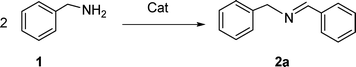
Copper(0)-catalyzed aerobic oxidative synthesis of imines from amines under solvent-free conditions†
Rajendra D.
Patil
and
Subbarayappa
Adimurthy
*
Analytical Science Division, Central Salt & Marine Chemicals Research Institute, (CSIR), G.B. Marg, Bhavnagar 364021, India. E-mail: adimurthy@csmcri.org; Fax: (+91)-278-2567562
First published on 13th April 2012
Abstract
A copper(0)-catalyzed direct synthesis of imines from amines under solvent-free aerobic conditions is described. The method is applicable for the synthesis of various imines from corresponding amines such as benzylic, aliphatic, cyclic secondary and heteroaromatic amines. Being solvent free, using air as a benign oxidant, and the easy separation and easy availability of the catalyst (copper powder) are the vital advantages of present protocol.
Imines are building blocks for the synthesis of nitrogen-heterocycles, fine chemicals and pharmaceuticals.1 Significant progress has been made in recent years in the synthesis of imines, including direct synthesis of imines from amines and condensation of amines with alcohols,2 self-condensation of primary amines with oxidants3 and oxidation of secondary amines.4 Most of these reactions are performed in solvent media. Solvents are perhaps the most active area for green chemistry research;5 they represent an important challenge because solvents often account for the vast majority of mass wasted in syntheses and processes.6 Recently, much attention has been paid to organic reactions carried out under solvent-free conditions7 or the use of greener solvents such as water,8 supercritical fluids9 (SCF) and ionic liquids.10 However, the ideal situation would be not to use any solvent because the decision to include an auxiliary always implies effort and energy to remove or regenerate it from a designated system.
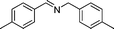
In continuation of our development of copper(I)-catalyzed synthetic methodologies11,12 and to overcome the drawbacks of by-product (aldehyde) formation during the imine synthesis,12 we have developed a novel strategy for the selective synthesis of imines under solvent-free conditions.13 Initially, the reaction of benzylamine 1 was subjected to oxidative imination using copper powder (0.5 mol%) as a heterogeneous catalyst under neat conditions at 90 °C in an open atmosphere and provided the corresponding benzylimine 2 in high yield (>99% GC yield) with 100% conversion (Table 1, entry 1). Attempts were made to use other copper catalysts including Cu supported on alumina14 (Cu/Al2O3) (Table 1, entries 2–9). The reaction does not proceed without catalyst (Table 1, entry 10). When the reaction was performed under argon and nitrogen (Table 1, entries 11 and 12) atmosphere, low conversions were observed. However, under an oxygen (balloon) atmosphere 33% imine 2a was observed along with undesired products (Table 1, entry 13). To understand the role of moisture in air, we carried out an experiment with a trace amount of water (without copper catalyst), it showed only 45% conversion (Table 1, entry 14). However, when the reaction was carried out under controlled conditions [copper powder, water, and oxygen (balloon)], 76% imine was observed (Table 1, entry 15). Notably, the presence of water increases the yield of imine and helps to avoid overoxidation of the amine (as seen in Table 1, entry 13). The selective conversion of benzylamine to imine with time was also monitored by GC-MS (see Table S1, ESI†). Therefore, the rest of the amines were subjected to this procedure (Table 1, entry 1) to synthesize various imines under solvent-free conditions using inexpensive and commercially available copper powder as a catalyst and in an open atmosphere (Table 2). As is evident from Table 2, the present catalytic system has a high degree of functional-group tolerance. Both electron-rich (para, meta, and ortho substituted) and electron-deficient substrates were well-tolerated, and produced good to excellent yields of imines (Table 2, entries 2–4). Similar yields were obtained from halo (F and Cl)-substituted benzylamines (Table 2, entries 5–9). These halo substituted imines are useful synthons for the synthesis of chiral amines.15 The chloro imine (Table 2, entry 7) was obtained in better yield (84%) compared to the literature method.16 The successful transformation of benzylamines into corresponding imines under neat conditions with as low a catalyst loading (0.5 mol%) prompted us to extend the generality of this method for the synthesis of other imines with different amine substrates. To our delight, we found the general applicability of this transformation for a wide range of amines such as the heteroaromatic amine pyridin-2-ylmethanamine (Table 2, entry 10), a secondary amine (Table 2, entry 11) and cyclic secondary amines (Table 2, entries 12 and 13). Alkyl primary amine substrates also produced corresponding imine in moderate yield (Table 2, entry 14). By using the newly established protocol, oxidative dimerization of para-methoxyaniline was unsuccessful due to the lack of α-H hydrogen (Table 2, entry 15). However, oxidative coupling of different amines such as para-methoxyaniline and benzyl aniline resulted in a good yield of un-symmetrical imine (Table 2, entry 16). To check the copper contamination in the product, we carried out an ICP-OES analysis, and the results showed a very low amount of copper in the product.17
| Entry | Catalyst (mol%) | Time/h | Conv. (%) | Yieldb (%) | Select. (%) |
|---|---|---|---|---|---|
| a Reaction conditions unless otherwise stated, benzylamine 1 (9.3 mmol), catalyst, T = 90 °C, open atmosphere. b Yield based on GC-area %. c Reactions performed under argon, nitrogen and oxygen atmosphere in entries 11, 12 and 13 respectively. d Rest constitutes unidentified oxidized product. | |||||
| 1 | Cu powder (0.5) | 20 | 100 | >99 | 100 |
| 2 | Cu wire (0.5) | 20 | 89 | 89 | 100 |
| 3 | Cu/Al2O3 (0.5) | 20 | 29 | 29 | 100 |
| 4 | CuCl (0.5) | 20 | 96 | 96 | 100 |
| 5 | CuCl2 (0.5) | 20 | 94 | 94 | 100 |
| 6 | CuI (0.5) | 18 | 100 | 89 | 89 |
| 7 | CuBr (0.5) | 18 | 78 | 73 | 94 |
| 8 | CuBr2(0.5) | 18 | 88 | 83 | 94 |
| 9 | CuO (0.5) | 20 | 09 | 09 | 100 |
| 10 | No catalyst | 20 | — | — | — |
| 11c | Cu powder (0.5) | 20 | 26 | 26 | 100 |
| 12c | Cu powder (0.5) | 20 | 22 | 22 | 100 |
| 13c | Cu powder (0.5) | 20 | 100 | 33d | 33 |
| 14 | H2O (50 μL) | 20 | 45 | 36 | 81 |
| 15 | Cu powder/H2O(50 μL)/O2(balloon) | 20 | 76 | 76 | 100 |
| Entry | Amine | Time/h | Imine | Yieldb (%) | Ref. |
|---|---|---|---|---|---|
| a Conditions: Amine (9.3 mmol), Cu (0.05 mmol), 90 °C, open atmosphere. b Isolated products. c GC yield. d 19% picolinamide and 49% remaining starting amine. | |||||
| 1 |
|
20 |
|
88 | 3a |
| 2 |
|
20 |
|
76 | 3d |
| 3 |
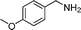
|
18 |
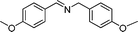
|
88 | 3d |
| 4 |
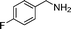
|
22 |
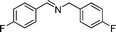
|
90 | 3a |
| 5 |
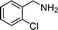
|
21 |
|
90 | 3a |
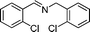
| 6 |
|
22 |

|
86 | 3a |
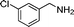
| 7 |
|
18 |
|
84 | 3d |
| 8 |
|
22 |
|
62 | 12 |
| 9 |
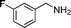
|
24 |
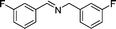
|
6 | 3a |
| 0 | |||||
| 10 |
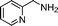
|
20 |
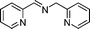
|
20c,d | 3c |
| 11 |
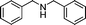
|
12 |
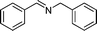
|
82 | 3a |
| 12 |

|
19 |
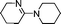
|
65 | 4d |
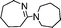
| 13 |
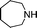
|
19 |
|
58 | 4d |
| 14 |

|
18 |
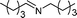
|
34c | 12 |
| 15 |

|
15 | NR | — | — |
| 16 |
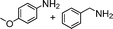 a a |
18 |
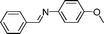
|
82 | 12 |
After completion of the reaction, copper powder (catalyst) was recovered through filtration and washed with diethyl ether, dried and its morphological changes studied using scanning electron microscopy (SEM) before and after the reaction (Fig. 1). The SEM image of copper-powder recovered after the reaction in an open atmosphere [Fig. 1(b)] clearly shows the formation of pits (indicated by arrow) on the copper surface due to oxidative corrosion (i.e. oxidation of copper by aerial oxygen) and it is easily understandable why it acts as an efficient oxidizing system for imine synthesis.
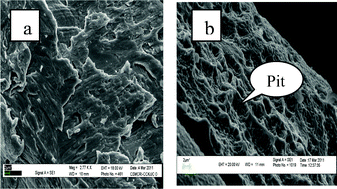 | ||
| Fig. 1 SEM images of copper powder (a) fresh; and after the reaction (b) in an open atmosphere. | ||
This study clearly explains the crucial role of atmospheric oxygen in the selective synthesis of imines with the present catalytic system. In the present study, copper powder is a more effective catalyst than its bulk metal (copper wire) counterpart (Table 1, entry 2). Therefore copper powder has been employed as a catalyst in the present work. The catalytic activities of Cu(0) in the forms of powder, turnings, or nanoparticles are useful in an aqueous environment.18 The surface area of the copper catalyst was determined by the BET method and found to be 0.9713 m² g−1. Mechanistic investigations of copper-catalyzed aerobic oxidative reactions are well studied in the literature.19 Although we do not isolate the intermediates, we propose a plausible reaction mechanism for this transformation as outlined in Scheme 1. The different copper catalysts (Cu powder, Cu wire, Cu supported on alumina, CuCl and CuCl2) gave only desired product, even though Cu supported on alumina gave lower conversion (Table 1, entries 1–5). These results imply that the reaction rate varies with the copper catalyst. Copper halides act as efficient catalysts, but the selectivity varies with the halide (Table 1 entries 4–8). In addition, the selectivity was not dependent on the valence state of copper. These results suggest that copper ions generated from copper powder might be active species for oxidative coupling reactions through the catalytic cycle consisting of copper(I) and copper(II) ions. Based on our observation and literature reports, we propose that the first step is the formation of complexes A and B with oxidative addition of copper to amine. Further oxidation of complexes A and B provides intermediate methanimine C.3k,12,16 In addition, it is also possible that water may form hydrogen bonds with amino groups which may lead to a peroxide intermediate,3m also subsequently leading to intermediate C. Finally the reaction of C with another molecule of amine provides imine (path 1)3n,3o,16 or partial hydrolysis of C (to aldehyde3l,16) followed by condensation with amine gives imine product (path 2). Since we have monitored the reaction with time and did not observe aldehyde (GC-MS), therefore we hypothesize that path 1 is likely to be more probable in the present study. A linear plot was obtained for the conversion of substrate with time, showing a slightly negative intercept (ESI†). This zero-order plot fits with the data better than a first order plot; suggesting that the observed reaction order does not depend upon substrate concentration. The rate limiting step is probably one of the following: (1) the transport of oxygen into the reaction liquid; (2) the activation of dioxygen into surface-bound oxygen atoms; or (3) the oxidation of the copper from a lower to a higher oxidation state. These results are consistent with a redox mechanism and it is known that copper is a redox catalyst.
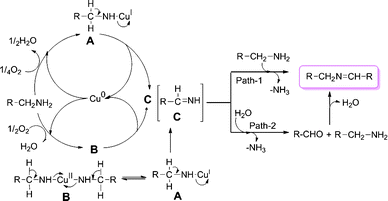 | ||
| Scheme 1 Probable mechanism for the copper-catalyzed aerobic oxidation of primary amines to imines. | ||
In conclusion, we have developed a novel copper-catalyzed protocol for the synthesis of imines, through oxidative imination. Particularly, the present protocol is highly useful for benzyl imine synthesis. Both symmetrical and cyclic imines can be conveniently prepared by this route. Notably, nominal catalyst loading, use of atmospheric air as an environmentally-friendly oxidant and mild reaction conditions are added advantages of the methodology.
Acknowledgements
We thank Mr. Hitesh, Mr. A.K. Das/Mr. H. Bramhabhatt, Mr. V. Agrawal, Mr. Rajesh Patidar and Mr. Jayesh Analytical Division of this institute for supporting NMR, LC/GC-MS, IR, ICP and BET and SEM analyses respectively. R.D.P. is thankful to CSIR, New Delhi, India, for the award of SRF. We are also thankful to the reviewers of this manuscript for their valuable and constructive comments.Notes and references
- (a) S. I. Murahashi and Y. Imada, in Transition Metals for Organic Synthesis, ed. M. Beller and C. Bolm, 2nd edn, Wiley–VCH, Weinheim, Germany, 2004, vol. 2, p. 497 Search PubMed; (b) J. P. Adams, J. Chem. Soc., Perkin Trans. 1, 2000, 125–139 RSC.
- (a) B. Gnanaprakasam, J. Zhang and D. Milstein, Angew. Chem., Int. Ed., 2010, 49, 1468–1471 CrossRef CAS; (b) C. G. Arellano, K. Yoshida, R. Luque and P. L. Gai, Green Chem., 2010, 12, 1281–1287 RSC; (c) M. S. Kwon, M. S. Kim, S. Park, W. Bosco, R. K. Chidrala and J. Park, J. Org. Chem., 2009, 74, 2877–2879 CrossRef CAS.
- (a) G. Chu and C. Li, Org. Biomol. Chem., 2010, 8, 4716–4719 RSC; (b) A. Berlicka and A. König, Photochem. Photobiol. Sci., 2010, 9, 1359–1366 RSC; (c) A. Dhakshinamoorthy, M. Alvaro and H. Garcia, ChemCatChem, 2010, 2, 1438–1443 CrossRef CAS; (d) S. Kodama, J. Yoshida, A. Nomoto, Y. Ueta, S. Yano, M. Ueshima and A. Ogawa, Tetrahedron Lett., 2010, 51, 2450–2452 CrossRef CAS; (e) K. Orito, T. Hatakeyama, M. Takeo, S. Uchiito, M. Tokuda and H. Suginome, Tetrahedron, 1998, 54, 8403–8410 CrossRef CAS; (f) A. H. Ėll, J. S. M. Samec, C. Brasse and J. E. Backvall, Chem. Commun., 2002, 1144–1145 Search PubMed; (g) M. Largeron, A. Chiaroni and M. B. Fleury, Chem.–Eur. J., 2008, 14, 996–1003 CrossRef CAS; (h) C. S. Yi and D. W. Lee, Organometallics, 2009, 28, 947–949 CrossRef CAS; (i) S. M. Landge, V. Atanassova, M. Thimmaiah and B. Torok, Tetrahedron Lett., 2007, 48, 5161–5164 CrossRef CAS; (j) X. Lang, H. Ji, C. Chen, W. Ma and J. Zhao, Angew. Chem., Int. Ed., 2011, 50, 3934–3937 CrossRef CAS; (k) M. -H. So, Y. Liu, C. –M. Ho and C. -M. Che, Chem.–Asian J., 2009, 4, 1551–1561 CrossRef CAS; (l) S. Furukawa, Y. Ohno, T. Shishido, K. Teramura and T. Tanaka, ACS Catal., 2011, 1, 1150–1153 CrossRef CAS; (m) L. Liu, S. Zhang, X. Fu and C. –H. Yan, Chem. Commun., 2011, 47, 10148–10150 RSC; (n) F. Su, S. C. Mathew, L. Mohlmann, M. Antonietti, X. Wang and S. Blechert, Angew. Chem., Int. Ed., 2011, 50, 657–660 CrossRef CAS; (o) Z. Hu and F. M. Kerton, Org. Biomol. Chem., 2012, 10, 1618–1624 RSC.
- (a) J. S. M. Samec, A. H. Ėll and J. E. Bäckvall, Chem.–Eur. J., 2005, 11, 2327–2334 CrossRef CAS; (b) S. I. Murahashi, Y. H. Okano, H. Sato, T. Nakae and N. Komiya, Synlett, 2007, 1675–1678 CrossRef CAS; (c) G. Jiang, J. Chen, J. S. Huang and C. M. Che, Org. Lett., 2009, 11, 4568–4571 CrossRef CAS; (d) B. Zhu and R. J. Angelici, Chem. Commun., 2007, 2157–2159 RSC; (e) K. C. Nicolaou, C. J. N. Mathison and T. Montagnon, Angew. Chem., Int. Ed., 2003, 42, 4077–4082 CrossRef CAS.
- P. T. Anastas, in Clean Solvent Alternative Media for Chemical Reactions and Processing, ACS Symposium Series 819, American Chemical Society, Washington, DC, 2002, ch. 1 Search PubMed.
- D. J. C. Constable, A. D. Curzons and V. L. Cunningham, Green Chem., 2002, 4, 521–527 RSC.
- F. M. Kerton, in Alternative Solvents for Green Chemistry, RSC Green Chemistry Book Series, Royal Society of Chemistry, Cambridge, UK, 2009, ch. 2, p. 23 Search PubMed.
- C. J. Li and T. H. Chan, in Comprehensive Organic Reactions in Aqueous Media, John Wiley & Sons, Inc., Hoboken, New Jersey, 2nd edn, 2007 Search PubMed.
- F. M. Kerton, in Alternative Solvents for Green Chemistry, RSC Green Chemistry Book Series, Royal Society of Chemistry, Cambridge, UK, 2009, ch. 8, p. 68 Search PubMed.
- R. D. Rogers and K. R. Seddon, in Ionic Liquids as Green Solvents, ACS Symposium Series, American Chemical Society, Washington, DC, 2003, p. 856 Search PubMed.
- S. Adimurthy, C. C. Malakar and U. Beifuss, J. Org. Chem., 2009, 74, 5648–5651 CrossRef CAS.
- R. D. Patil and S. Adimurthy, Adv. Synth. Catal., 2011, 353, 1695–1700 CrossRef CAS.
- In the case of benzylamine and its derivatives, after the completion of the reaction, the resultant mixtures were obtained as oily liquids (solids in entries 2 and 7, Table 2) along with a small amount of unidentified impurity. The reaction mixtures showed imine as a sole product by TLC as well as GC-MS analysis. The solid impurity was insoluble in diethyl ether used as a solvent for washing the residue after filtration. In this way the insoluble impurities were separated from the reaction mixture through filtration and washing with diethyl ether. Pure imines were obtained after removal of solvent (by 1H-NMR) (Table 2, entries 1–9) from the filtrate. However, other imines were purified by column chromatographic separation using neutral alumina.
- S. Bhadra, B. Sreedhar and B. C. Ranu, Adv. Synth. Catal., 2009, 351, 2369–2378 CrossRef CAS.
- (a) K. A. Nolin, R. W. Ahn and F. D. Toste, J. Am. Chem. Soc., 2005, 127, 12462–12463 CrossRef CAS; (b) Chiral Amine Synthesis, ed. T. C. Nugent, Wiley–VCH, Weinheim, Germany, 2010, p. 22 Search PubMed.
- B. Zhu, M. Lazar, B. G. Trewyn and R. J. Angelici, J. Catal., 2008, 260, 1–6 CrossRef CAS.
- General procedure for the synthesis of imines: (entry 1 Table 2): benzylamine (9.3 mmol) and copper(0) powder (0.05 mmol) were placed in a two-necked round-bottomed flask fitted with a reflux condenser. The resulting reaction mixture was heated to 90 °C in an open atmosphere for a period of 20 h. After completion of the reaction (TLC), the reaction mixture was allowed to reach room temperature and filtered through filter paper. The residue was washed with 10 mL diethyl ether and removal of solvent from the filtrate afforded pure imine in 88% (4.1 mmol) yield as a viscous liquid. The characterization data of the products were well matched with literature data.3a [Note: copper powder used in the above experiment was prepared by mechanically drilling a copper metal rod and used as such without any pre-treatment.] Determination of the copper content in N-(benzylidene)benzylamine using ICP-OES analysis (entry 1Table 2): the imine product obtained after work-up was converted to ash by heating it at 400 °C in a furnace during a period of 6 h followed by treatment with concentrated nitric acid (5 mL) to digest the reaction mixture. The mixture was then transferred to a standard 25 mL volumetric flask made up to the mark with water. The copper concentration of the sample was determined using ICP-OES analysis. The copper concentration was found to be 5.3 μmol.
- M. Meldal and C. W. Tornøe, Chem. Rev., 2008, 108, 2952–3015 CrossRef CAS and related references cited therein.
- (a) Q. Li, S. Fan, Q. Sun, H. Tian, X. Yu and Q. Xu, Org. Biomol. Chem., 2012, 10, 2966–2972 RSC; (b) S. Liao, K. Yu, Q. Li, H. Tian, Z. Zhang, X. Yu and Q. Xu, Org. Biomol. Chem., 2012, 10, 2973–2978 RSC; (c) N. H. Nguyen, B. M. Rosen, G. G. Lligadas and V. Percec, Macromolecules, 2009, 42, 2379–2386 CrossRef CAS; (d) Y. Maeda, T. Nishimura and S. Uemura, Bull. Chem. Soc. Jpn., 2003, 76, 2399–2403 CrossRef CAS; (e) G. J. Christian, A. Llobet and F. Maseras, Inorg. Chem., 2010, 49, 5977–5985 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedure and NMR spectra of compounds. See DOI: 10.1039/c2ra20339a/ |
| This journal is © The Royal Society of Chemistry 2012 |