Synthesis of 2,5-disubstituted thiophenes via metal-free sulfur heterocyclization of 1,3-diynes with sodium hydrosulfide†
Jialiang
Tang
a and
Xiaoming
Zhao
*ab
aDepartment of Chemistry, Tongji University, 1239 Siping Road, Shanghai, 200092, P.R. China. E-mail: xmzhao08@mail.tongji.edu.cn
bCollege of Environrmental Science and Engineering, Tongji University, 1239 Siping Road, Shanghai, 200092, P.R. China
First published on 25th April 2012
Abstract
Metal-free sulfur heterocyclization of various 1,3-diynes with sodium hydrosulfide under mild conditions has been realized. This method provides 2,5-disubsituted thiophenes in good to excellent yields.
2,5-Disubstituted thiophenes are of great importance with regards to synthetic intermediates and have found multiple applications in medicinal, agricultural, and materials chemistry.1
Traditional methods for the preparation of 2,5-disubstituted thiophenes are sulfur heterocyclization of the corresponding 1,4-dicarbonyl intermediates with sulfurating reagents such as P2S5,2 H2S/HCl,3 bis-(trimethylsilyl) sulfide,4 Steliou's reagent,5 and Lawesson's reagent.6 Functionalized thiophenes can be synthesized via ring closure of either 1-mercapto-1,3-butaidiene derivatives7 or thioacrylate ester and α-halocarbonyl compounds with use of excess triethylamine.8 Recently, an efficient synthetic approach to 2,5-disubstituted thiophenes has been developed through transition metal-catalyzed tandem S-alkenylation of 1,4-diiodo-1,3-dienes with potassium sulfide.9 In addition, 1,3-diynes are very important synthetic intermediates for the synthesis of five-membered heterocycles catalyzed by transition metals,10 however, synthesis of 2,5-disubsituted thiophenes from 1,3-diynes is less developed.11 As a result, new synthetic methods for the preparation of thiophenes from economic sulfur sources are highly desirable.
Herein, we first report the formation of 2,5-disubstituted thiophenes by metal-free sulfur heterocylization of 1,3-diynes with sodium hydrosulfide (NaHS), which produces 2,5-disubstituted thiophenes in good to excellent yields.
We aimed to optimize the reaction conditions for the heterocyclization of 1,3-diynes with sodium hydrosulfide (NaHS 2a) to form 2,5-disubstituted thiophenes. A preliminary reaction tested the reaction of 1a with NaHS 2a in the presence of CuI in DMF and 3a was obtained in good yield.10 Remarkably, in the absence of CuI, this reaction also gave thiophene 3a in good yield under similar conditions. Encouraged by these results, the effect of solvents on sulfur heterocyclization of 1,4-diphenylbuta-1,3-diyne 1a with NaHS 2a was studied. For example, a series of solvents including DMF, toluene, THF, THF–H2O (3/1), DCM, Et2O, CH3OH, CH3CN, and dioxane were screened (entry 1–9, Table 1). We found that DMF is the best choice of solvent for this reaction (entry 1, Table 1). CH3CN gave the desired product in 17% yield (entry 9, Table 1). Other solvents are ineffective for this heterocyclization reaction (entries 2–8, Table 1). This reaction was performed at a temperature range 0°C to 50 °C and either room temperature or 50 °C gave the best result after the completion of this reaction (entries 1, 10 and 11, Table 1). Variation of the ratio 1a/2a has a considerable influence on the efficiency of this reaction. A 1/3 ratio of 1a/2a resulted in the highest yield (99%, entries 1, 12 and 13, Table 1).
|
|
||||||
|---|---|---|---|---|---|---|
| Entry | Nu | Solvent | 1a/2a | T/°C | t (h) | Yield (%)b |
| a Reaction conditions: 1,4-diphenylbuta-1,3-diyne 1a (0.2 mmol), sulphide 2 in solvent (2.0 mL) at appropriate temp under air atmosphere. b Isolated yield. | ||||||
| 1 | NaHS | DMF | 1/3 | 25 | 1 | 99 |
| 2 | NaHS | Toluene | 1/3 | 25 | 12 | — |
| 3 | NaHS | THF | 1/3 | 25 | 12 | — |
| 4 | NaHS | THF/H2O | 1/3 | 25 | 12 | — |
| 5 | NaHS | DCM | 1/3 | 25 | 12 | — |
| 6 | NaHS | Et2O | 1/3 | 25 | 12 | — |
| 7 | NaHS | CH3OH | 1/3 | 25 | 12 | — |
| 8 | NaHS | Dioxane | 1/3 | 25 | 12 | — |
| 9 | NaHS | CH3CN | 1/3 | 25 | 12 | 17 |
| 10 | NaHS | DMF | 1/3 | 0 | 2 | 95 |
| 11 | NaHS | DMF | 1/3 | 50 | 0.2 | 99 |
| 12 | NaHS | DMF | 1/2 | 25 | 4 | 91 |
| 13 | NaHS | DMF | 1/1 | 25 | 3 | 63 |
| 14 | NaHS | DMF | 2/1 | 25 | 1 | 39 |
| 15 | Na2S | DMF | 1/3 | 25 | 2 | 98 |
| 16 | Na2S·9H2O | DMF | 1/3 | 25 | 3 | 99 |
| 17 | S | DMF | 1/3 | 25 | 48 | — |
Different types of sulfur reagents were further explored under the optimized reaction conditions and either sodium sulfide12 (Na2S) or sodium sulfide hydrate12a–d (Na2S·9H2O) gave 2,5-disubstituted thiophenes 3a in excellent yields (entry 15–16, Table 1). We found that this heterocyclization reaction completely failed when sulfur13 was employed as a nucleophile (entry 17, Table 1).
Having established the optimal conditions, we further tested the scope of 1,3-diynes 1 in order to explore the generality of this heterocyclization reaction. As shown in Table 2, phenyl- and all aromatic 1,3-diynes 1a–f with either electron-donating groups (e.g., 3-Me, 4-Me, 4-MeO, and 4-pentyl) or electron- withdrawing groups (e.g., 4-Br and 4-F) on the phenyl ring produced the corresponding 2,5-disubstituted thiophenes 3a–f in good to excellent yields (76–99%). 1,3-Diyne 1h, with a pentyl group on the phenyl ring, required a longer reaction time (entry 5). Thiophene 3e contains the 4-bromophenyl group, which could be employed in palladium-mediated couplings. It was noteworthy that thiophene 3h has been prepared in good yield, and is a liquid crystal.1d 1,4-Di(pyridin-2-yl)buta-1,3-diyne 1g afforded 2,5-di(pyridin-2-yl)thiophene 3g in 86% yield as well (entry 8).
|
|
||||
|---|---|---|---|---|
| Entry | R | t (h) | Product, 3 | Yield (%)b |
| a Reaction conditions: 0.2 mmol of 1,3-diyne 1, 0.6 mmol of NaHS 2a, and 2.0 mL of DMF at room temperature under air atmosphere. b Isolated yield. c This reaction was performed at 80 °C. | ||||
| 1 | C6H5 | 1 | a | 99 |
| 2 | 3-MeC6H4 | 2 | b | 99 |
| 3c | 4-MeC6H4 | 12 | c | 96 |
| 4c | 4-MeOC6H4 | 12 | d | 76 |
| 5 | 4-Pentylbenzene | 48 | h | 94 |
| 6 | 4-BrC6H4 | 1 | e | 98 |
| 7 | 4-FC6H4 | 1 | f | 99 |
| 8 | 2-Pyridyl | 1 | g | 86 |
Taking economic factors into consideration, we further explored metal-free sulfur heterocyclization of a range of 1,3-diynes 1 with Na2S·9H2O 2b under the optimized conditions (Table 3). All aromatic 1,3-diynes 1a–f with either electron-donating groups (e.g., 3-Me, 4-Me, 4-MeO, and 4-pentyl) or electron-atractive groups (e.g., 4-Br and 4-F) on the phenyl ring led to 2,5-disubstituted thiophenes 3a–f in excellent yields (91–99%). 1,4-Di(pyridin-2-yl)buta-1,3-diyne 1g gave 72% of 2,5-di(pyridin-2-yl)thiophene 3g (entry 8).
|
|
||||
|---|---|---|---|---|
| Entry | R | t (h) | Product | Yield (%)b |
| a Reaction conditions: 0.2 mmol of 1,3-diyne 1, 0.6 mmol of Na2S·9H2O 2b, and 2.0 mL of DMF at room temperature under air atmosphere. b Isolated yield. c This reaction was performed at 80 °C. | ||||
| 1 | C6H5 | 3 | 3a | 99 |
| 2 | 3-MeC6H4 | 8 | 3b | 99 |
| 3 | 4-MeC6H4 | 32 | 3c | 91 |
| 4c | 4-MeOC6H4 | 12 | 3d | 99 |
| 5c | 4-pentylbenzene | 3 | 3h | 99 |
| 6 | 4-BrC6H4 | 3 | 3e | 98 |
| 7 | 4-FC6H4 | 3 | 3f | 99 |
| 8 | 2-Pyridyl | 3 | 3g | 72 |
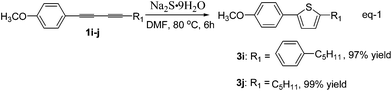
The synthesis of 2,5-disubstituted thiophenes from unsymmetrical 1,3-diynes 1 and Na2S·9H2O 2b was also investigated. As is known, arylated thiophenes could be prepared by C–H bond alkylation of thiophenes with iosoarenes,14 however, it is difficult to synthesize alkylated thiophenes by means of this method. Representative examples for sulfur heterocyclization of unsymmetrical 1,3-diynes 1 with Na2S·9H2O 2b are shown in Table 3. 1-(4-(4-Methoxyphenyl)buta-1,3-diynyl)-4-pentylbenzene (1i), which was synthesized by a known method,15 reacted with Na2S·9H2O 2b in DMF at 80 °C to form the corresponding thiophene 3i in 97% yield, which is a potential liquid crystal. In a similar synthetic sequence, 2-(4-methoxyphenyl)-5-pentylthiophene16 (3j) was prepared from 1-methoxy-4-(nona-1,3-diynyl)benzene (1j) in 99% yield.
In summary, we have developed a practical approach for the preparation of 2,5-disubsituted thiophenes via metal-free sulfur heterocyclization of a variety of 1,3-diynes with sodium hydrosulfide or sodium sulfide, which provided 2,5-disubsituted thiophenes in good to excellent yields. To the best of our knowledge, this is the first reported example of sodium hydrosulfide being used as the sulfur source for the synthesis of 2,5-disubsituted thiophenes. We are currently investigating applications of this methodology to generate other substituted thiophenes.
Acknowledgements
We gratefully acknowledge the Pu Jiang program of Shanghai (2010–2013), the NSFC (20 942 003), Key Laboratory of Fluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, and State Key Laboratory of Fine Chemicals, Dalian University of Technology (KF1006) for generous financial support.References
- (a) S. Gronowitz, A. B. Hornfeldt, Thiophenes, Elsevier, Oxford, 2004. Search PubMed; (b) J. B. Press, In The Chemistry of Heterocyclic Derivatives. Thiophene and its Derivatives, ed. Gronowitz, S., Wiley, New York, 1984, Vol. 44 (Part 1), pp. 353–456. Search PubMed; (c) J. B. Press, In Heterocyclic Chemistry. Thiophene and its Derivatives; Gronowitz, S., Ed.; Wiley: New York, 1990; Vol. 44 (Part 4), pp. 397–502. Search PubMed; (d) N. L. Campbell, W. L. Duffy, G. I. Thomas, J. H. Wild, S. M. Kelly, K. Bartle, M. O'Neill, V. Minter and R. P. Tuffin, J. Mater. Chem., 2002, 12, 2706 RSC; (e) L. Y. Lin, C. W. Lu, W. C. Huang, Y. H. Chen, H. W. Lin and K. T. Wong, Org. Lett., 2011, 13, 4962 CrossRef CAS; (f) T. S. Osdene, P. B. Russell and L. Rane, J. Med. Chem., 1967, 10, 431 CrossRef CAS; (g) P. S. Bailey and H. H. Hwang, J. Org. Chem., 1985, 50, 1779 CrossRef; (h) C. Kim, M. C. Chen, Y. J. Chiang, Y. J. Guo, J. Youn, H. Huang, Y. J. Liang, Y. J. Lin, Y. W. Huang, T. S. Hu, G. H. Lee, A. Facchetti and T. J. Marks, Org. Electron., 2010, 11, 801 CrossRef CAS.
- (a) C. Paal, Ber., 1885, 18, 367 CrossRef; (b) L. Knorr, Ber., 1885, 18, 299 CrossRef.
- (a) B. P. David and D. W. Boykin, J. Med. Chem., 1977, 20, 1219 CrossRef; (b) E. Campaigne, Chem. Rev., 1946, 39, 1 CrossRef CAS.
- F. Freeman, M. Y. Lee, H. Lu, X. Wang and E. Rodriguez, J. Org. Chem., 1994, 59, 3695 CrossRef CAS.
- F. Freeman, D. S. H. L. Kim and E. Rodriguez, J. Org. Chem., 1992, 57, 1722 CrossRef CAS.
- (a) K. Kaleta, B. T. Makowski, T. Soós and R. Dembinski, Org. Lett., 2006, 8, 1625 CrossRef; (b) V. M. Sonpatki, M. R. Herbert, L. M. Sandvoss and A. J. Seed, J. Org. Chem., 2001, 66, 7283 CrossRef CAS; (c) A. A. Kiryanov, P. Sampson and A. J. Seed, J. Org. Chem., 2001, 66, 7925 CrossRef CAS; (d) C. E. Hewton, M. C. Kimber and D. K. Taylor, Tetrahedron Lett., 2002, 43, 3199 CrossRef CAS; (e) D. R. Shridhar, M. Jogibhukta, P. S. Rao and V. K. Handa, Synthesis, 1982, 1061 CrossRef CAS.
- (a) E. Campaigne and R. E. Cline, J. Org. Chem., 1956, 21, 39 CrossRef CAS; (b) E. Campaigne and W. O. Foye, J. Am. Chem. Soc., 1952, 17, 1405 CAS.
- T. J. Katz and N. Acton, J. Am. Chem. Soc., 1969, 91, 208 CrossRef.
- (a) W. You, X. Y. Yan, Q. Liao and C. J. Xi, Org. Lett., 2010, 12, 3930 CrossRef CAS; (b) B. Gabriele, R. Mancuso, E. Lupinacci, L. Veltri, G. Salerno and C. Carfagna, J. Org. Chem., 2011, 76, 8277 CrossRef CAS.
- (a) V. Lavallo, G. D. Frey, B. Donnadieu, M. Soleihavoup and G. Bertrand, Angew. Chem., Int. Ed., 2008, 47, 5224 CrossRef CAS; (b) S. Kramer, J. L. H. Madsen, M. Rottlander and T. Skrydstrup, Org. Lett., 2010, 12, 2758 CrossRef CAS; (c) Q. W. Zheng and R. M. Hua, Tetrahedron Lett., 2010, 51, 4512 CrossRef CAS.
- (a) K. T. Potts, S. A. Nye and K. A. Smith, J. Org. Chem., 1992, 57, 3895 CrossRef CAS; (b) F. Freeman, H. Y. Lu and Q. B. Zeng, J. Org. Chem., 1994, 59, 4350 CrossRef CAS.
- Sodium sulfide (Na2S) has been successfully used as the sulfur source for the preparation of thiophene derivatives, for details, see: (a) C. Maeda, T. Yoneda, N. Aratani, M. C. Yoon, J. M. Lim, D. Kim, N. Yoshioka and A. Osuka, Angew. Chem., Int. Ed., 2011, 50, 5691 CrossRef CAS; (b) T. Kowada, T. Kuwabara and K. Ohe, J. Org. Chem., 2010, 75, 906 CrossRef CAS; (c) B. Baytekin, S. S. Zhu, B. Brusilowskij, J. Illigen, J. Ranta, J. Huuskonen, L. Russo, K. Rissanen, L. Kaufmann and C. A. Schalley, Chem.–Eur. J., 2008, 14, 10012 CrossRef CAS; (d) F. Morisaki, M. Kurono, K. Hirai and H. Tomioka, Org. Biomol. Chem., 2005, 3, 431 RSC; (e) S. Kozhushkoc, T. Haumann, R. Boese, B. Knieriem, S. Scheib, P. Bauerle and A. Meijere, Angew. Chem., Int. Ed. Engl., 1995, 34, 781 CrossRef; (f) J. Alzeer and A. Vasella, Helv. Chim. Acta, 1995, 78, 177 CrossRef CAS; (g) T. Kitamura, C. H. Lee, Y. Taniguchi, Y. Fujiwara, Y. Sano and M. Matsumoto, Mol. Cryst. Liq. Cryst. Sci. Technol., Sect. A, 1997, 293, 239 CrossRef CAS.
- T. Okamoto, K. Kudoh, A. Wakamiya and S. Yamaguchi, Org. Lett., 2005, 7, 5301 CrossRef CAS.
- (a) J. Wang, M. A. Seefeld and J. Luengo, Tetrahedron Lett., 2011, 52, 6346 CrossRef CAS; (b) K. Ueda, S. Yanagisawa, J. Yamaguchi and K. Itami, Angew. Chem., Int. Ed., 2010, 49, 8946 CrossRef CAS; (c) H. Q. Do, R. M. K. Khan and O. Daugulis, J. Am. Chem. Soc., 2008, 130, 15185 CrossRef CAS; (d) T. Okazawa, T. Satoh, M. Miura and M. Nomura, J. Am. Chem. Soc., 2002, 124, 5286 CrossRef CAS; (e) M. A. Pena, I. Perez, J. P. Sestelo and L. A. Sarandeses, Chem. Commun., 2002, 2246 RSC.
- K. Yin, C. J. Li, J. Li and X. S. Jia, Appl. Organomet. Chem., 2011, 25, 16 CrossRef CAS.
- Thiophene 3j, a known liquid crystal, has been synthesized by palladium-catalyzed coupling of 2-bromo-5-pentylthiophene with 4-methoxyphenyl boronic acid in good yield, for details, see: WO 2000060027[P], 2000..
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and analysis data for new compounds. See DOI: 10.1039/c2ra20326j |
| This journal is © The Royal Society of Chemistry 2012 |