fe(CN)6]4−/[Fe(CN)6]3− (with concentration of 5 mM in 1 M KCl), which is reversible in most of the usual electrodes. The theoretical separation between the cathodic and anodic peak should be ΔEp = 59 mV, however, on the carbon paste electrodes—even those of optimal composition—the experimentally observed potential difference is usually higher (from 100 up to 150 mV2,40). Behind the breakpoint, the excess of the binder manifests itself (in electrochemical properties) as an enormous increase of the ΔEp, being accompanied by the sudden increase of the resistance and, vice versa, the drop of conductivity.Such behaviour was found also in the case of all CNTPEs tested: the position of “electrochemical” and “resistivity” breakpoints (Fig. 3) corresponds well to each other (see also Table 1), analogously like in the case of ordinary CPE.40
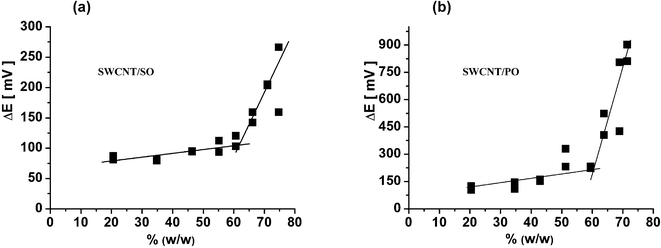 | ||
| Fig. 3 Dependence of the peak separation (from CV measurements) on the content of binder in % (w/w) for mixtures: (a) “SWCNT/SO” type and (b) “SWCNT/PO” type. Details of CV measurement are described in the experimental part. | ||
When comparing the average electrochemical behaviour of CPEs and CNTPEs, the peak separation, ΔEP = EP(A) − EP(C) , on the CV for the reversible system at the CNTPEs is lower (with 90–120 mV) than the ΔEP of a “standard” CPE (with 110–150 mV).
The nanotubes and their bundles in the paste mixture serve most probably as “nanowires”, enabling better conductivity than mutually touching carbon particles in CPE. This remarkable electrochemical merit, together with the advantageous carbon-to-pasting liquid ratio (saving the nanotube material) and suitable mechanical properties (compactness and lack of oil leakage) makes the CNTPEs a very promising electrode material. In addition to this, CNTs, as the objects of defined structure and composition, are convenient for aimed and highly effective chemical modification.2–5,59
Finally, the often discussed effect of nanotube-material purification and treatment on the electrochemical behaviour of such material has also been studied. For purification of nanotubes (SW and MW1030), the previously reported procedure9 was used. For electrochemical treatment (the so-called anodization or activation, resp.) the already proven procedure32 was applied. The results obtained by both procedures have, however, shown that there was no significant improvement in the peak separation.
3.4 Studies on ageing effect
The CV measurements reflect also the character and condition of the electrode surface which should offer—after the relevant renewing procedure—stable results at least for several weeks or even months. This part of study was therefore focused on the stability of prepared CNTPEs, as well as upon the ageing effect.2,5,35The above-mentioned series of CVs with all pastes was repeated after a period of five days, in order to check the eventual changes in composition. The CNTPEs made of single-walled nanotubes did not exhibit any significant change in the peak separation even after five days for both paraffin and silicon oils, respectively. In the case of multi-walled nanotubes paste mixtures, however, the choice of the binder had played a principal role: whereas the CNTPEs of both types of MW-CNTs containing paraffin oil as the binding liquid (i.e., MW-CNT1030/PO and MW-CNT4060/PO) showed no trend in the peak separation even after five days, the silicon oil-containing mixtures (MW-CNT1030/SO and MW-CNT4060/SO) exhibited the effect of so-called “self-homogenization”,5,17,35 while the electrode material acquired, after some time, somewhat better electrochemical properties.
In relevance to this, it was observed (cf.Fig. 2) that those pastes where silicon oil (SO) served as a binder have exhibited a lower resistance and thus better electrochemical properties. Even if the paraffin oil (PO) is generally being used for CPEs more frequently, for the CNTPEs silicone fluids seem to be more suitable.
3.5 Consistency and composition of CNTPEs
One of the rules for successful preparation of a CPE is the choice of an optimal ratio between the carbonaceous material and binding liquid, ensuring a proper consistency. When a very low amount of binder (around 5%) is used, the nanotube particles start to crumble and the electrode material is not ultimately compact. On the other hand, the selection of too high content of the binder (behind the breakpoint) may cause the electrode to start to “bleed”.36 Thus, the remaining interval suitable for use (from 10 to 50% of the binder) is unexpectedly large.Nevertheless, even here some differences were remarkable—at the lower limit of this region (i.e., 10–30% of the oil) the surface got hard, exhibiting rather composite character with more difficult renewal of the surface. The typical consistency of the paste made from CNTs has risen at 40–50% (w/w) of the pasting liquid which can then be taken as the recommended composition for practical use.
4. Conclusions
In this article, the measurements of the ohmic resistance of carbon paste mixtures (RCP) made from various types of carbon nanotubes (CNTPEs) have been shown to be a good tool for the characterisation of this electrode material. The entire study has been carried out with a sextet of CNTPEs, when using three different types of nanotubes (namely: one single-walled and two multi-walled CNTs) and two pasting liquids (either traditional mineral oil, PO, or silicone oil, SO).Characteristic dependences of RCP on the carbon-binder ratio (having the shape of an ice-hockey stick) have been obtained for various types of CNTPEs. This result is analogous to the previous study with CPEs.40 In the case of CNTPEs, a surprising substantial shift of the breakpoint towards higher proportion of the binding liquid was ascertained. The explanation is based on fundamental physical differences between classic and nano materials, namely in adhesion/adsorption abilities.
The most important relevance of these plots is their role as indicators for finding optimal composition of the paste. It can be generally concluded that for the practical use the composition just before the breakpoint should be chosen, where the resistance is still low, but the consistency has the character of the compact paste, the surface of which is easy to be renewed. Hence, the optimal carbon–binder ratio for CNTPEs is about 50% (w/w) CNTs with the same amount of binding oil, whereas for traditional CPEs about 80% (w/w) graphite and 20% (w/w) silicone oil is convenient. The fibre-like microstructure and the bundle character of the CNTs change not only the mechanical properties of the paste, but also improve the electrochemical quality.
Based on the presented studies with various CPEs and CNTPEs it is possible to conclude, that systematic measurements of the ohmic resistance can be recommended as effective and a very simple procedure for the experimental characterisation of every new type of carbon paste-based electrodes, providing consistent and at least equivalent information to rather time-consuming testing by means of cyclic voltammetry with model redox systems.
According to the often discussed theme of residual metallic catalyst impurities in CNTs, the purification has to be considered prior to preparation of each new CNTPE. In this contribution, we purified the CNTs using a recommended procedure. In our case, however, the purification did not change the experimental response.
Other types of new CPEs—namely, GCPEs and CILEs—as well as CPEs with various proportions of graphite/CNTPEs are currently under study.
Acknowledgements
The autors thank Dr Martin Kalbáč (J. Heyrovský Institute) for measurements of Raman spectra. The financial support of the research centre program (projects No. LC 06035, LC 510, and KONTAKT MEB091139) Ministry of Education, Youth and Sports of the Czech Republic) are gratefully acknowledged.References
- R. N. Adams, Anal. Chem., 1958, 30, 1576 CrossRef CAS.
- K. Kalcher, I. Švancara, R. Metelka, K. Vytřas and A. Walcarius, in The Encyclopedia of Sensors, Ed. C. A. Grimes, E. C. Dickey, and M. V. Pishko, American Scientific Publishers, Stevenson Ranch, 2006, vol. 4, 283–429 Search PubMed.
- I. Švancara, K. Vytřas, K. Kalcher, A. Walcarius and J. Wang, Electroanalysis, 2009, 21, 7 CrossRef.
- I. Švancara, A. Walcarius, K. Kalcher and K. Vytřas, Cent. Eur. J. Chem., 2009, 7, 598 CrossRef.
- I. Švancara, K. Kalcher, A. Walcarius, K. Vytřas, in Electroanalysis with Carbon Paste Electrodes, ed. C. Lochmuller, Taylor Francis/CRC Press, Boca Raton, 2012, in press Search PubMed.
- I. Švancara, M. Hvízdalová, K. Vytřas, K. Kalcher and R. Novotný, Electroanalysis, 1996, 8, 61 CrossRef.
- J. Wang, Ü.A. Kirgöz, J.-W. Mo, J.-M. Lu, A. N. Kawde and A. Muck, Electrochem. Commun., 2001, 3, 203 CrossRef CAS.
- P. J. Britto, K. S. V. Santhanam and P. M. Ajayan, Bioelectrochem. Bioenerg., 1996, 41, 121 CrossRef CAS.
- F. Valentini, A. Amine, S. Orlanducci, M. L. Terranova and G. Palleschi, Anal. Chem., 2003, 75, 5413 CrossRef CAS.
- M. D. Rubianes and G. A. Rivas, Electrochem. Commun., 2003, 5, 689 CrossRef CAS.
- H.-T. Liu, P. He, Z.-Y. Li, Y. Liu, J. Li, L.-Z. Zheng and J-H. Li, Electrochem. Solid-State Lett., 2005, 8, 17 CrossRef.
- H.-T. Liu, P. He, Z.-Y. Li, C.-N. Sun, L.-H. Shi, Y. Liu, G.-Y. Zhu and J.-H. Li, Electrochem. Commun., 2005, 7, 1357 CrossRef CAS.
- R. T. Kachoosangi, G. G. Wildgoose and R. G. Compton, Electroanalysis, 2007, 19, 483 CrossRef.
- S. Iijima, Nature, 1991, 354, 56 CrossRef CAS.
- P. Wasserscheid and T. Welton, in Electrochemical Properties of Ionic Liquids, VCH-Wiley Publishers, Weinheim, 2003 Search PubMed.
- I. Svancara, J. Zima and K. Schachl, Sci. Pap. Univ. Pardubice, Ser. A, 1998, 4, 49 CAS.
- I. Svancara and K. Schachl, Chem. Listy, 1999, 93, 490 CAS.
- C. Olson and R. N. Adams, Anal. Chim. Acta, 1960, 22, 582 CrossRef CAS.
- C. Olson and R. N. Adams, Anal. Chim. Acta, 1963, 29, 358 CrossRef CAS.
- Gy. Farsang, Acta Chim. Acad. Sci. Hung., 1965, 45, 163 CAS.
- R. Landsberg and R. Thiele, Electrochim. Acta, 1966, 11, 1243 CrossRef CAS.
- R. Neeb, I. Kiehnast and A. Narayanan, Fresenius' Z. Anal. Chem., 1972, 262, 339 CrossRef CAS.
- J. Lindquist, J. Electroanal. Chem., 1974, 52, 37 CrossRef CAS.
- P. Söderhjelm, J. Electroanal. Chem., 1976, 71, 109 CrossRef.
- M. E. Rice, Z. Galus and R. N. Adams, J. Electroanal. Chem., 1983, 143, 89 CrossRef CAS.
- C. Urbaniczky and K. Lundström, J. Electroanal. Chem., 1984, 176, 169 CrossRef CAS.
- N. A. Ulakhovich, E. P. Medyantseva and G. K. Budnikov, Zh. Anal. Khim., 1993, 48, 980 CAS.
- C. A. H. Chambers and J. K. Lee, J. Electroanal. Chem., 1967, 15, 309 CrossRef.
- J. Wang, B. K. Deshmukh and M. Bonakdar, J. Electroanal. Chem., 1985, 194, 339 CrossRef CAS.
- B. K. Deshmukh, Indian J. Chem., A, 1987, 26, 315 Search PubMed.
- D. K. Gosser Jr., in Cyclic Voltammetry: Simulation and Analysis of Reaction Mechanisms, VCH Publishers, New York, 1993 Search PubMed.
- K. Ravichandran and R. P. Baldwin, Anal. Chem., 1984, 56, 1744 CrossRef CAS.
- F. N. Albahadily and H. A. Mottola, Anal. Chem., 1987, 59, 958 CrossRef CAS.
- E. D. Kingsley and D. J. Curran, Anal. Chim. Acta, 1988, 206, 385 CrossRef CAS.
- I. Švancara and K. Vytřas, Anal. Chim. Acta, 1993, 273, 195 CrossRef.
- K. Kalcher, Electroanalysis, 1990, 2, 419 CrossRef CAS.
- K. Kalcher, J.-M. Kauffmann, J. Wang, I. Švancara, K. Vytřas, C. Neuhold and Z. Yang, Electroanalysis, 1995, 7, 5 CrossRef CAS.
- L. Gorton, Electroanalysis, 1995, 7, 23 CrossRef CAS.
- T. Mikysek, A. Ion, I. Švancara, K. Vytřas and F.G. Banica, in Sensing in Electroanalysis, Ed. K. Vytřas and K. Kalcher, Univ. Press Centre, Pardubice, 2005, vol. 1, pp. 19–27 Search PubMed.
- T. Mikysek, I. Švancara, M. Bartoš, K. Kalcher, K. Vytřas and J. Ludvík, Anal. Chem., 2009, 81, 6327 CrossRef CAS.
- D. G. Davis and M. E. Everhart, Anal. Chem., 1964, 36, 38 CrossRef CAS.
- A. L. Beilby and B. R. Mather, Anal. Chem., 1965, 37, 766 CrossRef CAS.
- L. S. Marcoux, K. G. Prater, B. G. Prater and R. N. Adams, Anal. Chem., 1965, 37, 1447 CrossRef.
- G. M. Schmid and G. W. Bolger, Clin. Chem., 1973, 19, 1002 CAS.
- D. E. Ormonde and R. D. O'Neill, J. Electroanal. Chem., 1990, 279, 109 CrossRef CAS.
- J. P. Kulys, P. Klitgaard and H. E. Hansen, Mater. Sci. Eng., C, 1996, 4, 39 CrossRef.
- K. Vytřas, I. Švancara, in Sensing in Electroanalysis, Ed. K. Vytřas, K. Kalcher, Univ. Press Centre, Pardubice, 2007, vol. 2, pp. 7–22 Search PubMed.
- K. Grennan, A. J. Killard and M. R. Smyth, Electroanalysis, 2001, 13, 745 CrossRef CAS.
- M. Pumera and Y. Miyahara, Nanoscale, 2009, 1, 260 RSC.
- M. Pumera, Langmuir, 2007, 23, 6453 CrossRef CAS.
- I. Švancara, R. Metelka and K. Vytřas, in Sensing in Electroanalysis, ed. K. Vytřas and K. Kalcher, Univ. Press Centre, Pardubice, 2005, vol. 1, pp. 7–18 Search PubMed.
- CZ Pat., 301 714, 2010 Search PubMed.
- P. T. Araujo, I. O. Maciel, P. B. C. Pesce, M. A. Pimenta, S. K. Doorn, H. Qian, A. Hartschuh, M. Steiner, L. Grigorian, K. Hata and A. Jorio, Phys. Rev. B, 2008, 77(241403/1) CAS.
- A. Jorio, P. T. Araujo, S. K. Doorn, S. Maruyama, H. Chacham and M. A. Pimenta, Phys. Status Solidi B, 2006, 243, 3117 CrossRef CAS.
- M. Kalbáč, L. Kavan, M. Zukalová and L. Dunsch, Carbon, 2004, 42, 2915 CrossRef.
- M. Kalbáč and L. Kavan, Carbon, 2010, 48, 832 CrossRef.
- M. Kalbáč, Y. P.Hsieh, H. Farhat, L. Kavan, M. Hofmann, J. Kong and M. S. Dresselhaus, Nano Lett., 2010, 10, 4619 CrossRef.
- S. Grigoras and T. H. Lane, in Silicone Based Polymer Science; Adv. Chem. Ser. 224, ed. J. M. Zeigler, F. W. G Fearon, American Chemical Society, Washington , 1990 Search PubMed.
- E. Nazaruk, K. Sadowska, J. F. Biernat, J. Rogalski, G. Ginalska and R. Bilewicz, Anal. Bioanal. Chem., 2010, 398, 1651 CrossRef CAS.
Footnote |
| † This article is part of a web theme in Analyst and Analytical Methods on Future Electroanalytical Developments, highlighting important developments and novel applications. Also in this theme is work presented at the Eirelec 2011 meeting, dedicated to Professor Malcolm Smyth on the occasion of his 60th birthday. |
| This journal is © The Royal Society of Chemistry 2012 |
