Some approaches for high performance polymer based membranes for gas separation: block copolymers, carbon molecular sieves and mixed matrix membranes
M.G.
Buonomenna
*a,
W.
Yave
b and
G.
Golemme
a
aDepartment of Material and Chemical Engineering, University of Calabria, Rende (CS), INSTM Consortium, Italy 87036. E-mail: mgbuono@unical.it; mgbuonomenna@gmail.com
bSchlossligasse 14, 79576 Weil am Rhein, Germany
First published on 15th August 2012
Abstract
The intrinsic characteristics of a membrane process, i.e. efficiency and operational simplicity, high selectivity and permeability for the transport of specific components, compatibility between different membrane operations in integrated systems, low energetic requirements, easy control and scale-up, and high operational flexibility, might be decisive factors in imposing membrane processes in most gas separation fields. This review highlights the research hardware in membrane gas separation, i.e. the membrane material. Emphasis has been devoted to three interesting and important membrane classes for gas separation: block copolymer membranes, carbon membranes and mixed matrix membranes.
1 Introduction
Gas separation occupies a central position in the chemical feedstock industry:1 oxygen and nitrogen enrichment of air, hydrogen recovery, natural gas separation and removal of volatile compounds from effluent streams are all current applications. Gas separation by membranes has acquired great economic importance because of its energy efficiency. Some typical examples of membrane gas separations are summarized in Table 1. In particular, the non-cryogenic nitrogen production and hydrogen purification are already present at the industrial level.2| Gas to be separated | Gas pair | Application |
|---|---|---|
| a HC = hydrocarbons. | ||
| H2 | H2/N2 | Hydrogen recovery from ammonia purge gas |
| H2/HCa | Refinery hydrogen recovery | |
| H2/CO,CO2 | Synthesis gas ratio adjustment | |
| H2/CO2 | Fuel cells | |
| Air | O2/N2 | Oxygen-enriched air for combustion |
| Acid gases | CO2/CH4 | Natural gas sweetening |
| H2S/CH4 | Sour gas sweetening | |
| CO2/N2 | Digester gas treatment | |
| Drying | H2O/HCa | Hydrocarbon drying |
| H2O/air | Air drying | |
| Hydrocarbons | HC/air | Pollution control; stack gas or solvent recovery |
| HC/N2 | Upgrading low-BTU gas | |
| Helium | He/HCa | Helium recovery from gas wells |
| He/N2 | Helium recovery from diving air | |
A MIT (Massachusetts Institute of Technology) study of 2007 predicts that global CO2 emissions from coal combustion will increase from 9 GTon/year in 2000 to 32 GTon/year in 2050 (55% of projected global CO2 emissions at that time). Therefore, CO2 separation by membranes is a great challenge for scientists worldwide.
One way to reduce CO2 emissions from the atmosphere is carbon capture and sequestration (CCS), i.e. CO2 is captured from power plant emissions and sequestrated underground in geological structures for long periods of time. Membrane processes have been suggested previously for CO2 capture from flue gas. Hendricks et al.3 reported as early as 1989 that commercial gas separation membranes available at that time were not competitive with absorption techniques for flue gas treatment.
Recent feasibility studies by Favre and co-workers have rekindled interest in using membranes for CO2 capture from flue gas.4,5 Another interesting analysis has been carried out by Merkel et al.6 The results for H2 and CO2 selective membranes show that by using state-of-the-art membranes (CO2/H2 selectivity 15.5, H2/CO2 selectivity 5.9), the current requirements concerning CO2 purity and CO2 separation degree cannot be fulfilled. A CO2/H2 selectivity of 150 for a single CO2 selective membrane would be needed to obtain power plant efficiency losses below 10% points with separation degrees above 85%. For a cascade concept the needed CO2/H2 selectivity would have to be of the order of 60 to achieve the same values. For H2 selective membranes with a H2/CO2 selectivity of 50, separation degrees of 85% at efficiency losses below 10% points can be reached.
Natural gas processing represents the largest market for industrial gas separation processes, and equipment and membrane-based removal of natural gas contaminants is growing faster than any other segment of the membrane gas separation business.2
The oxygen-enriched air produced by membranes has been used in various fields, including chemical and related industries, the medical field, food packaging, etc. In industrial furnaces and burners, for example, injection of oxygen-enriched air (25–35% oxygen) leads to higher flame temperatures and reduces the volume of parasite nitrogen to be heated; this means lower energy consumption. Industrial nitrogen is used in the chemical industry to protect fuels and oxygen-sensitive materials. Membranes today dominate the fraction of the nitrogen market for applications of less than 50 tons/day and relatively low purity (0.5–5% O2).2 What advances have there been in terms of research of membrane materials for the gas separations listed in Table 1?
There are various and interesting opportunities because a vast array of potential materials can be considered as membranes. In this review, the attention has been focused on three promising classes of membrane materials, which are attractive for the gas separation membrane market in terms of performance, reproducibility, processability and cost: carbon molecular sieves (CMSs), block copolymers and mixed matrix membranes (MMMs).
In the case of CMSs, one major disadvantage that hinders their commercialization is their brittleness, meaning they require careful handling. This may be prevented to a certain degree by optimizing precursors and preparation methods. The cost of carbon-based membranes is 1 to 3 orders of magnitude greater per unit area than polymeric membranes. Only when they achieve a much better performance than polymeric membranes might this high investment cost be justified. Several studies report selectivities and permeabilities for CMSs well in excess of the performance of polymeric membranes for difficult cases where the size of the molecules to be separated is very small, such as the CO2/N2, the O2/N2 and the C2 and C3 alkene/alkane pairs. CMS membranes are prepared by pyrolyzing polymers. Unlike zeolites, which have a well-defined pore size distribution, CMSs have been typically plagued by larger pore size distributions, which allow large and condensable molecules to enter and plug the pore network. This traditional picture of CMSs is changing in the last few years thanks to a controlled oxidation technique that is able to finely tune the pore size; this breakthrough is giving a new impetus to the research on CMS membranes.7
Block copolymers have gained increasing interest for gas separations involving CO2 because they show outstanding separation properties. In particular, these materials have become of great interest in nanotechnology, precisely due to their ability to self-assemble in a variety of ordered nanostructures.8 The self-assembly process of block copolymers on a substrate is known as the “bottom-up” approach to nanofabrication.9 By using block copolymers with different structures, i.e. diblock, triblock and multiblock copolymers, the nanostructure and the properties of these materials can be exquisitely tuned for several specific applications. For instance, the fabrication of nanometric thin membranes from a block copolymer was recently reported.10 The membranes had thicknesses of less than 100 nm and showed extremely high performance in the separation of carbon dioxide.
MMMs comprise rigid permeable or impermeable particles, such as zeolites, carbon molecular sieves, silica, carbon nanotubes and metal organic frameworks, dispersed in a continuous polymeric matrix. In this approach, using properties of both polymer and inorganic phases, membranes with enhanced permeability, selectivity, mechanical strength, thermal and chemical stability and processability can be prepared.11
The separation performance of homogeneous polymeric films is limited by an upper bound trade-off curve relating permeability and selectivity,12 while the performance of block copolymers, carbon molecular sieves and mixed matrix membranes can overcome this trade-off curve.
In this review, the separation performance studies of membrane materials based on pure advanced polymers, like block copolymers, CMSs and MMMs for gas separation will be critically reviewed. The outlook of research and development to fully exploit the potential usage of these new membrane materials will be given.
2 Gas separation transport mechanism: theory
Gas transport through dense polymeric membranes is governed by eqn (1):| Ji = P/l(pil − pi0) | (1) |
The most commonly used membranes in the gas separation processes are polymeric and nonporous. The separation is based on a solution–diffusion mechanism and the permeability P, defined in eqn (2), represents the ability of molecules to permeate through a membrane:
| P = SD | (2) |
Selectivity is a measurement of the membrane's ability to separate the components of a mixture. The ideal selectivity (αij) is the ratio of the permeabilities of single gases and it is defined by eqn (3).
| αij = Pi/Pj = (Di/Dj) × (Si/Sj) | (3) |
The mechanism assumes that each molecule is sorbed by the membrane at one interface, transported by diffusion across the membrane through the voids between the polymeric chains (which make up the so called free volume), and desorbed at the other interface. According to the solution–diffusion model, the permeation of molecules through the membranes is controlled by two major parameters: the diffusion coefficient (D) and the solubility (S).13 The diffusion coefficient is a measure of the mobility of the individual molecules passing through the voids between the polymeric chains in a membrane material. The solubility equals the ratio of sorption uptake normalized by the partial pressure at which sorption takes place.14
Recently, intense research has been directed towards the development of membranes that have more favorable trades-off between selectivity and permeability, such as carbon molecular sieves,15 zeolites and metal–organic frameworks16 as membrane materials.
Commercial gas separation membranes are composed of synthetic polymeric materials. Polymers provide a range of desirable properties that are important for gas separation processes including low cost, high permeability, good mechanical stability, and easy processability.17
Highly permeable rubbers are not very selective and discriminate between gases mainly on the basis of their solubility differences. For separation of permanent gases, less permeable glassy polymers are chosen instead, because of their size selectivity. Glassy polymers have stiffer polymer backbones and therefore let smaller molecules such as H2 and He pass through more quickly, and larger molecules such as hydrocarbons permeate the membrane slowly. To increase the membrane selectivity, either the diffusivity or the solubility needs to be enhanced; however, polymers that are more permeable are generally less selective and vice versa. Polymeric membranes suffer from a trade-off between their permeability and selectivity. This trade-off was illustrated first by Robeson in 1991 when he plotted polymer selectivity versus permeability, and it later became known as Robeson plots, characterized by “upper-bound trade-off” lines. Owing to improvements during the last decade, Robeson revisited the upper bound curves in 2008.12
The trade-off line for each gas pair depicts the permeability–selectivity relation for conventional polymers, and this linear relation has been rationalized in terms of kinetic diameters, free volume and solubility coefficients of gases.
A substantial amount of research effort has been directed to overcoming the limit imposed by the upper bound. Glassy polymers provide high selectivity, but the gas permeability is usually low.
3 Strategies to overcome the upper bound
Common glassy polymers used as membrane materials include polysulfones, polyimides, polyaramides, polycarbonates, polyphenylene oxides and cellulose derivatives.One of the proposed strategies to alter the trade-off relation was the chemical modification of classical polymers. Sridhar et al.18 prepared and modified poly(phenylene oxide) (PPO) membranes using chlorosulfonic acid. The CO2/N2 selectivity (27.2) for sulfonated PPO membranes was 2.2 times higher than for PPO membranes, while the CO2 permeability (43.7) decreased by a factor of 2.4. Due to the permeability decrement after the chemical modification, PPO membranes lay well below the upper bound line.
Another strategy is polymer blending. Kapantaidakis et al.19 prepared membranes by blending polyethersulfone (PES) and polyimide (PI) in different ratios: (20/80), (50/50), (80/20) w/w blends. No significant improvement was observed in selectivity. The ideal selectivity of the CO2/N2 gas pair was reported as 40 for the PES/PI (20/80) blend and as 39 for PES/PI (80/20), both being close to the selectivity values of single polymers. Polymer blend membranes of poly(bisphenol A-co-4-nitrophthalic anhydride-co-1,3-phenylenediamine) (PBNPI) and polyphenylsulfone (PPSU) in different weight ratios were prepared and investigated for H2, CO2 and CH4 separations. No improvements in selectivity, but a continuously enhanced permeability has been observed for these gases with increasing PPSU content.20 Asymmetric membranes based on Matrimid® and polysulphone blends with improved permeance and stability, without selectivity increase compared to Matrimid®, have been observed in binary gas (CO2/CH4) mixture separations.21 Simple modifications of the polymer structure often only lead to a trade-off, as in the examples reported above for polymer blends: one of the parameters is improved, while the other is simultaneously negatively affected.22 This behavior can be explained by the following equations (eqn (4) and (5)).
| αij=logβi/j − λijlogPi | (4) |
| βi/j= Si1+λij/Sjexp{−λij[b − f[(1 − a)/RT]} | (5) |
The theory developed by Freeman23 justifies the empirical principle of obtaining the best permeability/selectivity properties, up to a limit, i.e. one needs to create a polymeric structure with a stiff backbone (which enhances mobility selectivity at the expense of diffusivity) whilst also disrupting inter-chain packing (to improve permeability). This principle is taken to the extreme with PIMs (polymer of intrinsic microporosity).24 Alentiev and Yampolskii25 showed that the trade-off behavior could be predicted on the basis of a free volume model. Both Freeman23 and Alentiev and Yampolskii25 indicated that one way to surpass the upper bound is by enhancing the solubility selectivity (Si/Sj): even if the ratio Si/Sj is unchanged, a significant improvement can be achieved through an overall increase in S, and hence P.
The upper bound is based on homogeneous polymer films, and several approaches involving heterophase membranes have been demonstrated to exceed the upper bound: a) surface modifications, b) facilitated transport, c) block copolymers, d) MMMs, e) carbon molecular sieve membranes (CMSMs).
Among the methods of membrane surface modifications, UV modification, ion beam surface carbonization,26 and surface fluorination27 are investigated. Facilitated transport membranes rely on a chemical reaction occurring between the gas of interest and a component of the membrane (carrier). The reacted species are readily carried across the membrane, whereas diffusion of non-reactive gases is inhibited. The active carrier is generally basic in nature, given that carbon dioxide is acidic. The driving force for gas transport remains the partial pressure difference across the membrane; however the facilitator carrier increases both the permeability and selectivity of the membrane through the increased loading. The facilitator carrier can be either fixed-sited within the polymeric matrix or mobile.28
More recent patents have focused on hydrogel films from cross-linked hydrophilic polymers, such as polyvinyl alcohol, polyvinylacetate, polyvinyl pyrrolidone, polyethylene oxide, polyacrylamide, blends and copolymers, cast on permeable supports.29,30 These hydrogels have high water absorbing power and can therefore accommodate considerable loading of the carrier species. Copolymer composite membranes take advantage of the different swelling potential of each polymer component, whereas cross-linking agents are used to generate a strong polymer matrix. As a consequence, even though the membranes are thin, the high water content hydrogel film can retain its shape upon being subjected to pressure. Hence, they can function as a separation membrane with a long service life by exhibiting good water-retention and weatherability.31 Fixed carriers are generally polyallylamine, polyethylenimine and polyvinylamine.32–35
Mobile carriers are basic compounds, and are often a combination of hydroxide salts, organic ammonium salts, aminoacids, carbonates, alkanolamines and polydentate ligands, such as EDTA.36–38
An extensive treatise of all the approaches to exceed the upper bound is far beyond the scope of this review and further reading of the relevant publications on the subjects of surface modification and facilitated transport, which are not the object of this paper, are recommended. The next paragraphs are focussed on the main aspects and recent advances in the interesting membrane classes of block copolymers, carbon molecular sieve membranes and MMMs.
4 Block copolymer membranes
4.1 Overview of block copolymers
Many years ago scientists and technologists exploited the mixing of two different homopolymers to prepare superior polymeric materials. The polymer mixtures or alloys were denominated polymeric blends; those blends however always presented separated phases (macrodomains) due to the immiscibility of polymers.39 One of the greatest challenges in that time was thus to solve the phase separation or immiscibility that results from the low entropy of mixing two different polymer chains.40 One way of reducing the immiscibility was by using a compatibilizer with a chemical similarity to each homopolymer, i.e. surface-active block copolymers coming from two different monomers.40–42 Those compatibilizers had the feature of self-assembling in the interface of separated phases, thus the size of the macrodomains was reduced. This strategy helped to create heterogeneous materials with improved mechanical,43 barrier,44 thermal45 and other properties.46,47In parallel, and once the features (nanostructure) of block copolymers were known, it was suggested that they could become advanced materials with better properties than blends, so they received more attention due to their ability to self-assemble, especially in the last 20 years.39,48
Today it is well-known that block copolymers exhibit amphiphilic behaviour, because they are single polymer chains containing two or three different homopolymers covalently bound to each other (Fig. 1), thereby having the ability to form ordered nanostructures, and they are mostly synthesized by living anionic polymerization.49 Their properties mainly depend on morphology, i.e. nature, size of the nanostructure and its alignment; the morphology in turn depends on the degree of polymerization of each segment, entire molecular weight, polydispersity of the copolymer and interaction between segments.50,51
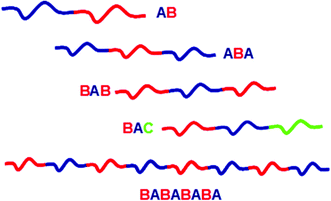 | ||
| Fig. 1 Schematic representation of linear block copolymers: AB di-block, ABA, BAB and ABC tri-block and AB multi-block copolymers | ||
The phase separation in block copolymers is microscopic, i.e. the size of the separated phases is nanometric. The phase behaviour of block copolymers and the properties of complex nanodomains have been extensively studied; the readers hence are encouraged to read the following reviews.52–54 Here, we only present a short overview of nanostructures formed by these block copolymers.
The simplest block copolymers are di-block copolymers, they are the most studied and a phase diagram has been constructed by Matsen and Bates.55 Four morphologies have been identified, which are body-centered-cubic packed spheres, hexagonal closed packed cylinders, double gyroids, and alternating lamellae (Fig. 2). The morphologies that represent different phases are governed by the Flory–Huggins56,57 interaction parameter χ, the volume fraction of the blocks and the total degree of polymerization, i.e. these factors direct the equilibrium phase. Nevertheless, it is important to point out that recent studies noticed that not only the degree of polymerization, composition and interaction between segments (thermodynamics) direct the final nanostructure, but so do the kinetics.58,59
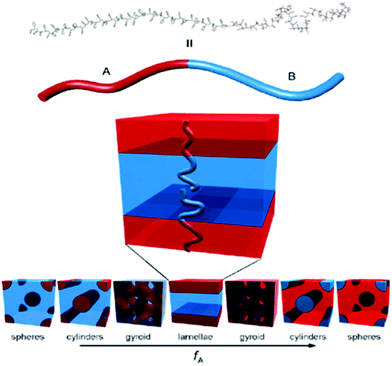 | ||
| Fig. 2 Di-block copolymer morphologies as a function of volume fraction of each segment. Reproduced with permission from ref. 52. Copyright of Elsevier B. V. | ||
The morphology of a tri-block copolymer is more complex than that of a di-block; this is due to the number of independent molecular parameters, i.e. the addition of a chain segment, either the same as an already existing segment or a completely different one, and thus two types of tri-block copolymers can be identified: ABA and ABC types. Because their morphology is complex, all of them are difficult to show in a short overview, hence only some of the most elaborate nanostructures are shown in Fig. 3A. With the addition of more segments of either A or B, the copolymers become multi-block copolymers, so their morphology is even more complex and can show unexpected nanostructures. Typical multi-block copolymers are those commercially known as thermoplastics; they are engineering materials and have multiple and important applications.60,61Fig. 3B shows a possible morphological structure of multi-block copolymers; they are two separated micro-phases where amorphous and crystalline structures coexist within each phase. In addition to these phases there is also a microphase (interface) between the amorphous and crystalline structures, this has different properties compared to the separated microphases and can also influence the final properties of the block copolymer.62
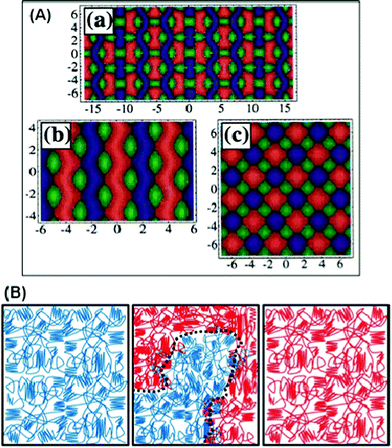 | ||
| Fig. 3 Some typical morphologies of a tri-block copolymer theoretically obtained (A)64 and two semi-crystalline separated micro-phases in multi-block copolymers (B) where the blue (left) and red (right) represent the homopolymers. Reproduced with permission from ref. 64. Copyright of American Physical Society. | ||
In general, block copolymers may constitute a combination of flexible and rigid segments, and they can be hydrophobic and/or hydrophilic; therefore depending on the final application, the type and the volume fraction of each segment must be correctly chosen and well-controlled during the synthesis.
4.2 Block copolymers as membrane materials for gas separation
As block copolymers present defined and exquisite nanostructures, they were always of great interest for nanopatterning as a “bottom-up” approach. By using this technique, advanced functional nanostructures and nanodevices can be fabricated,63,64 but nowadays block copolymers have many applications in other fields too, and membrane technology is one where they have potential as high separation performance materials.Solubility, diffusivity and permeability of gases through block polymers are important from both the academic and the practical point of view. Knowledge of these properties and their relationships with the nanostructure are the basis to manufacture tailored materials for packaging, perm-selective membranes, protective coating and fibers, among others.
Odani et al.65 were perhaps among the first to report on the permeation and diffusion of gases through block copolymers. They investigated poly(styrene-butadiene-styrene) (SBS) block copolymers, and styrene rod-like domains dispersed in butadiene and alternating styrene lamellae domains have been obtained by thermal treatment. Gas permeation and diffusion were correlated with the nanodomain structure (lamellae) by using parallel and series models. Knight and Lyman66 were also among the first, but they studied the effect of chemical structure and fabrication variables on the gas permeability of several block copoly(ether-urethane-urea) and copoly(ether-urethane) membranes. The report showed that the type and length of the chains affected the gas permeability in both copolymer systems. The gas permeability variation was also related to the degree of microphase separation and the nature of chain packing. D. R. Paul67 in that time developed mixing rules to relate the gas permeation in homogeneous multi-component polymers (block copolymers) to that in pure component polymers. The sorption and diffusion of propane in block copolymers of polydimethylsiloxane (PDMS) and poly(bisphenol-A carbonate) were also studied in that period by Barrie and Williams.68 The results were interpreted in terms of composition and length of blocks, i.e. in terms of models where the microdomains are dispersed in a continuous phase. The Cohen group69,70 has reported several studies on semi-crystalline block copolymers and their gas permeation. For the studied block copolymers, they found that the orientation of the nanodomains strongly influenced the effective gas permeability.
Although the first reports about block copolymers as a membrane material for gas separation were published in the last decade, the self-assembly of block copolymers as a tool to fabricate tailor-made separation membranes is only now being exploited. Block copolymers are however being mostly studied in the fabrication of nanopore membranes, and it is known that for the fabrication of nanopore membranes, the manipulation of the self-assembly process is a great challenge to control the formation of the cylindrical pore structure perpendicular to the surface. Some research has shown that di- and tri-block copolymers form defined nanopores,58,71,72 and since the size of the pores is uniform they would become the membranes of the future; we say “membranes of the future” because at the moment there is not any technique or method to fabricate these membranes on a square meter scale, and the price of block copolymers is still very high. Recently, Peinemann's group58 reported the development of a method to produce membranes on a large scale. The method consists of the combination of two processes: the formation and stabilization of micelles and the well-known phase inversion process.
With respect to the block copolymers for gas separation membranes, the self-assembly process has not been really exploited, only the microphase separation of block copolymers was well correlated with the gas transport.65,69,73 In this section it will be shown that membranes with a high gas separation performance may be obtained by the self-assembly of block copolymers, on the basis of old reports and recent advances related to block copolymers and membranes. The purpose is to construct strategies to design membranes with tailored properties.
4.3 Di-block and tri-block copolymer membranes
As reported, di- and tri-block copolymers are of great interest for the nanoporous membrane fabrication due to their feature of forming cylindrical and bicontinuous gyroid nanodomains, but these copolymers were studied to a lesser extent for gas separation membranes. Nevertheless, few reports on the correlation of the gas transport properties and the nanostructure geometry can be found in the literature. Barrie and Munday,74 for example, in 1983 studied a di-block copolymer containing PS and polydimethysiloxane (PDMS). They reported the dependence of permeability at different temperatures on the composition of the copolymer in terms of models for transport in a continuous phase with an impermeable dispersed phase. Tri-block copolymers such as SBS were already being reported as permeable membranes in 1975.65Fig. 4 depicts the geometry for a SBS tri-block copolymer; although this report is old, the authors tried to explain the permeability and diffusivity of inert gases by a simple model of a parallel array of elements (nanodomains formed by polystyrene and polybutadiene). They found that a styrene rod-like membrane dispersed in butadiene exhibited greater permeability and diffusivity than that in alternating styrene lamellae domains, and in conclusion they stated that the permeation and diffusion of inert gases are primarily governed by the rubbery polybutadiene matrix.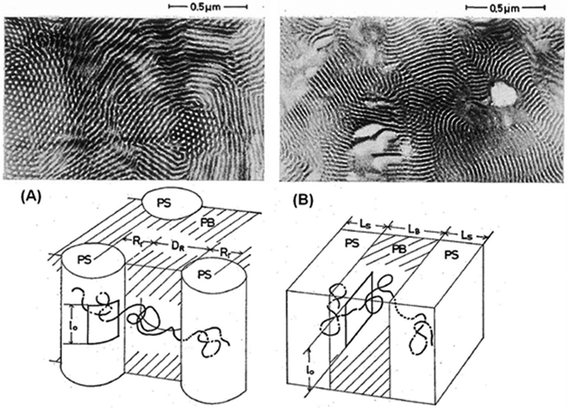 | ||
| Fig. 4 Morphologies of a tri-block copolymer membrane of ABA type (SBS); (A) styrene rod-like domains dispersed in butadiene and (B) alternating styrene lamellae domains. Reproduced with permission from ref. 65. Copyright of Institute for Chemical Research, Kyoto University | ||
The Cohen group from MIT also investigated di-, tri- and tetra-block copolymers as permeable or barrier films.69,70,76 One of their reports presents a simple model to describe the gas transport through di-block copolymer nanodomains; they proposed, as an upper bound, the lamellae aligned parallel (with respect to the permeation direction) and, as a lower bound, the lamellae aligned in series.76 They also calculated that the gas permeation through misoriented lamellae materials and the permeability values were in good agreement with the experimental data. Thus they suggested that materials with tailored permeability and barrier properties can be developed by taking into account the upper and lower bounds. In addition to the nanodomain structure, they also studied how the crystalline structure affects gas permeation (segments that crystallize). Premnath from MIT also77 developed a model for permeability through block copolymer membranes, i.e. for systems with perfect orientation in both parallel and series, as well as for random orientation. Kofinas (a former student of the Cohen) group78,79 also investigated the relationship of different nanodomain orientations and gas permeation in semi-crystalline di- and tri-block copolymers; they generated confined nanopores within the block copolymer domains and increased the gas flux without altering the selectivity.
As seen, the relatively old reports already showed that the self-assembly process of block copolymers can be used as a powerful tool to develop advanced membrane materials. In the last few years only a few papers were published on gas separation membranes from di- and tri-block copolymers. Lokaj et al.80 reported the synthesis and gas permeability of block copolymers composed of poly(styrene-b-acrylonitrile) and polystyrene, i.e. by using the di-block copolymer as a macroinitiator, a tri-block copolymer of the ABA type was obtained. The prepared film showed high O2/N2 selectivity (>6), so this work showed that the gas selectivity can be improved by adding a third segment to di-block copolymers. In 2004, Patel and Spontak73 explored the properties of a poly(styrene-b-ethylene-oxide-b-styrene) tri-block copolymer and its blend with polyethylene glycol (PEG) as a reverse selective membrane for CO2 separation. The membrane design as CO2 selective was based on the unusually high affinity between CO2 and ethylene oxide; thus by adding PEG, the CO2 permeability was enhanced, and a composition-dependent transition from alternating lamellar to polyether continuous morphology was evidenced. Li et al.81 synthesized similar copolymers to those used by Patel and Spontak (PS-PEG-PS); they obtained copolymers with similar molecular weights but different fractions of the crystalline PEG segment. Although the copolymers were not studied as gas separation membranes, the studies showed that the PEG domain can be tuned in terms of the crystalline/amorphous ratio as a gas sensing material. These two works clearly suggest that by tuning the morphology of the tri-block copolymers containing PEG, the affinity between copolymers and CO2 or certain vapours can be enhanced.
The use of PEG as a hydrophilic phase in block copolymers is important for several industrial applications. A polyacrylonitrile-PEG-polyacrylonitrile tri-block copolymer was for example synthesized and characterized as a pervaporation membrane.82 The authors reported that the dehydration of a mixture of acetone with 5% water is considerably improved by using block copolymers; the performance of the membrane mainly depended on PEG content and size of the segment. The transport properties were correlated with the micro-phase separation of the block copolymer and the water solubility in PEG at different temperatures. The effect of nanoscale morphology on ethanol transport through SBS block copolymer membranes was also reported.83,84 The ethanol selectivity and total flux can be optimized by controlling the size of the block copolymer nanodomains.
In a recent report, the self-assembly of SBS and its behaviour as a membrane for gas separation has been thoroughly studied.75 Structural differences of SBS in the copolymer membranes, obtained by manipulation of the self-assembly of block copolymer in solution, have been characterized by means of AFM, TEM and transport properties of three gases (CO2, N2 and CH4). The unexpected CH4/N2 ideal selectivity of 7.2 (the highest value ever reported in the literature for block copolymers), with a CH4 permeability of 41 Barrer, has been attributed to the controlled and improved micro-phase separation of block copolymer, i.e. the hexagonal array of columnar polystyrene cylinders normal to the membrane surface resulted in a membrane with superior gas separation properties. In addition to the unexpected CH4/N2, the CO2/N2 ideal selectivity of 50, coupled with a CO2 permeability of 289 Barrer, makes the SBS a good candidate for the preparation of membranes for the post-combustion capture of carbon dioxide. The synthesis and gas transport properties of rigid block copolyaramides were also reported.85 The block copolymers containing a highly gas permeable rigid segment and a barrier aramide presented lower permeability values than that in the homopolymer with the higher gas permeability, but the gas transport was well described by a parallel arrangement of nanodomains; hence those models developed in the last century were validated.
Robeson86 recently reported a review combining two of the major research areas of Prof. Paul: polymer blends and gas separation membranes. In that review, the morphology of phase separated polymer blends is highlighted as an important variable during the design of materials to enhance the transport properties. We mention this report here to show that the morphology of blends is the same as in block copolymers, i.e. series and parallel models, Maxwell’s model and the continuous model can be used to study the mass transport. The relationship between the transport properties and the morphology are analogous in both systems; the differences, however, are the size of the separated phases (for blends are macro-phases and for block copolymers are micro-phases), and thus this review could be helpful in analysing the gas transport through block copolymer membranes.
It is also worth mentioning the work reported by Querelle et al.87 They used a reactive block polymer precursor, and membranes with a bicontinuous nanostructure were obtained. The strategy was to cross-link one phase, thus improving the plasticization effect. The membrane was evaluated for CO2 separation from CH4. Although the permeabilities remained the same, the selectivity was slightly reduced; thereby they demonstrated that by this means, cross-linked bicontinuous block copolymer membranes resisted plasticization. One of the latest reports on tri-block copolymers for gas separation was published by Gu and Lodge.88 They synthesized a novel tri-block copolymer based on styrene and ionic liquids (IL), i.e. a polystyrene-poly(ionic liquid)-polystyrene named as PS-PIL-PS. The work was inspired by the enhanced transport of PILs developed by Noble’s group.89 The developed block copolymer membrane as an ion gel showed enhanced gas transport properties, and the authors pointed out that the thermoreversible nature of this ion gel material offers advantages during processing (solvent-free). The membrane exhibited a high permeability and high CO2 selectivity over N2 and CH4.
4.4 Multi-block copolymer membranes
Many years ago (around 1950) researchers from all over the world were looking for novel polymeric materials with similar properties to those of natural rubber, and thus thermoplastic elastomers were developed. In fact, the above described SBS tri-block copolymer has been developed as a thermoplastic elastomer. Thanks to the advances in polymer synthesis, the segmented or multi block copolymers (linear) have become commercial (thermoplastics and thermoplastic elastomers), and these copolymers have been used in several applications. In membrane technology these multi block copolymers have been mostly studied as gas separation membranes and membranes for fuel cells.90–93Multi-block copolymer membranes are composed of copolymers containing flexible and rigid segments, which in turn form the soft and hard phases. Generally, the rigid segment gives mechanical and thermal stability to the material, and it is usually dispersed in the soft phase formed by the flexible segment. An example of a multi block copolymer nanostructure is shown in Fig. 5. The hard phase can be formed by a glassy amorphous polymer with a high glass transition temperature (Tg) or a semi-crystalline polymer, and the soft phase can be formed by a semi-crystalline or amorphous polymers with a low Tg and a relatively low melting temperature (Tm). For gas separation membranes, the segments must be carefully selected before synthesizing a novel polymer, i.e. the combination of both segments should result in high gas permeability, high selectivity and good mechanical and thermal stability.
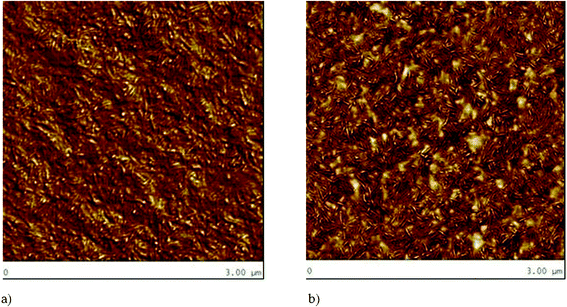 | ||
| Fig. 5 Typical multi block copolymer nanostructures (AFM) showing the hard and soft separated phases; (a) poly(ethylene oxide)-poly(buthylene terephthalate) and b) poly(ethylene oxide)-poly(buthylene terephthalate/PEG blend. As seen, the degree of phase separation is different between them, and the hard semi-crystalline poly(buthylene terephthalate) as rod-like domains dispersed in the poly(ethylene oxide) soft phase is clearly distinguished, especially in the blends. Reproduced with permission from ref. 94. Copyright of American Chemical Society. | ||
The most studied multi-block copolymers are polyurethanes, poly(ether-ester)s and poly(ether-amide)s. Polyurethane based membranes were studied in the 1980s as gas separation membranes,95,96 but nowadays these kinds of copolymers are still being investigated due to their versatility in terms of synthesis. Freeman’s group, for example, recently reported the synthesis of a series of poly(urethane-urea)s containing poly(ether)s.97 They tried to synthesize a membrane with improved gas separation properties as a CO2-selective membrane; they correlated the fractional free volume with the molecular weight of polyethers as well as the gas permeability. Gomes et al.98 also investigated polyurethane membranes, but in this case containing ether-siloxane segments. They reported that these copolymers could have potential applications in the separation of n-C4H10/CH4. In order to improve the gas permeability or selectivity, nanocomposite membranes from polyurethanes were also prepared;99 however, it was unsuccessful because the gas permeability decreased with increasing selectivity or vice-versa (typical trade-off of gas separation membranes).
Poly(ether-amides)s containing poly(ethylene oxide) (PEO) as well as poly(ether-ester)s and polyurethanes were investigated as highly CO2-selective materials. In recent years, the number of published papers on the development of membranes from block copolymers containing PEO has greatly grown. Since PEG or PEO (i.e. ethylene oxide units) was discovered as a functional group with a high affinity for CO2,100 almost all work related to multi-block copolymers containing PEO has been focused on membrane fabrication for CO2 separation. Poly(ethylene oxide-b-amide) has been one of the most studied block copolymers as a CO2-selective membrane. Although this copolymer was developed in 1972 as a thermoplastic elastomer (Pebax), only in 1990 was it investigated as a membrane material for gas separation.90 However, due to solubility problems, study of this copolymer was not any more intensive; thus new multi-block copolymers were explored. Among these are the poly(ether ester)s, especially the poly(ethylene oxide)-poly(butylene terephthalate), known commercially as Polyactive.101 This multiblock copolymer however presented solubility problems too, hence the studies were also limited.
Pebax membranes form nanostructures of soft and hard phase;102 the typical hard phases structure has a size of around 6 nm and the longest around 17 nm. Gas permeability generally increases with the size of the polyether domains, but imperfect microphase separation exhibits better gas permeation. The size of the nanodomains of Pebax with a high polyether content depends on the used solvent during casting and on the water content in the membrane.
In recent years, interest in improving the separation performance of Pebax started again thanks to its affinity for CO2; thereby membranes for CO2 capture are being developed by using this copolymer. Sridhar et al.103 cross-linked Pebax and evaluated its performance for CO2/CH4 separation. The gas permeance was decreased to a great extent and the selectivity increased (expectedly), the interesting fact was that CO2/CH4 selectivity reached up to 47 (more than twice than that for pristine Pebax). Hamouda et al.104 and Sridhar et al.105 studied Pebax membranes filled with silver nanoparticles and reported the water uptake properties and CO2/CH4 separation. Car et al.106,107 reported the modification of Pebax by PEG; they used a simple binary mixture as the solvent (water/ethanol) and studied the gas separation properties with respect to the resulting morphology. They used PEG to increase the number of ethylene oxide units; hence the polymer–CO2 affinity was enhanced. Thanks to these reports, Pebax copolymer was again considered as a potential membrane material, and later, several reports have been focused on the improvement of this copolymer. Sijbesma et al.108 for example investigated the water permeation and stability under flow of the flue gas; they suggested that polymeric membranes are viable to directly separate CO2 from the flue gas. Louie et al.109 studied the effect of Pebax coating on reverse osmosis (RO) membranes, the RO membranes had defects and by using a thin film of Pebax the gas permeation was greatly decreased but the selectivity was enhanced two-fold. They also suggested that by treating Pebax with different solvents, the morphology can be changed. In general, the separation performance of the Pebax membrane mainly depends on the nature of the block copolymer (Pebax is a trade name of different poly(ether-amide)s), i.e. as a CO2-selective membrane it depends on PEO content.
Because of their rubber-like properties, Pebax membranes are improved due to their solubility selectivity. But later a report demonstrated that not only is this fact important but so is diffusivity selectivity; and thus it was suggested that by increasing the free volume of Pebax, the CO2 permeability and selectivity can be simultaneously enhanced.110 Pebax was also blended with PEG-DME (di-methyl ether), and they were investigated following the strategy of increasing the free volume.111 The DME end group of PEG hindered PEO crystallization and enhanced the micro-phase separation; thus the CO2 permeability was increased up to 8-fold, and the CO2/H2 selectivity was simultaneously enhanced from 9 to 15. Another similar work was reported by Reijerkerk et al.,112 but instead of PEG-DME they used PEG-PDMS, which improved the Pebax gas permeation due to the presence of PDMS.
The incorporation of quaternary ammonium compounds into the Pebax was also investigated; since the ammonium compounds have a high affinity for CO2, the gas solubility can be greatly enhanced.113 The gas permeation with a humid feed revealed that these blend membranes have extremely high permeability (the CO2 permeability was increased up to 35 fold) without selectivity loss. Pebax/multiwalled carbon nanotube (MWNT) hybrid membranes were also investigated;114 the authors characterized the hybrid membranes by different techniques to understand the effect of MWNTs on the polymer–MWNT interaction, crystallinity, cross-linking degree and gas separation performance. Of interest was the strategy used, i.e. manipulation of the free volume. A Pebax/polyhedral oligosilsesquioxane hybrid membrane was also prepared and investigated for pervaporation,115 but it could also be used for gas separation when water vapour is present.
Recently, a complex Pebax/AgBF4 thin nano-hybrid membrane was developed;116 the thickness of the thin film was between 100 and 200 nm and the propylene/propane separation was explored. Zeolites were also incorporated into the Pebax membranes and better permeability was observed too.117
A tubular Pebax/ceramic hybrid nanocomposite membrane was also fabricated and examined in terms of separation performance.118 Although the idea of using a ceramic porous support is something interesting, it did not really improve the membrane performance of Pebax; the microphase separation led to the same nanostructure observed when a polymeric porous support is used (hard phase dispersed in a soft amorphous phase).
The use of poly(ether ester)s as a CO2-selective membrane is also of great interest.119 Poly(ethylene oxide)-poly(butylene terephthalate) block copolymer has been extensively studied by Peineman’s group,94,120,121 but the first studies of this multi-block copolymer as a gas separation membrane were undertaken by Mulder's group.101 This multi-block copolymer is highly CO2-selective, and the permeability is similar to or a little bit higher than that for Pebax. Through a detailed investigation, tailor-made membranes were fabricated from this copolymer,120 and after understanding the membrane behaviour and morphology, a new CO2-philic polymer was synthesized. The nanostructured blend membranes from this copolymer exhibited extremely high separation performance, i.e. the CO2-permeability was increased up to 5-fold without any loss of selectivity.94 The authors reported that by choosing the right smart additive, the separation performance of the membrane can be greatly improved. Based on this multi-block copolymer, nanometric thin film membranes with high gas flux and permeability were manufactured.121 Later, it was demonstrated that by a suitable molecular manipulation of this block copolymer and fabrication process, super permeable membranes with a constrained thickness can be developed for CO2 capture.122
Other poly(ether ester) multi-block copolymers were also explored and some of them had better gas permeability and selectivity than Pebax and Polyactive. Simmons123 for example patented a series of poly(ester-ether)s as a membrane for gas separation. However, the reported permeability values are higher than those of the commercial poly(ester-ether)s, e.g. for poly(ethylene oxide)-poly(butylene terephthalate) with ∼75% PEG (1500 g mol−1), the CO2 permeability is higher than 220 Barrer, a similar commercial polymer (Polyactive) has a CO2 permeability less than 120 Barrer.120 Another example is the CO2 permeability for Pebax, which is reported as ∼150 Barrer, but the CO2 permeability for the used Pebax reported by other authors is between 70 and 120 Barrer depending on the solvent used for casting.90,106 Different gas permeation properties (a fact that could depend on the method of processing) can only result due to the different morphologies (separated microphases). As the morphology is a factor that controls the gas transport through block copolymers, it could explain the differences in permeability. Therefore, one can conclude that block copolymers might self-assemble in different ways, due to the different methods used for the preparation of the films.
Block copolymers containing PEO and PPO soft phase and di-amide as the hard phase were also investigated.124–126 One paper reported by those authors suggested that uniformity of the crystal structure (size) in the hard phase improves the gas separation performance.126 Although this could be true to a certain extent, completely amorphous phases in both the hard and soft phase are preferred; thus the hard phase that is considered as impermeable becomes a little permeable due to the presence of flexible segments inter-dispersed between the rigid segments.94 Poly(tetramethylene) as the hard phase was also investigated in poly(ether-ester)s as CO2-selective membranes.127 It was interesting that polymers with the same features as Polyactive resulted in being more permeable; this could be explained in terms of chemical structure. A poly(ether-ester) containing poly(tetramethylene) could have a high gas permeability due to the number of methylenes in its glycol moiety; it is called odd-numbered polyester. Even-numbered polyesters are PBT and poly(ethylene terephthalate) (PET), hence a detailed study of poly(ether-ester)s with PET would demonstrate this hypothesis. Block copolymers with polyimide as the hard phase were also reported.128 Wessling's group at the University of Twente recently reported an overview of the advances in multi block copolymers and on the limit of gas separation performance.129Fig. 6 shows the modified plot of Robeson for CO2/N2 separation, where different multi-block copolymers are included.
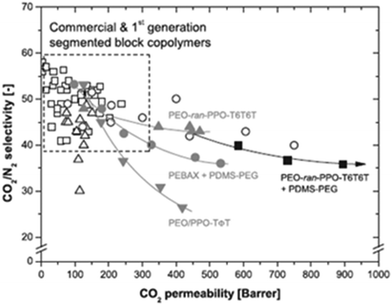 | ||
| Fig. 6 Modified Robeson plot, CO2/N2 selectivity as a function of CO2 permeability. Reproduced with permission from ref. 129. Copyright of Elsevier B.V. | ||
4.6 Design and strategy for optimal gas separation membrane fabrication
The versatility of block copolymers in terms of chemical structure and synthesis gives many opportunities to design novel membranes with outstanding properties. Nevertheless, for designing a required membrane material for gas separation, one must simply analyze eqn (2) and consider the following aspects before choosing the segments for the synthesis; (1) the sorption of gas molecules on the membrane surface (upstream), (2) the diffusion of gas through the membrane and (3) the desorption of gas from the membrane (downstream), i.e. by choosing segments that give high sorption properties, high free volume and good mechanical and thermal properties, tailor-made block copolymer membrane might be developed. The three factors described above are well represented by the diffusion–solution model for gas transport through polymeric membranes.130As presented in eqn (3), the overall selectivity can also be split into diffusivity selectivity and solubility selectivity. Therefore, during the design of new block copolymers, we must think about improving both selectivities, as well as the gas permeability. By enhancing both properties simultaneously, one can achieve a breakthrough in membrane science and technology.
In block copolymer membranes, different factors govern the mass transport, such as the chemical nature, the molecular weight and the content of each segment. Those factors are the most important parameters to be controlled during the synthesis. In addition to these parameters, the processability or solubility of the block copolymer in simple solvents must be also taken into account. Generally, the gas transport occurs through the amorphous soft phase; the hard phase is considered to be impermeable and does not contribute to the gas transport, but the importance of the hard phase is due to the physical cross-linking, i.e. it gives mechanical and thermal stability to the material. However, by modifying or manipulating the impermeable hard phase, one can also get some permeability through this phase, which would improve the total permeability.94
The self-assembly process of block copolymers was a forgotten aspect during membrane fabrication, i.e. the self-assembly process was not considered a tool to direct the desired morphology that could give high separation properties. The manipulation of block copolymer self-assembly by chemical or physical means may lead to desired nanostructures that give high permeability and high selectivity. Clear examples of improving the gas separation performance of block copolymers by manipulating their self-assembly are described for example in ref. 75, 122, 132.
The use of di-block copolymers containing PEO or functionalized PEO, which form cylindrical nanostructures perpendicular to the surface (as reported in the literature),131,132 can be used to embed carbon nanotubes, hence instead having PEO only as the permeable phase, the carbon nanotubes embedded within this phase parallel to the direction of gas transport would enhance the gas transport, resulting in a super-permeable nanohybrid membrane. It was reported that carbon nanotubes can be functionalized with PEG or PEO,133 thus it could be easily accommodated into the PEO phase. The challenges would be however to develop carbon nanotubes with controlled sizes, and strategies to embed the carbon nanotubes into the PEO phase. The size (length) of nanotubes should not be more than 100 nm and they should be open. As PEO has a high affinity for CO2 and the nanotubes exhibited unexpected transport properties,134 super-permeable membranes for CO2 separation might be easily developed. Research is therefore called upon to investigate this kind of nanohybrid material. This hypothesis is shown in Fig. 7, and upon analyzing eqn (2) it is simple to see that the solubility of CO2 due to PEO, and diffusivity due to the nanotubes, might be enhanced, thus resulting in high permeability. This hypothesis can be also applied to tri-block copolymers and other segments, depending on the gas mixture to be separated.
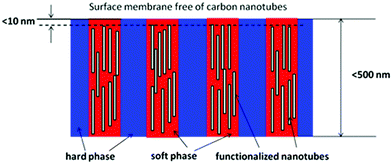 | ||
| Fig. 7 Di-block copolymer with a cylindrical nanostructure plus embedded carbon nanotubes; the soft segment could be PEO and the nanotubes could be functionalized with PEG to have a desired dispersion. In addition, the top surface should be free of nanotubes (a condition). Thus the selectivity would not be lost. As a CO2 selective membrane, this hypothetical membrane would be highly selective due to the presence of PEO, and super-permeable due to the nanotubes. | ||
The addition of other inorganic compounds is also a way to improve the gas separation performance of block-copolymer membranes. The incorporation and well-controlled dispersion of nanoparticles are being extensively studied,135,136 hence the developed strategies can be also used to design new membranes. Instead of using inorganic nanoparticles and nanotubes, the use of organic or biological compounds that have a high affinity for certain gases and can self-assemble within one phase are also interesting.137,138
Another way to improve the gas separation membranes by using block copolymers could be the use of organic–inorganic block materials, i.e. a polymer segment bonded to an inorganic material. In fact, Malenfant et al.139 investigated the self-assembly of organic–inorganic block copolymers. They reported that this new hybrid block copolymer enables the formation of ordered nanostructures with a tunable morphology and composition. This new block copolymer would be interesting to investigate as a membrane material. Through the polymer segment (soft or hard phase, i.e. rubber or glassy polymer), the gas molecules would permeate and the inorganic phase would give a high mechanical and thermal stability. As the inorganic particles form clusters with nanopores (between particles) of less than 2 nm, this phase of the material would also exhibit a high permeability, especially for condensable gases such as CO2 and n-butane. For instance, Yave et al. suggested that TiO2 nanoparticles form clusters (agglomeration of nanoparticles) with small pores through which the n-butane permeated faster than methane due to its condensability; thus the permeability and selectivity in the mixed gas was greatly increased.140 A recent report by Lau and Chung supports this hypothesis;141 they studied nanohybrid membranes containing nanoparticles that agglomerated, and the agglomeration formed small nanopores that enhanced the CO2 permeability.
The synthesis of new block copolymers with three or four different segments could be also another option to overcome the state-of-the-art of gas separation membranes. Either the selectivity or the permeability can be improved by adding a new segment. The addition of a third or fourth segment that creates more free volume and improves the solubility of gases would be a challenge. This new segment can even interpenetrate through each segment or self-assemble in a way that enhances the gas permeability and selectivity. An example would be to develop well defined tri-block copolymers containing PEO, PDMS and a rigid segment. Due to the PEO, the CO2 solubility would be high, the PDMS would contribute to the permeability and the rigid segment would give the mechanical and thermal stability. An additional example would be to develop a di-block copolymer containing PEO and PDMS. A variety of CO2-selective materials can be developed by manipulating the self-assembly and cross-linking the PDMS. Therefore, and in general, the synthesis of block copolymers containing segments that give a high free volume and high solubility to a certain gas is a topic to be investigated.
5 Carbon molecular sieve membranes
Very selective polymers are not permeable enough, and highly permeable polymers are not very selective. The permeability and the selectivity of polymeric membranes for gas separation is limited by the so called upper bound in a log–log plot of selectivity vs. permeability, also known as a Robeson diagram.12,22,142–145 Inorganic membranes are not bound to this restriction, due to the fact that the size of the pores is fixed and true molecular sieving is possible in this case. Examples are zeolites, amorphous silica, metal organic framework (MOF)146 and carbon molecular sieve (CMS) membranes. With respect to crystalline molecular sieves and MOFs, the pore size of amorphous oxides and CMS membranes is not determined by the lattice constraints of a crystalline material: even though a wide pore size distribution can easily be obtained as a consequence of unsuitable preparation conditions, on the other hand one single precursor can yield several different amorphous oxides or CMSs characterized by different pore sizes and size distributions, depending on the preparation protocol and procedure. In particular, CMSs are versatile materials, more permeable than zeolites, yet with sieving ability, the properties of which may be finely tuned to prepare membranes able to accomplish difficult separations. In fact, several studies report selectivities and permeabilities well in excess of the performance of polymeric membranes for difficult cases where the size of the molecules to be separated is very small, such as the C2 and C3 alkene/alkane pairs, the CO2/N2 and the O2/N2 separations.In the following section, a non-exhaustive description of the structure, precursors, pyrolysis methods, pre- and post-treatments and separation performances of CMS membranes for gas separation will be given. Details and extensive treatments can be found in the excellent reviews present in the literature.147–152
5.1 Structure of CMSs and CMS membranes for gas separation
Carbon molecular sieves are produced during the early stages of decomposition of polymeric precursors, and they are classified as amorphous because they do not display a well defined crystalline structure. However, small crystalline regions may coexist with amorphous char, organized in disordered aggregates with very little or no long range order.148 The graphene flakes in the small crystalline regions are often defective due to the lack of sp2 carbons far from the edge, can be haphazardly folded or crumpled together to form a so-called turbostratic structure, and in general the degree of order is so poor that the materials are considered essentially isotropic. Mesoporous carbon membranes are not the subject of the present description. Carbon molecular sieve membranes for gas separation usually have a structure consisting of slit-like cavities of 1–2 nm size (micropores) connected by pore restrictions (ultramicropores) of smaller size. This structure is usually modelled (Fig. 8) as an array of ink-bottle slit-like pores connected by ultramicropores that are able to discriminate between the molecules on the basis of size.148,153 As evidenced in Fig. 8 (c), the dimensions of these cavities, included the ultramicropore size dC, are not precisely defined, as is the case in crystalline molecular sieves, but are rather described by size distributions. The basic validity of these assumptions has been confirmed by a recent modelling study on a commercial material (Shirasagi CMS 3 K-161) which indicates that the investigated CMS is a structurally heterogeneous material composed of stacks of slit-shaped, turbostratic carbon nanopores of different sizes embedded in an amorphous carbon matrix.154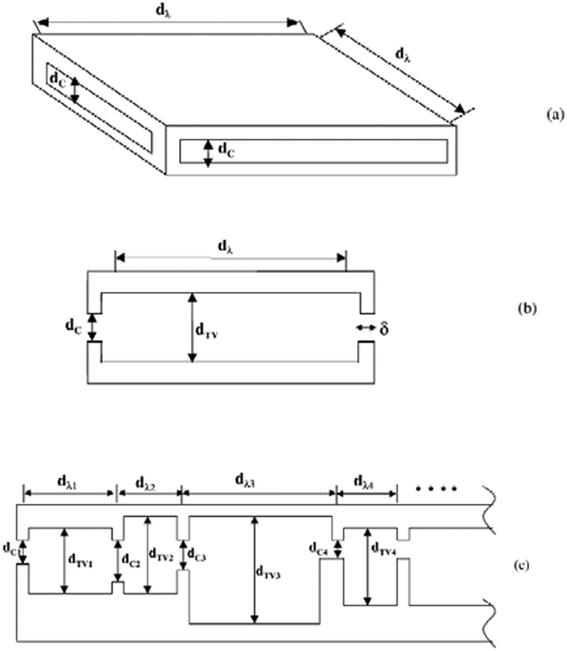 | ||
| Fig. 8 (a) A schematic representation of a 3D slit-like cavity in a CMS membrane. (b) Cross-section through (a) showing the micropore size (dTV), the ultramicropore size (dC), the jump length dimension (dλ) and the negligible “window thickness” dimension (δ). (c) Representation of the fact that dTV, dC and dλ are actually described by distributions. Reproduced with permission from ref. 153. Copyright of Elsevier B. V. | ||
5.2 Preparation of CMS membranes
In a typical CMS membrane preparation a polymeric precursor is pyrolysed in a vacuum or in an inert purge, usually N2, by slowly increasing the temperature, in most cases up to 500–800 °C, holding at that temperature for a certain thermal soak time, and then cooling down. The pyrolysis gases evolved leave behind pores, and at the same time the precursor forms the rigid turbostratic structures described in the previous section, along with a severe shrinking (up to 70% in some cases) of the membrane.Several parameters can be modified in order to finely tune the structure and the performance of CMS membranes: the nature of the precursor polymer, the pyrolysis temperature, the heating rate, the thermal soak time at the maximum temperature and the pyrolysis atmosphere, pre- and post-treatments.
Before we give a detailed description of some of these factors, it is useful to give a basic picture of the carbonization process for Matrimid, a typical polyimide precursor, in vacuum or in an inert atmosphere. The only species released up to 400 °C is water. Large molecules (50–240 Dalton) are only released from 400 to 600 °C, and this gives rise to the formation of micropores. From 550 °C, significant amounts of methane and carbon oxides are given off due to the degradation of imide and benzene rings. The decomposition rigidifies the structure of the precursor, and the release of small molecules reasonably gives rise to the formation of the ultramicropores. From 700 °C upwards, the release of H2 becomes significant, and leads to the formation of an amorphous carbon material155 and eventually to graphitic structures. The micropores formed during the low temperature pyrolysis of Matrimid (400–600 °C) shrink at higher temperatures, as evidenced by X-ray diffraction data and gas permeation experiments. The rule of thumb is that higher pyrolysis temperatures bring about a decrease of gas permeability, as well an increase of the gas selectivity in favor of small molecules.149
The role of the pyrolysis atmosphere has been studied by several researchers. Vacuum pyrolysis of PIs yields more selective but less permeable membranes than inert gas purge pyrolysis,155–157 and this effect has been explained with an increased heat and mass transfer from the gas phase, which accelerates the carbonization reaction and produces a more open porous matrix.156 At low flow rates of inert sweep gas, the non volatile by-products are not removed quickly enough, and they can presumably degrade further, to leave carbon deposits on the surface: this may explain the sharp reduction of flux observed in these cases.156
The role of small amounts of oxygen in the inert sweep gas has been investigated by Koros and co-workers.159–161 During the pyrolysis of Matrimid membranes, oxygen impurities (3–100 ppm in Ar) reduce both the permeance and the CO2/CH4 selectivity of the resulting CMS membranes. For a 6FDA/BPDA-DAM polyimide, instead, the increase of O2 concentration in the Ar sweep from 4 to 30 ppm brings about a reduction of fluxes but with an increase in CO2/CH4 selectivity, whereas 50 ppm O2 provoke a reduction of flux and selectivity. According to the authors, the oxidation at ppm levels of O2 takes place preferentially at the more reactive sp2 carbon edges of the selective pores, and as a consequence the size of the ultramicropores (dC in Fig. 8 (a)) is reduced. The oxidation process is controlled by the concentration of oxygen in the inert atmosphere.162 This oxidative process represents a powerful tool for finely tuning the pore size and the selective transport of CMS membranes.
One strategy for the improvement of the productivity of CMS membranes is the introduction in the precursor membrane of porogens, i.e. species which serve as templates for the formation of microporosity in carbon. This can be accomplished by blending with labile polymers (e.g. polyvinylpirrolidinone, PVP), which completely gasify during the pyrolysis step, by co-polymerization with labile monomers or by the introduction of thermally labile groups (e.g. –SO3H, –SO3−, alkyl) or ions (NO3−), which decompose.
During the pyrolysis of sulfonated PIs,169 the sulfur containing groups have already decomposed at 450 °C; when instead the loss of a sulfone moiety requires the breakage of two covalent bonds, the maximum peaks for the release of sulfur oxides are observed at or below 500 °C.162 In sulfonated poly(2,6-dimethylphenylene oxide) (PPO) the loss of the sulfonic acid group takes place at around 200 °C.163 PVP has been used as a porogen in blends with PIs,170,171 PPO172–174 and cellulose.175,176 Silica containing CMS membranes prepared by pyrolysis of poly(imide-co-dimethylsiloxane) on alumina supports demonstrated good separation factors for the He/N2, H2/N2, CO2/N2 and O2/N2 pairs:177,178 larger polydimethylsiloxane blocks in the co-polymer caused an increase in permeability at the expense of selectivity.179
Another strategy for the improvement of the productivity of CMS membranes is the dispersion in the precursor of cations that catalyse the thermal decomposition of the polymer. Trivalent cations (mainly Fe3+) introduced as nitrates proved the most effective species for the improvement of the CO2/CH4 and O2/N2 separation factor of cellulose derived carbon membranes, and MgO increased the H2/CO2 selectivity by reducing the CO2 permeability.180
Chung and co-workers produced flat CMSMs by cross-linking a polyetherketone with 2,6-bis(4-azidobenzylidene)-4-methylcyclohexanone and pyrolyzing at low temperature (450–650 °C).181–182 The decomposition of the bis-azide blended with the polymer produces extremely reactive nitrene intermediates, which are able to bind to the surrounding chains and stabilize the thermally labile polyetherketone. The membranes prepared at 550 °C exhibited excellent propene/propane ideal selectivity of 44 with a propane permeability of 48 Barrer, which decreased to 32 and 3.6 Barrer, respectively, with real mixtures.181 Similar membranes prepared at 800 °C displayed a CO2 permeability of 280 Barrer and ideal CO2/CH4 selectivity of 164.182
The pre-oxidation stage at 400 °C was accompanied by the decomposition of the sulfone group contained on the aromatic diamine comonomer, giving rise to sulfur oxides in the gas phase. Yoshimune and Haraya pre-oxidised sulfonated poly(phenylene oxide) hollow fibres in air at 260 °C for two hours before pyrolysis under vacuum, obtaining very thin (280 nm) and flexible CMS hollow fibres with high CO2/CH4 and H2/CH4 separation factors (118 and 392, respectively), and CO2 permeance in excess of 10−8 mol m−2 s−1 Pa−1.168 Also in this case the sulfur containing groups decomposed almost completely during the pre-oxidation stage.
Another interesting pretreatment which can improve the selectivity of polyimide P84 derived CMSMs is the soaking of the precursor membrane in a linear alcohol (the best effects were given by ethanol) prior to pyrolysis.183 The experimental evidence indicates that the weakening of the intramolecular cohesion forces caused by the sorption of ethanol allows a more effective structural re-organization of the polymer chains leading to narrower pores.
A similar method (propene187,188 or methane189 CVD and air oxidation at 250–350 °C) has been adopted by Blue Membranes GmbH for their honeycomb carbon membranes described in a later section.
Pyrolytic carbon deposition of benzene had been also used for the enhancement of the sorption selectivity of activated carbon fibres (ACFs) for pressure swing absorption.190–192 At a deposition temperature of 900 °C, benzene already pyrolysed in the gas phase to form larger molecules that could not enter the micropores, so that carbon deposited mainly on the external surface of the fibres and produced fibres with limited sorption capacity and poor CO2/CH4 sorption selectivity.191
At 727 °C, instead, the experimental evidence indicated that benzene could be sorbed as such into the micropores before pyrolysis, restricting the size of the micropores: as a consequence the sorption amount of CO2 reduced slightly with respect to the pristine ACFs, whereas a very sharp reduction of CH4 was observed, with high CO2/CH4 sorption selectivity.191
Morooka and co-workers used propene CVD at 650 °C on their supported CMSMs derived from polyimide to reduce the pore size and to increase the O2/N2 and CO2/N2 selectivity. It turned out that an optimum CVD time was two minutes: at longer times both permeances and selectivity were reduced.193
Another popular post-treatment method is oxidation. Morooka and co-workers treated their carbon membranes with 10% and 20% O2 for 3 h at 300 °C, obtaining a generalized increase of permeance and almost no change in selectivity. The treatment in air at 100 °C for 30 days led to a decrease in permeability with an increase in selectivity; since the former performance could be recovered after heating in N2 at 600 °C, the authors raised the hypothesis that the 100 °C oxidation produces surface oxides that reduce the size of the ultramicropores.194
In both cases, the change in elemental composition of the membranes caused by the oxidation at 100–300 °C was modest, suggesting an increase in the micropore volume of the membrane, with almost no change in the pore size distribution.195
The post-oxidation of PPO derived CMSMs supported on alumina (air at 100–400 °C), instead produced a broadening of the pore size distribution and a drop in all selectivity values.196–198
BPDA-ODA polyimide (PI) and PEI derived CMS membranes were coated with PPO and pyrolyzed again at 600 °C.199 The second pyrolysis caused the expansion of the pores of the pristine CMS membranes, and at the same time the decomposition of the thermally labile PPO produced the deposition of carbon at the mouth of the pores. As a result, more permeable and more selective membranes were formed. In particular, the PI derived membrane showed outstanding CO2 and H2 permeability (1320 and 1450 Barrer, respectively) coupled with very high CO2/N2 (156), CO2/CH4 (157) and H2/CH4 (172) selectivity.
5.3 CMS membrane modules
The brittleness of carbon molecular sieve membranes is a major problem for the preparation and durability of a module. Carbon Membranes Ltd. (Israel) was the first company to produce high quality hollow fibre membrane modules on an industrial scale.184 The hollow fibres, 1 m long, had a diameter of 170 μm and a thickness of 9 μm (Fig. 9, left).186 The packing density was about 2000 m2 m−3 and each module contained 4 m2.188 Carbon Membranes Ltd. started the commercialization of its membranes and modules at the end of the 1990s, but ceased its activity in 2001.Blue Membranes GmbH, based in Wiesbaden, Germany, was the second company to develop and construct a CMSM module of a quite different concept,188,189 in which the membrane and the module are produced at the same time. Bulky ceramic supports severely limit the maximum membrane packing density of the CMSM that can be obtained, and also interfere with the shrinking of the pyrolizing material and introduce mechanical stresses that reduce the resistance of the membrane: the choice of a sheet of paper reinforced with ceramic fibres greatly reduced these risks. The paper was first impregnated with a mixture of a phenolic resin and an epoxy glue dissolved in 2-butanone; then a diagonal pattern was stamped on the dry composite, so that the direction of the wavy motif had a diagonal orientation with respect to the sheet side (Fig. 9a, right). The rippled sheet was then pleated (Fig. 9b, right), creating flow channels for both the feed and the permeate. Next, alternate pairs of folds were sealed at their edges, as shown in Fig. 9c (right), isolating in this way the feed from the permeate side. The pockets that result at this stage of the preparation process resemble the pockets to be glued on the central permeate collector tube in a spiral wound module; in this case, however, no spacer was needed to separate the facing membranes within the pockets. After curing at moderate temperature to bond the points of contact of the sheets, the nascent membranes were pyrolyzed under nitrogen up to 780 °C, then underwent a CVD treatment followed by air oxidation, as described in section 5.2.3, to finely tune the gas separation properties. The structure of the membranes, 50–100 μm thick, comprised a macroporous support with a separating layer of 10–30 μm. The resulting membranes had a high packing density—up to 2500 m2 m−3—and were housed in modules containing up to 10 m2 of membrane area.188
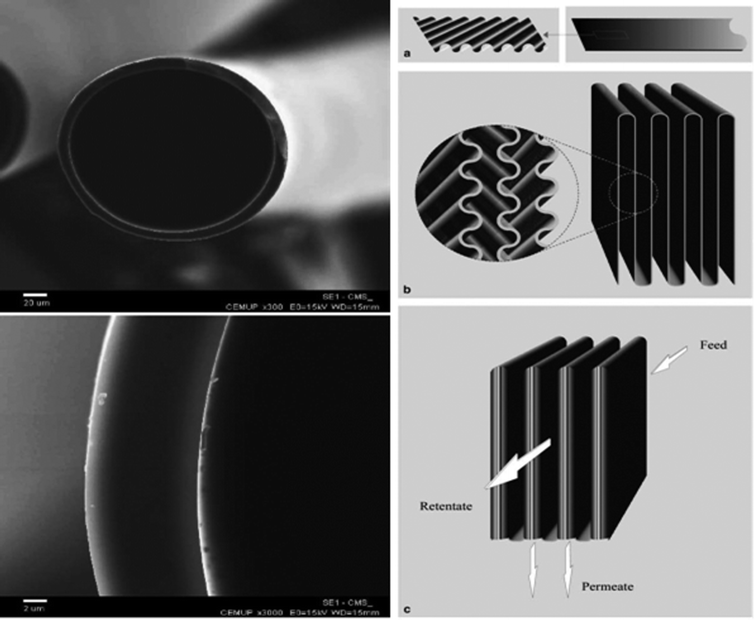 | ||
| Fig. 9 SEM pictures of a hollow fibre manufactured by Carbon Membranes Ltd (left). Reproduced with permission from ref. 186. Copyright of Elsevier B.V. Scheme of the preparation of the Blue Membranes GmbH honeycomb CMSM module (right). Reproduced with permission from ref. 188. Copyright of Elsevier B.V. | ||
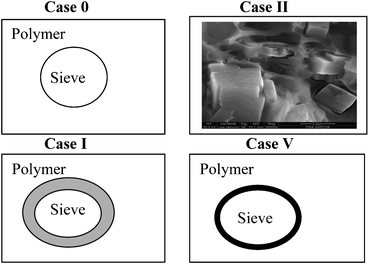 | ||
| Fig. 10 Mixed matrix membrane morphologies. | ||
6 MMMs
MMMs are composed of homogeneously interpenetrating polymeric and inorganic particles. Significant improvement in separation properties compared to neat polymers is expected for the resultant mixed matrix membranes (MMMs).200 Generally, the literature has focused on the incorporation of inorganic particles like zeolites, carbon molecular sieves and non-porous silica particles201 as a dispersed inorganic phase in a polymer matrix. Inorganic particles added to a polymer matrix can have three effects on the permeability: they can act as molecular sieves altering the permeability, they can disrupt the polymeric structure increasing the permeability too and they can act as barriers reducing permeability.202 However, it has been found that the performance of MMMs is not a simple addition of the intrinsic properties of the individual phase. Many variables may seriously affect MMM performance, making it difficult to understand. Currently, the major concerns in research on MMMs are a suitable combination of polymers and particles, the physical properties of the inorganic fillers (e.g., particle size and particle agglomerations), and the polymer/particle interface morphologies.2036.1 Molecular design and key advances in MMMs: choice of the polymer and of the filler
Many variables may affect the performance of MMMs, such as a suitable combination of polymers and particles, the physical properties of the inorganic fillers (particles size, surface area, aspect ratio) and their physical-chemical properties (hydrophilicity, hydrophobicity). Even though the selection of an appropriate inorganic filler was the major concern in the development of MMMs, it has been found that the choice of a suitable polymer as hosting matrix is crucial for MMM performance. In Table 2 an overview of some polymers used for the preparation of MMMs is reported. The transport properties are strongly dependent on the adhesion between the filler and the hosting matrix. Both rubber and glassy polymers are used. The most used rubber polymer is PDMS. Usually the fillers are added before the crosslinking step to enhance the adhesion with the polymeric matrix. It has been shown that successful mixed matrix materials can be formed easily by using flexible polymers, i.e. those with a relatively low glass transition temperature. For example, poly(vinyl acetate) (PVAc) has a low glass transition temperature (35 °C) and for high zeolite loading (e.g. zeolite 4A) the priming of the zeolites before their entrapment in the polymeric solutions is enough to obtain defect free membranes. Priming the zeolites involves adsorbing a layer of polymer onto the surface of the sieve. For rigid polymeric materials i.e. with high Tg, for example PES, Psf, Matrimid PI, 6FDA-6FpDA-DABA, Teflon AF and Hyflon AD (Table 2), the priming step is not sufficient to obtain good adhesion between the polymer and the sieve. Attempts at developing MMMs based on glassy polymers may be classified as fabrication methods with and without modifying the filler surface. The methods used without modifying the filler surface include the use of compatibilizers and the preparation protocols applying high processing temperatures during membrane formation.205 Mahajan et al.206 maintain the polymer flexibility during the membrane formation by applying processing temperatures close to the Tg of polymeric materials, coupled with the use of a non-volatile solvent. In fact, the addition of plasticizers (or compatibilizers) may lower the intrinsic gas separation performance of polymeric materials. Moore and Koros207 summarize the relationships between MMM morphologies and transport properties in five cases. Case 0 represents the ideal case. Case I consists of the presence of a rigidified polymer layer; cases II and III have voids at the interface with the effective void thickness of different size; cases IV and V are both caused by sorption of a strongly held molecule. In particular, in case IV, the strongly held molecule completely prevents the penetrants of interest from entering the zeolites, whereas in case V, the penetrants of interest enter through the zeolite at a slower rate than usual. In Fig. 10, cases 0, I, II and V are represented.| Polymer | Structure | Polymer | Structure |
|---|---|---|---|
| ABS |
|
PDMS |
|
| Teflon® AFs |
|
CA |
|
| Hyflon® ADs |
|
PC |
|
| 6FDA-6FpDA-DABA |
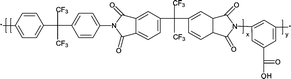
|
Matrimid PI |
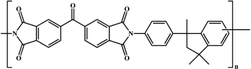
|
| PMP |
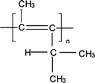
|
PTMSP |
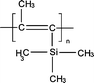
|
| PES |
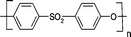
|
PVAc |
|
| PSf |
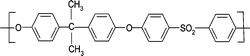
|
PEI |
|
The addition of a proper silane coupling agent on the zeolite surface may reduce the voids between the unmodified zeolite and the polymer phases.
In Fig. 11 this aspect is illustrated for the preparation of MMMs based on a glassy polymer with high Tg (polyimide, PI84) and surface modified SAPO-34 molecular sieves.
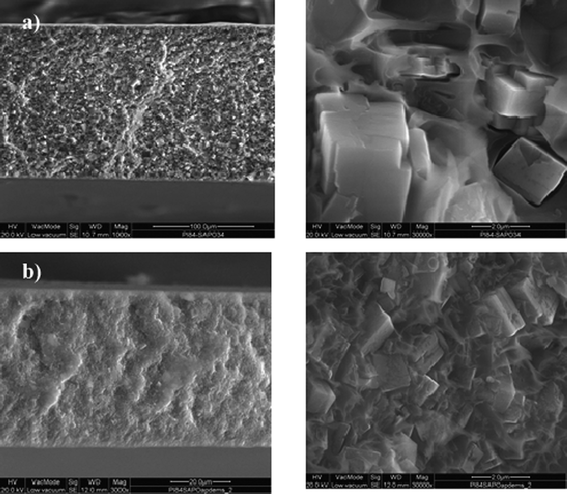 | ||
| Fig. 11 SEM pictures of MMMs based on PI84 and SAPO-34 molecular sieves functionalized with two types of silane agents containing (a) phenetyl and (b) aminopropyl groups. Overall (left) and magnification (right) of the membrane cross section. | ||
The successful compatibilization between the inorganic and organic phases is due to the presence of amino groups on the surface functionalized SAPO-34 molecular sieves: they react with the imino ring of the polyimide, favoring the interaction with the hosting polymeric matrix.208
In Table 3, an overview of the most used porous fillers for MMMs is given. They are materials with excellent gas separation properties, including zeolites and carbon molecular sieves (CMSs). There are many types of filler other than zeolites and CMSs that have been employed as filler: ordered mesoporous silica, silica spheres, carbon nanotubes and metal organic frameworks (MOFs).
| Filler | Category | Pore size (Å) | Structure |
|---|---|---|---|
| 3A, 4A, 5A (LTA) | Zeolite | 3.2–4.3 | 3D |
| ITQ-29 (LTA; Si/Ge = 100-∞) | Zeolite | 4.2 | 3D |
| SAPO-34 (CHA) | Zeolite | 3.8 | 3D |
| SSZ-13 (CHA) | Zeolite | 3.8 | 3D |
| ZSM-2 (FAU) | Zeolite | 7.4 | 3D |
| Silicalite-1 (MFI) | Zeolite | 5.5 | 3D |
| ZSM-5 (MFI) | Zeolite | 5.5 | 3D |
| Beta (BEA) | Zeolite | 7.6 × 6.4 | 3D |
| Y (FAU) | Zeolite | 7.4 | 3D |
| CMS | CMS | 4–5 | 2D |
| MWCNTs | CNTs | 20 | 3D |
| SWCNT | CNT | 20 | 3D |
| ZIF-90 | MOF | 3.5 | 3D |
| ZIF-8 | MOF | 3.4 | 3D |
| MOF-5 | MOF | 6.7 | 3D |
| HKUST-1 | MOF | 9.0 | 3D |
| Cu-DHBC-BPY | MOF | 3.6 × 4.2 | 2D |
| Cu-BPY-HFS | MOF | 8.0 × 8.0 | 2D |
| Cu-TPA | MOF | 5.2 | 2D |
| [Cu3(BTC)2] | MOF | 9 | 3D |
| MIL-53(Al) | MOF | 13.0, 7.9 | 3D |
| SBA-15 | Ordered mesoporous silica | 40–140 | 2D |
| MCM-41 | Ordered mesoporous silica | 15–200 | 1D |
| Hollow silicalite-1 spheres | Zeolite macrostructures | 3D | |
| Halloysite nanotubes | Mineral clays | 100–1000 | 3D |
Non-porous fillers have been proposed and used for MMMs in various studies (Table 4). The most used fillers are silica nanoparticles. The introduction of impermeable particles modifies the packing structure of the polymer matrix. In particular, most of the polymers coupled with non-porous fillers are high free volume polymers (PMP, PTMSP, Teflon AFs). The presence of fumed silica nanoparticles further increases both the size of the larger holes and their fraction, as demonstrated by positron annihilation lifetime spectroscopy (PALS).209,210
| Filler | Polymer | Ref. |
|---|---|---|
| Fumed silica nanoparticles | Teflon AF1600 | 212 |
| Fumed silica nanoparticles | Teflon AF2400 | 213,209 |
| Fumed silica nanoparticles | PSf | 214 |
| Fumed silica nanoparticles | PIM-1 | 215 |
| Fumed silica nanoparticles | PTMSP | 210,216,217 |
| Fumed silica nanoparticles | PMP | 218,219 |
| TiO2 | PMP | 140 |
| TiO2 | Poly(amide-imide) | 219,220 |
| Modified fumed silica nanoparticles | PDMS | 221–223 |
The high free volume reduces the importance of diffusivity selectivity (see section 2, Gas separation transport mechanism: theory), so that solubility selectivity becomes dominant for the overall separation process (reverse selectivity). As we know, in size-selective polymer dense membranes, small molecules preferentially permeate relative to larger ones. However, in membranes with reverse-selective properties, the larger one preferentially permeates in a gas mixture. The presence of nano-sized particles in the polymeric matrix increases the accessible free-volume compared to neat polymer. The resultant MMMs present an improvement in both permeability and selectivity for the larger molecules, with a mechanism very similar to that of microporous carbons, for which selective surface flow and solubility selectivity prevail.211
6.2 Gas transport models for MMMs
Different theoretical models are used to describe the gas permeabilities in MMMs.The Maxwell model, originally developed for the estimation of electrical conductivity of composite materials,224 can be adapted to permeability as:
 | (6) |
Where Pr is the relative permeability of the species, P is the effective permeability of the species in MMM, Pm is the permeability of the matrix (continuous phase), ϕ is the volume fraction of the fillers, λdm is the permeability ratio Pd/Pm (Pd is the permeability of the species in the dispersed phase).
This model predicts well for a spherical filler with a low-moderate concentration (ϕ < 0.2). It does not take into account the effects of particle distribution, particle shape or aggregation of particles.
A model developed by Cussler is similar in form, but considers a staggered array of high aspect ratio particles (eqn (7)).225
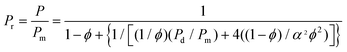 | (7) |
Where α is the filler aspect ratio (the ratio of the longest to the shortest dimension of the filler). Cussler defines α as half this ratio and his equation differs accordingly. Hence, the Cussler model takes into account the increased tortuosity of the path of the diffusing gas because of the non-spherical shape of the fillers.
The Cussler model is reasonably successful for predicting MMM performance when the aspect ratio is large and the volume is moderate to large (ϕ > 0.2).225,226 However, there are many combinations of volume fractions and aspect ratios for which neither the Maxwell nor the Cussler models are successful for predicting MMM performance. In eqn (8) a modification of the Cussler model manages to fill much of this gap:
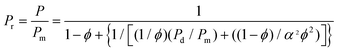 | (8) |
The above models assume ideal contact between the filler particles and the matrix. More often, as reported by Moore and Koros207 the contact between the particles and the matrix phase is defective; dewetting of polymer chains from the filler surface often occurs, resulting in void space between the two phases (Fig. 10, case II). Another possible situation is that the polymer in direct contact with the filler surface becomes rigidified in comparison to the bulk polymer phase (Fig. 10, case I). Therefore, the permeability of a species in the interfacial region surrounding the filler particles is often significantly different from the permeability in the bulk polymer matrix. Mahajan and Koros227 proposed a three-phase Maxwell model (3MM): the Maxwell model is first applied to a combined sieve and interphase region to estimate an effective permeability (PI). In eqn (9), ϕs is the volume fraction of the sieve phase in the combined sieve and rigidified interfacial matrix layer phase. This effective permeability is then used in the Maxwell model, along with bulk polymer permeability, to estimate the effective permeability of a three-phase system (eqn (10). The 3MM model can predict, in the case of sieve-in-a-cage (SIAC) and leaky interface defects, the dimension of interfacial voids.
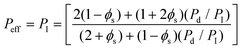 | (9) |
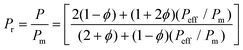 | (10) |
Other models, in addition to those reported above, have been proposed and evaluated by Hashemifard et al.204 by using the parameters that reflect the morphology and separation characteristics of the MMMs (matrix or polymer permeability, inorganic permeability, MMM permeability, inorganic loading, inorganic particle size and interphase thickness), the Lewis-Nielsen model, the modified Lewis-Nielsen model and the Felske model.204 Generally, the only parameter that is challengeable is inorganic permeability. Sheffel and Tsapatsis226 proposed a semi-empirical approach for predicting the performance of mixed matrix membranes. The predictions of this approach were compared to experimental results for two cases studies, PTMSP/silicalite-1 membrane for the separation of normal and iso-butane and the separation of carbon dioxide and methane by MMMs containing CHA-type molecular sieves.
Fig. 12 indicates how to choose a matrix material in order to optimize mixed matrix membrane selectivity.
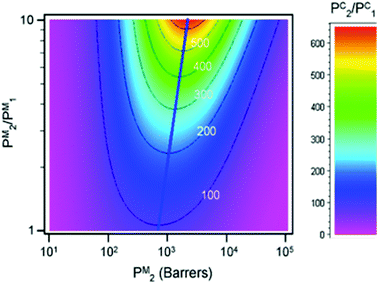 | ||
| Fig. 12 Prediction of the selectivity P2C/P1C of a mixed matrix membrane containing fillers with an aspect ratio of α = 50 and loading of ϕ = 30% as a function of matrix permeability (P2M) and selectivity (P2M/P1M). The colours (right) represent the selectivity of MMMs that would result from forming a membrane based on a matrix with a given permeability (x-axis) and selectivity (y-axis). Reproduced with permission from ref. 226. Copyright of Elsevier B.V. | ||
The Robeson upper bound curve12 is a guideline about which matrix materials are actually experimentally available. If the Robeson line and the selectivity ridgeline of Fig. 12 (the blue line) intersect at a point where the Robeson line is populated with polymeric materials, this point will provide guidance about which polymer to choose.
For example, for MMMs based on zeolite 4A, the gas separation performance with polymeric matrix as PVAc228 and Ultem PEI229 indicates that the higher intrinsic selectivity of Ultem produces MMMs more selective than those based on PVAc. A highly permeable polymer matrix may make the filler useless, because the majority of gas diffusion will occur through the phases with lower transport resistance instead of the filler phase possessing a higher separation performance.
Of interest in evaluating the importance of the physical properties of the zeolite and of the combination polymer/zeolite is the example proposed by Sheffel and Tsapatsis: CO2/CH4 separation by means of membranes containing CHA-type molecular sieves (see next paragraph for recent experimental works for this separation).226Fig. 13 shows the predictions for a mixed matrix membrane containing 30 vol% CHA molecular sieves with an aspect ratio of 30 operating at a feed pressure of 104 kPa.
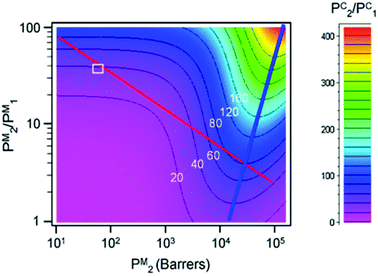 | ||
| Fig. 13 Predictions of the CO2/CH4 selectivity (P2C/P1C) of a mixed matrix membrane containing dispersed CHA crystals at a loading of 30 vol% and an aspect ratio of α = 30 as a function of matrix permeability (P2M) and selectivity (P2M/P1M). Reproduced with permission from ref. 226. Copyright of Elsevier B.V. | ||
The upper bound curve CO2/CH4, represented by Robeson12 is shown as a red line.
Fig. 13 shows that a polymer with a CO2 permeability of 3 × 104 Barrer and a selectivity of 4 makes possible an improvement in selectivity up to 80. For this particular separation, the necessity of using large aspect ratio particles (α = 30) is mandatory; without them the selectivity improvements are perhaps negligible. The second condition is that the MMMs operate at conditions that maximize the effective permeability of the zeolite, i.e. at high operating pressures.
However, the experimental results obtained with the membranes based on a cross-linked polymer and SSZ13 reported by Hillock et al.230 did show enhanced selectivity and permeability with a filler loading and an aspect ratio different from those proposed by the model; theoretical models need to include a more accurate picture of the experimental membrane geometry, perhaps including interfacial effects.
In Table 5 the experimental combinations (polymer/filler) for CO2/CH4 separation are reported; all are based on SAPO-34 (CHA structure) to evaluate the role played by the polymer matrix. Other polymer/filler combinations based on inorganic additives different from SAPO-34 are reported in the next paragraph.
| Entry # | Polymer | Separation performance MMMs | Separation performance neat polymer | Ref. | ||
|---|---|---|---|---|---|---|
| Permeability CO2 (Barrer) | Ideal selectivity | Permeability CO2 (Barrer) | Ideal selectivity (α) | |||
| 1 | PES | 5.12 | 24.9 | 2.88 | 29.4 | 231 |
| 2 | PES | 1.53 | 37.4 | 2.88 | 29.4 | 231 |
| 3 | PPZ | 48 | 17.5 | 71 | 15.3 | 232 |
| 4 | Poly(RTIL) | 477 | 19 | 9.2 | 39 | 233 |
| 5 | Poly(RTIL)-[C2 mim][Tf2N] | 77 | 30 | 44 | 27 | 233 |
Karatay et al.231 used PES as a polymeric matrix and evaluated the presence of a compatibilizer, 2-hydroxy 5-methyl aniline (HMA) (entry 2, Table 5) to improve the adhesion between the filler and the polymer: only by using HMA the ideal selectivity of MMMs (37.4) improves compared to neat polymer (29.4).
In the case of the PPZ matrix (entry 3, Table 5), which is more permeable to CO2 and less selective than PES (selectivity CO2/CH4 15.3), a slight improvement has been observed by adding the molecular sieves (17.5), confirming the general consideration that the gas diffusion occurs through the phases with lower transport resistance, instead of the filler phase, which possess higher separation performance.
Polymerizable room-temperature ionic liquid ((Poly(RTIL)) material has been used by Hudiono et al.233 with and without room-temperature ionic liquid (RTIL) additives (entries 4 and 5, Table 5). The addition of RTIL in the composite membrane facilitates interaction between the polymer and the inorganic particles of SAPO-34; the selectivity from 19 (entry 4, Table 5) without the compatibilizer increases up to 30 (entry 5, Table 5). Compared to PES and PPZ, the composite membranes show a better performance than the corresponding neat polymer for both permeability and selectivity. The increase in permeability is due to more rapid gas diffusion in the RTIL.
6.3 MMMs: recent progresses
In the present section, some recent advances of MMMs based on conventional fillers (i.e. zeolites and CMS) (Table 6) and unconventional fillers (Table 7) are reported. An overview of interesting examples of MMMs based on non porous fillers is reported above in Table 4. Recent reviews on MMMs by Aroon et al.,234 Zornoza et al.,235 provide additional examples and information.| Entry# | Filler | Polymer | Gas pair | Separation performancea | Ref. | |
|---|---|---|---|---|---|---|
| Permeability separated species (Barrer) | Ideal selectivity (α) | |||||
| a In brackets the separation performance for the neat polymer. | ||||||
| 1 | SSZ-13 | PDMC | CO2/CH4 | 148 (66.9) | 38.9 (36.4) | 236 |
| 2 | 4A | PVAc | CO2/CH4 | 4.33 (2.15) | 49.4 (33.5) | 237 |
| 3 | FAU/EMT | 6FDA-ODA | CO2/CH4 | 17.6 (16.5) | 80 (53.2) | 238 |
| 4 | ITQ-29 | PSf | H2/CH4 | 21.9 (97.7) | 118 (18) | 239 |
| 5 | Hollow silicalite-1 spheres | PSf | H2/CH4 | 15.4 (11.8) | 80.3 (58.9) | 240 |
| 6 | Hollow silicalite-1 spheres | PI | H2/CH4 | 38.4 (30.4) | 180 (132) | 240 |
| 7 | Hollow silicalite-1 spheres | Psf | O2/N2 | 2.3 (1.6) | 6.9 (4.7) | 240 |
| 8 | Hollow silicalite-1 spheres | PI | O2/N2 | 2.8 (1.9) | 8.5 (5.5) | 240 |
| 9 | CMS | PSf Udel P-1700 | O2/N2 | 6.52 (1.58) | 6.05 (5.5) | 241 |
| Entry | Filler | Polymer | Gas pair | Separation performancea | Ref. | |
|---|---|---|---|---|---|---|
| Permeability separated species (Barrer) | Ideal Selectivity (α) | |||||
a In brackets the separation performance for the neat polymer.
b The MMMs were characterized with a gas mixture CO2/CH4 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and the selectivity is not ideal. 1 and the selectivity is not ideal.
|
||||||
| 1b | ZIF-90 | 6FDA-DAM | CO2/CH4 | 720 (390) | 37 (24) | 245 |
| 2 | ZIF-8 | Matrimid | CO2/CH4 | 8.8 (9.8) | 81 (43.6) | 246 |
| 3 | ZIF-8 | PPEES | O2/N2 | 1.56 (1.03) | 6.2 (5.4) | 247 |
| 4 | ZIF-8 | PPEES | H2/N2 | 17.5 (7.9) | 70.0 (41.6) | 247 |
| 5 | MWCNTs | PES | CO2/CH4 | 6.79 (10.98) | 250.13 (51.26) | 251 |
| 6 | MWCNTs | PES | O2/N2 | 3.19 (2.13) | 10.65 (2.56) | 251 |
| 7 | MWCNTs | PES | CO2/N2 | 4.5 (2.75) | 21.8 (20.73) | 252 |
| 8 | MWCNTs | PEBAX1657 (crosslinked) | CO2/N2 | 17.5 (13.4) | 83.2 (55.8) | 114 |
| 9 | MWCNTs | PEBAX1657 (crosslinked) | O2/N2 | 1.05 (1.19) | 7.1 (4.95) | 114 |
| 10 | MWCNTs | PEBAX1657 (crosslinked) | H2/N2 | 7.18 (5.83) | 82.5 (24.8) | 114 |
| 11 | MWCNTs | PI | CO2/CH4 | 37.31 (16.83) | 16.5 (10.9) | 253 |
Ward and Koros236 prepared and evaluated MMMs based on SSZ-13 molecular sieves and crosslinkable polyimide (PDMC) for CO2/CH4. For unmodified filler (loading 25 wt%) a permeability enhancement of 129% and a decline in selectivity of 4.7% over neat PDMC are reported. The modification of the crystal surface (which presents sylanol groups) consisted in chemical linking to the polymer backbone during the steps of polymerization and polymer crosslinking. MMMs with 25 wt% surface modified crystals exhibit enhancements in both permeability and selectivity of 121% and 6.9%, respectively (entry 1, Table 6). The analysis of interfacial defects has been carried out by using the three-phase Maxwell model described by Mahajan and Koros.227
The CO2/CH4 separation has been investigated too by Adams et al.237 by using another combination zeolite/polymer, i.e. zeolite 4A and PVAc, respectively (entry 2, Table 6). High particle loading has been used (50 vol %). A selectivity increase of 47% with a lack of significant permeability has been observed.
Overall, it is shown that low performance, low cost polymers, like PVAc, typically not used for gas separation membranes, may be considered in real applications for gas separation.
An excellent combination polymer/zeolite has been proposed and investigated by Nik et al.238(entry 3, Table 6) for CO2/CH4; FAU/EMT intergrowth zeolites grafted with 3-aminopropylmethyldiethoxysilane (APMDES) in a polymeric matrix of 6FDA-ODA polyimide. The ideal selectivity for MMM prepared with the higher loading of aminosilane crystals (0.33 mmol g−1) is 80.0 with an enhancement of selectivity and permeability compared to neat polymer of 50.4% and 6.7%, respectively.
ITQ-29 crystals (entry 4, Table 6) were introduced, at different loadings (4, 8 and 12 wt%), into a commercial polysulfone matrix for the separation of H2/CH4, comparing the use of two solvents (tetrahydrofuran and dichloromethane) for the MMMs preparation.239
The cubic zeolite particles were highly dispersed but sparse for a 4 wt% ITQ-29 loading. This dispersibility decreased at the loading of 8 wt%, with agglomeration at the bottom of the MMM.
Promising results have been obtained for the 4 wt% ITQ-29/polysulfone membrane: the highest H2 permeability (21.9 Barrer) and a separation factor of 118. MMMs using the novel molecular sieves have potential industrial applications for key separations in energy saving processes, such as hydrogen recovery from flue gases.
Hollow silicalite-1 spheres (Fig. 14),240 obtained by hydrothermal synthesis from solid mesoporous silica spheres, seeded with silicalite 1 nanocrystals, have been used as fillers for two polymeric matrices, polysulfone Udel and polyimide Matrimid (entries 5–8, Table 6). For both the gas separations investigated, i.e. H2/CH4 and O2/N2, improved selectivity and permeability compared to neat polymers have been obtained.
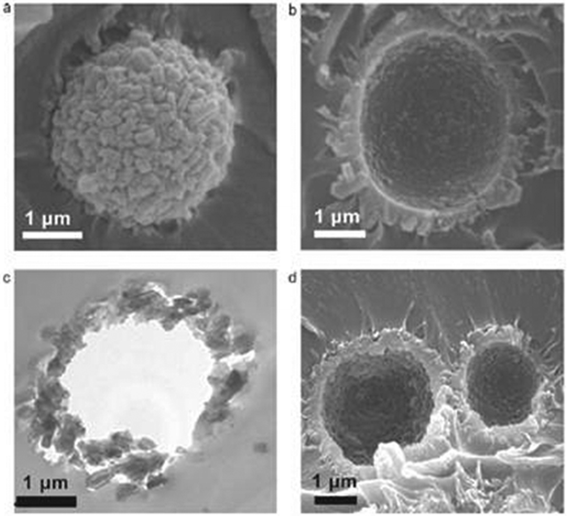 | ||
| Fig. 14 Cross section SEM or TEM images (a–c) 8 wt% HZSPSF MMMs; (d) 8 wt% HZSPI MMM. Reproduced with permission from ref. 240. Copyright of Elsevier B.V. | ||
Carbon molecular sieve particles in a polymeric matrix of polysulfone have been explored for gas separation (O2/N2) by Rafizah and Ismail (entry 9, Table 6).241
The CMS particles were treated with poly(vinyl pyrrolidone) kollidone 15 (PVP K-15) to improve the adhesion to the polysulfone matrix. An enhancement of both the selectivity (10%) and permeability (313%) has been obtained.
For untreated CMS particles a decrease in selectivity of 33% has been observed, confirming the crucial role of the crystal treatment.
Recent advances have shifted towards the addition of new fillers, namely MOFs, carbon nanotubes and layered silicates for the polymer matrix.
MOFs are among the most sophisticated nanostructured materials: their chemical environment can be fine-tuned by selecting the appropriate building blocks242 and/or by post-synthetic modification.243 The structural diversity of ZIFs is shown in Fig. 15.244
 | ||
| Fig. 15 The single crystal X-ray structures of ZIFs. (Left and center) In each row, the net is shown as a stick diagram (left) and as tiling (center). (Right) The largest cage in each ZIF is shown with ZnN4 tetrahedra in blue, and, for ZIF-5, InN6 octahedra in red. H atoms are omitted for clarity. Reproduced from ref. 244 (www.pnas.org). | ||
For each structure, the metal center is solely coordinated by the N atoms of imidazolate to give overall neutral frameworks. The five-membered imidazolate ring serves as the bridging unit between the Zn(II), Co(II), or In(III) centers and imparts an angle of 145° throughout the frameworks via coordinating N atoms in the 1,3-positions of the ring. The organic components of ZIFs provide for organically lined cages and channels rather than a silicate oxide surface as in zeolites.
Submicrometer-sized crystals ZIF-90 crystals were synthesized by non-solvent-induced crystallization and used for the fabrication of MMMs based on poly(imide)s as polymer matrices (entry 1, Table 7).245
ZIF-90 has a sodalite cage-like structure with 0.35 nm pore windows, through which size exclusion of CH4 (0.38 nm) from CO2/CH4 mixtures is possible. In addition, the imidazole linker in ZIF-90 contains a carbonyl group, able to facilitate the transport of CO2.
MMMs made with 6FDA-DAM (a highly permeable polymer) showed substantial enhancements in both CO2 permeability (85%) and selectivity (54%), for the gas mixture 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
Interestingly, the CO2/CH4 mixed-gas selectivity was higher than the ideal selectivity measured by pure-component gas permeation, presumably because of the selective sorption and diffusion of CO2 in the crystals.
The performances observed by Bae et al.245 of ZIF-90 MMMs based on polymers as Ultem and Matrimid (with the same filler loading of 15 wt %), are lower than those observed for 6FDA-DAM. The Maxwell model (applicable to systems with filler loading <20 vol%) predicts that when the gas permeability of the filler is much larger than that of the polymer matrix, there will be no enhancement in selectivity, even if the dispersed filler is highly selective. The CO2 permeability of ZIF-90 was found to be approximately 8000 Barrer. The CO2 permeability for both the polymers Ultem and Matrimid is in the range 1–10 Barrer, whilst for 6FDA-DAM it is 390 Barrer.
However, an improvement in selectivity by using Matrimid as the polymeric matrix and ZIF-8 as the filler, has been observed by Ordoñez et al. (entry 2, Table 7).246 ZIF-8 exhibits, like ZIF-90, the sodalite topology and its pore aperture is 3.4 Å. In this case, higher loading of the filler (50 wt% and 60 wt% ) than that used by Bae et al.245 is responsible for the different behavior. The observed enhancement of the ideal selectivities of the gas pairs containing small gases, such as H2/O2, H2/CO2, H2/CH4 and CO2/CH4 demonstrates a transition from a polymer-driven to a ZIF-8 controlled gas transport process. The control experiment using as-synthesized ZIF-8 crystals with filled pores did not show a transition at 50 wt%. These results suggest that additive loading >50 wt% may be required to observe this effect in MMMs.
Diaz et al. (entries 3 and 4, Table 7)247 fabricated MMMs based on ZIF-8 (with loadings of 10, 20 and 30 wt %) using as a polymeric matrix poly(1,4-phenylen ether-ether-sulfone (PPEES). The permeability of all gases increases as the ZIF-8 loading increases, without affecting the ideal selectivity in a significant way, confirming the results of Bae et al.245 Only the selectivity of the MMMs (30 wt %) for the gas pairs O2/N2 and H2/N2 is close to that marked by the Robeson upper-bound for this pair of gases.
Carbon nanotubes (CNTs) have been considered to be potential fillers for mixed matrix membranes due to their outstanding mechanical, thermal and electrical properties.248
CNTs can be synthesized as singular tubes called single walled carbon nanotubes (SWCNTs) or as a series of shells of different diameters spaced around a common axis, called multi-walled nanotubes (MWCNTs) (Fig. 16).
 | ||
| Fig. 16 Single walled carbon nanotube (left) and multi walled carbon nanotube (right)249 (http://en.wikipedia.org/wiki/Carbon_nanotube) | ||
Recent studies have shown that membranes incorporating CNTs show promise for high selectivity and throughput.250
CNTs possess several possible adsorption sites, such as interstitial channel sites with a high binding energy and pore sites with a large surface area. Moreover, for MWCNTs, the interlayer spaces between the graphite walls provide possible adsorption sites for small molecules exhibiting appreciable adsorption selectivities.
Gas permeation in CNTs was found to be several orders of magnitude faster than in other fillers such as zeolite.
Ismail et al. fabricated and characterized MMMs composed of functionalized MWCNTs and PES. In particular, the MWCNTs were functionalized with 3-aminopropyltriethoxysilane (APTES) to facilitate the dispersion of the fillers in the PES matrix.
The positive effect of the functionalization on the membrane performance (for CO2/CH4 and O2/N2) can be recognized on the basis of the comparison to the analogue MMMs prepared with MWCNTs unfunctionalized (1 wt % of loading): CO2/CH4 selectivity of 30.9 vs. 19.6 and O2/N2 selectivity of 6.2 vs. 2.6. The effect of CNTs loading in the range of 0.5–3.0 wt % has been evaluated. The analysis of the MMMs morphology revealed that at 0.5 wt % MWCNTs loading, the nanotubes were well dispersed throughout the PES matrix and a good adhesion of MWCNTs particles with PES matrix also was observed, while at 2 wt % and 3.0 wt % of MWCNTs loading, the nanotubes tended to agglomerate and formed interface void. The highest CO2/CH4 and O2/N2 selectivities were observed for a loading of 0.5 wt % (entries 5 and 6, Table 7),251i.e. 250 and 10.65, respectively. Beyond this loading, a decrease of selectivity with an enhancement of permeability was observed.
Ge et al. (entry 7, Table 7)252 explored PES as a hosting polymeric matrix for MMMs based on MWCNTs. MWCNTs were functionalized via H2SO4/HNO3.
The MMMs have been fabricated via the phase inversion method, exploring a loading range of 1–10 wt%. The gas permeability increased with nanotube loading of 1–5 wt% without loss of selectivity.
Only for the gas pair CO2/N2, a slight improvement in selectivity compared to the neat polymer is observed.
The combination Pebax 1657/MWCNTs (loading range of 0–5 wt %) has been evaluated for MMMs for gas separation by Murali et al. (entries 8–10, Table 7).114
Un-functionalized MWCNTs have been used for the fabrication of the MMMs. The polymeric matrix has been crosslinked by using 2,4-toluylene diisocyanate. Characterization by XRD and FTIR showed both physical and chemical interactions within the matrix. Optimization of the gas performance has been realized with a loading of 2 wt% for CO2/N2 and H2/N2; enhancement of the selectivity of 49% and 233% has been observed, respectively. In the case of O2/N2, the selectivity improved by 43% (entry 9, Table 7). By contrast, the un-crosslinked MMMs exhibited high flux with low selectivity.
Aroon et al. evaluated the effect of chitosan as a functionalization agent for MWCNTs.253 Polyimide (PI) has been used as the polymeric matrix.
The addition of raw MWCNTs to the PI matrix drastically decreases the permeability for both CO2 and CH4 gases, compared to neat PI, with an improvement of selectivity of 61%. These contrasting effects collocate the performance of the MMMs below the Robeson's upper bound.
In contrast, by using functionalized MWCNTs, an improvement in both the selectivity (51.4%) and permeability (122%) for the gas pair CO2/CH4 has been observed. In both the cases, i.e. raw and functionalized MWCNTs, a loading of 1 wt% was used. For the first case, the decrease in gas permeability can be attributed to the impermeable behaviour of raw MWCNTs (closed ended); in the second case, the increase of permeability with an enhancement in selectivity implies open ended nanotubes with good adhesion between the nanotubes and the polymeric matrix.253
7 Conclusions
The prediction of R. Baker in 2002,254i.e. that the growth of membrane gas separation technology will take place in the refinery, petrochemical and natural gas industries, is confirmed in the present. Success in these sectors will require membrane materials with high performance (high flux and selectivity), that are able to operate at 70–100 °C. High-cost (in terms of research investment) materials such as block copolymers, CMS and MMMs will displace traditional polymers, such as cellulose–acetate, polysulfone, polyaramide. Carbon membranes 10–100 times more expensive than the polymeric membranes for the separation of interest can only be used in applications in which polymeric membranes completely fail to make the separation and where the competition is distillation.References
- Membrane gas separation, ed. Y. Yampolskii and B. Freeman, Wiley, Chichester, 2010, ISBN 978-0-470-74621-9 Search PubMed.
- (a) R. Baker and K. Lokhandwala, Natural Gas Processing with Membranes: An Overview, Ind. Eng. Chem. Res., 2008, 47, 2109–2121 CrossRef CAS; (b) Membrane Engineering for the treatment of gases, ed. E. Drioli and G. Barbieri, RSC, 2011, ISBN 978-1-84973-348-9 Search PubMed.
- C.A. Hendricks, K. Blok, W.C. Turkenburg in The recovery of carbon dioxide from power plants, ed. P. A. Okken, R. J. Swart, S. Zwerver, Climate and Energy, Kluwer Academic Publishers, Dordrecht, 1989 Search PubMed.
- R. Bounaceur, N. Lape, D. Roizard, C. Vallieres and E. Favre, Membrane processes for post combustion carbon dioxide capture: a parametric study, Energy, 2006, 31, 2556–2570 CrossRef CAS.
- E. Favre, Carbon dioxide recovery from post-combustion processes: can gas permeation membranes compete with absorption?, J. Membr. Sci., 2007, 294, 50–59 CrossRef CAS.
- T. C. Merkel, H. Lin, X. Wei and R. Baker, Power plant post-combustion carbon dioxide capture: An opportunity for membranes, J. Membr. Sci., 2010, 59, 126–139 CrossRef.
- M. Kiyono, P. J. Williams and W. J. Koros, Effect of pyrolysis atmosphere on separation performance of carbon molecular sieve membranes, J. Membr. Sci., 2010, 359, 2–10 CrossRef CAS.
- S. Park, O. Yavuzcetin, B. Kim, M. T. Tuominen and T. P. Russell, A simple top-down/bottom-up approach to sectored, ordered arrays of nanoscopic elements using block copolymers, Small, 2009, 5, 1064–1069 CrossRef CAS PubMed.
- S. Ham, C. Shin, E. Kim, D. Y. Ryu, U. Jeong, T. P. Russell and C.J. Hawker, Microdomain orientation of PS-b-PMMA by controlled interfacial interactions, Macromolecules, 2008, 412, 6431–6437 CrossRef.
- W. Yave, A. Car, J. Wind and K.-V. Peinemann, Nanometric thin film membranes manufactured on square meter scale: Ultra-thin films for CO2 capture, Nanotechnology, 2010, 21, 395301–395308 CrossRef PubMed.
- T. S. Chung, L. Y. Jiang, Y. Li and S. Kulprathipanja, Mixed matrix membranes (MMMs) comprising organic polymers with dispersed inorganic fillers for gas separation, Prog. Polym. Sci., 2007, 32, 483–507 CrossRef CAS.
- L. M. Robeson, The upper bound revisited, J. Membr. Sci., 2008, 320, 390–400 CrossRef CAS.
- B. D. Freeman, I. Pinnau, Polymeric Materials for Gas Separations, In Polymer Membranes for Gas and Vapor Separations, ed. B. D. Freeman and I. Pinnau, American Chemical Society, Washington DC, 1999, 733, 1–27 Search PubMed.
- W. J. Koros and G. K. Fleming, Membrane-based Gas Separation, J. Membr. Sci., 1993, 83, 1–80 CrossRef CAS.
- D. Q. Vu, W. J. Koros and S. J. Miller, Mixed matrix membranes using carbon molecular sieves. I. Preparation and experimental results, J. Membr. Sci., 2003, 211, 311–34 CrossRef CAS.
- M. C. McCarthy, V. Varela-Guerrero, G. V. Barnett and H. K. Jeong, Synthesis of Zeolitic Imidazolate Framework Films and Membranes with Controlled Microstructures, Langmuir, 2010, 26, 14636–14641 CrossRef CAS PubMed.
- N. Du, H. B. Park, M. M. Dal-Cin and M. D. Guiver, Advances in high permeability polymeric membrane materials for CO2 separations, Energy Environ. Sci., 2012, 5, 7306–7322 CAS.
- S. Sridhar, B. Smith, M. Ramakrishn and T. M. Aminabhavi, Modified poly(phenylene oxide) membranes for the separation of carbon dioxide from methane, J. Membr. Sci., 2006, 280, 202–209 CrossRef CAS.
- G. C. Kapantaidakis and G. H. Koops, High Flux polyethersulfone-polyimide blend hollow fiber membranes for gas separation, J. Membr. Sci., 2002, 204, 153–171 CrossRef CAS.
- T.-H. Weng, H.-H. Tseng and M.-Y. Wey, Preparation and characterization of PPSU/PBNPI blend membrane for hydrogen separation, Int. J. Hydrogen Energy, 2008, 33, 4178–4182 CrossRef CAS.
- S. Basu, A. Cano-Odena and I. F.J. Vankelecom, Asymmetric membrane based on Matrimid® and polysulphone blends for enhanced permeance and stability in binary gas (CO2/CH4) mixture separations, Sep. Purif. Technol., 2010, 75, 15–21 CrossRef CAS.
- L.M. Robeson, Correlation of separation factor versus permeability for polymeric membranes, J. Membr. Sci., 1991, 62, 165–185 CrossRef CAS.
- B. D. Freeman, Basis of permeability/selectivity tradeoff relations in polymeric gas separation membranes, Macromolecules, 1999, 32, 375–380 CrossRef CAS.
- P. M. Budd, N. B. McKeown and D. Fritsch, Polymers of Intrinsic Microporosity (PIMs): High Free Volume Polymers for Membrane Applications, Macromol. Symp., 2006, 245, 403–405 CrossRef.
- A. Alentiev, Y. Yampolskii, J. Kostina and G. Bondarenko, New possibilities for increasing the selectivity of polymer gas separating membranes, Desalination, 2006, 199, 121–123 CrossRef CAS.
- K. C. Khulbe, C. Feng and T. Matsuura, The art of surface modification of synthetic polymeric membranes, J. Appl. Polym. Sci., 2010, 115, 855–895 CrossRef CAS.
- M. Anand, J. P. Hobbs, I. J. Brass in Surface fluorination of polymers in organofluorine chemistry: principles and commercial applications, ed. R. E. Banks, B. E. Smat, J. C. Tatlow, Plenum Press, New York, 1994, 469–481 Search PubMed.
- M. Mulder, Basic Principles of Membrane Technology, Kluwer Academic Publishers, Dordrecht, 1991, ISBN 0-7923-0978-2 Search PubMed.
- Ho, W.S.W.: US20006099621 (2000).
- Ho, W.S.W.: WO06050531 (2006).
- C. A. Scholes, S. E. Kentish and G. W. Stevens, Carbon Dioxide Separation through Polymeric Membrane Systems for Flue Gas Applications, Recent Patents on Chemical Engineering, 2008, 1, 52–66 CrossRef CAS.
- H. C. Ferraz, L. T. Duarte, M. Di Luccio, T. L. M. Alves, A. C. Habert and C. P. Borges, Recent achievements in facilitated transport membranes for separation processes, Braz. J. Chem. Eng., 2007, 24, 101–118 CrossRef CAS.
- R. D. Noble, Facilitated transport mechanism in fixed site carrier membranes, J. Membr. Sci., 1987, 60, 297–306 CrossRef.
- Y. Cai, Z. Wang, C. Yi, Y. Bai, J. Wang and S. Wang, Gas transport property of polyallylamine–poly(vinyl alcohol)/polysulfone composite membranes, J. Membr. Sci., 2008, 310, 184–196 CrossRef CAS.
- M. Sandru, T.-J. Kim and M.-B. Hägg, High molecular fixed-site-carrier PVAm membrane for CO2 capture, Desalination, 2009, 240, 298–300 CrossRef CAS.
- M.-B. Hägg and R. Quinn, Polymeric Facilitated Transport Membranes for Hydrogen Purification, MRS Bull., 2006, 31, 750–755 CrossRef.
- M. Teramoto, N. Takeuchi, T. Maki and H. Matsuyama, Facilitated transport of CO2 through liquid membrane accompanied by permeation of carrier solution, Sep. Purif. Technol., 2002, 27, 25–31 CrossRef CAS.
- J. Won, D. B. Kim, Y. S. Kang, D. Ki Choi, H. S. Kim and C. K. Kim, An ab initio study of ionic liquid silver complexes as carriers in facilitated olefin transport membranes, J. Membr. Sci., 2005, 260(1), 37–4 CrossRef CAS.
- A.-V. Ruzette and L. Leibler, Block Copolymers in Tomorrow's Plastics, Nat. Mater., 2005, 4, 19–31 CrossRef CAS PubMed.
- A. J. Ryan, Polymer science: Designer polymer blends, Nat. Mater., 2002, 1, 8–10 CrossRef CAS PubMed.
- K. R. Shull and E. J. Kramer, Mean-Field Theory of Polymer Interfaces in the Presence of Block Copolymers, Macromolecules, 1990, 23, 4769–4779 CrossRef CAS.
- A. N. Wilkinson, A. J. Ryan, Polymer Processing Structure Development, Kluwer, Dordrecht, The Netherlands, 1998 Search PubMed.
- N. Equiza, W. Yave, R. Quijada and M. Yazdani-Pedram, Use of SEBS/EPR and SBR/EPR as Binary Compatibilizers for PE/PP/PS/HIPS Blends: A Work Oriented to the Recycling of Thermoplastic Wastes, Macromol. Mater. Eng., 2007, 292, 1001–1011 CrossRef CAS.
- D. Jarus, A. Hiltner and E. Baer, Barrier properties of polypropylene/polyamide blends produced by microlayer coextrusion, Polymer, 2002, 43, 2401–2408 CrossRef CAS.
- C. Chen and F.S. Lai, Processability and thermal properties of blends of high density polyethylene, poly (ethylene terephthalate), and ethyl vinyl acetate compatibilizer, Polym. Eng. Sci., 1994, 34, 472–476 CAS.
- H. Pernot, M. Baumert, F. Court and L. Leibler, Design and properties of co-continuous nanostructured polymers by reactive blending, Nat. Mater., 2002, 1, 54–58 CrossRef CAS PubMed.
- Y. Cao, J. Zhang, J. Feng and P. Wu, Compatibilization of immiscible polymer blends using graphene oxide sheets, ACS Nano, 2011, 5, 5920–5927 CrossRef CAS PubMed.
- T. P. Lodge, Block Copolymers: Past Successes and Future Challenges, Macromol. Chem. Phys., 2003, 204, 265–273 CrossRef CAS.
- O.W. Webster, Living Polymerization Methods, Science, 1991, 251, 887–893 CrossRef CAS PubMed.
- T. Smart, H. Lomas, M. Massignani, M.-V. Flores-Merino, L. R. Perez and G. Battaglia, Block copolymer nanostructures, Nanotoday, 2008, 3, 38–46 CrossRef CAS.
- M.W. Matsen and F.S. Bates, Origins of complex self-assembly in block copolymers, Macromolecules, 1996, 29, 7641–7644 CrossRef CAS.
- S.B. Darlin, Directing the self-assembly of block copolymers, Prog. Polym. Sci., 2007, 32, 1152–1204 CrossRef.
- S. Foester and M. Antonietti, Amphiphilic block copolymers in structure-controlled nanomaterials hybrids, Adv. Mater., 1998, 10, 195–217 CrossRef.
- M.C. Orilall and U. Wiesner, Block Copolymer based Composition and Morphology Control in Nano-Structured Hybrid Materials for Energy Conversion and Storage: Solar Cells, Batteries, and Fuel Cells, Chem. Soc. Rev., 2011, 40, 520 RSC.
- M.W. Matsen and F.S. Bates, Unifying weak- and strong-segregation block copolymer theories, Macromolecules, 1996, 29, 1091–1098 CrossRef CAS.
- P. J. Flory, Thermodynamics of high polymer solutions, J. Chem. Phys., 1942, 10, 51–61 CrossRef CAS.
- M. L. Huggins, Theory of Solutions of High Polymers, J. Am. Chem. Soc., 1942, 64, 1712–1719 CrossRef CAS.
- S. P. Nunes, R. Sougrat, B. Hooghan, D. H. Anjum, A.R. Behzad, L. Zhao, N. Pradeep, I. Pinnau, U. Vainio and K.-V. Peinemann, Ultraporous films with uniform nanochannels by block copolymer micelles assembly, Macromolecules, 2010, 43, 8079–8085 CrossRef CAS.
- W. Yave, Membranes from Block Copolymers, in Advanced Materials for Membrane Preparation, ch. 10, pp. 148–162, ed. M.G. Buonomenna and G. Golemme, Bentham Science Publishers, 2012 Search PubMed.
- J. Giboz, T. Copponnex and P. Mele, Microinjection molding of thermoplastic polymers: a review, J. Micromech. Microeng., 2007, 17, R96 CrossRef CAS.
- M. Muller, O. Kulikov, K. Hornung and M.H. Wagner, The use of thermoplastic elastomers as polymer processing aids in processing of linear low density polyethylene, Polym. Sci., 2010, 52, 1163–1170 Search PubMed.
- J.M. Zielinski and R.J. Spontak, Confined single-chain model of microphase-separated multiblock copolymers, Macromolecules, 1992, 25, 653–662 CrossRef CAS.
- R.F. Saraf and H.K. Wickramasinghe, US Patent 6,218, 175, B1, 2001 Search PubMed.
- Y. Bohbot-Raviv and Z.-G. Wang, Discovering New Ordered Phases of Block Copolymers, Phys. Rev. Lett., 2000, 85, 3428–3431 CrossRef CAS PubMed.
- H. Odani, K. Taira, N. Nemoto and M. Kurata, Solubilities of inert gases in styrene-butadiene-styrene block copolymers, Bull. Inst. Chem. Res., 1975, 53, 409–423 CAS.
- P.M. Knight and D.J. Lyman, Gas permeability of various block copolyether-urethanes, J. Membr. Sci., 1984, 17, 245–254 CrossRef CAS.
- D.R. Paul, Gas transport in homogeneous multicomponent polymers, J. Membr. Sci., 1984, 18, 75–86 CrossRef CAS.
- J.A. Barrie, M.J.L. Williams and H.G. Spenser, Gas transport in heterogeneous polymer blends : III. Alternating block copolymers of poly(bisphenol-a carbonate) and polydimethylsiloxane, J. Membr. Sci., 1984, 21, 185–202 CrossRef CAS.
- J. Csernica, R.F. Badduor and R.E. Cohen, Gas permeability of a polystyrene/polybutadiene block copolymer with oriented lamellar domains, Macromolecules, 1987, 20, 2468–2471 CrossRef CAS.
- J. Csernica, R.F. Badduor and R.E. Cohen, Gas permeability of a polystyrene/polybutadiene block copolymer possessing a misoriented lamellar morphology, Macromolecules, 1989, 22, 1493–1496 CrossRef CAS.
- K.-V. Peinemann, V. Abetz and P. F. W. Simon, Asymmetric superstructure formed in a block copolymer via phase separation, Nat. Mater., 2007, 6, 992–996 CrossRef CAS PubMed.
- S. H. Kim, M. J. Misner, M. Kimura, T. Xu and T. P. Russell, Highly Oriented and Ordered Arrays from Block Copolymers via Solvent Evaporation, Adv. Mater., 2004, 16, 226–231 CrossRef CAS.
- N. P. Patel and R. J. Spontak, Gas-transport and thermal properties of a microphase-ordered poly(styrene-b-ethylene oxide-b-styrene) triblock copolymer and its blends with poly(ethylene glycol), Macromolecules, 2004, 37, 2829–2838 CrossRef CAS.
- J. A. Barrie and K. Munday, Gas transport in heterogeneous polymer blends: I. Polydimethylsiloxane-g-polystyrene and polydimethylsiloxane-b-polystyrene, J. Membr. Sci., 1983, 13, 175–195 CrossRef CAS.
- M. G. Buonomenna, G. Golemme, C. M. Tone, M. P. De Santo, F. Ciuchi and E. Perrotta, Adv. Funct. Mater., 2012, 22, 1759–1767 CrossRef CAS.
- P. Kofinas, R. E. Cohen and A. F. Halasa, Gas Permeability of E/EP Semicrystalline Diblock Copolymers, Polymer, 1994, 35, 1229–1235 CrossRef CAS.
- W. Premnath, A model for the permeation of gases in block copolymer membranes with lamellar morphology, J. Membr. Sci., 1996, 110, 133–137 CrossRef.
- P. L. Drzal, A. F. Halasa and P. Kofinas, Microstructure Orientation and Nanoporous Gas Transport in Semicrystalline Block Copolymer Membranes, Polymer, 2000, 41, 4671–4677 CrossRef CAS.
- P. Kofinas and P. L. Drzal, Melt Processing of Semicrystalline E/EP/E Triblock Copolymers Near the Order–Disorder Transition, Rubber Chem. Technol., 1999, 72, 918–928 CrossRef CAS.
- J. Lokaj, L. Brožová, P. Holler and Z. Pientka, Synthesis and gas permeability of block copolymers composed of poly(styrene-co-acrilonitrile) and polystyrene blocks, Collect. Czech. Chem. Commun., 2002, 67, 267–278 CrossRef CAS.
- R. J. Li, J. R. Xu, M. Q. Zhang, M. Z. Rong and Q. Zheng, The role of crystalline phase in triblock copolymer PS–PEG–PS based gas sensing materials, Polymer, 2005, 46, 11051–11059 CrossRef.
- Q. Zhao, J. Qian, Q. An, Z. Zhu, P. Zhang and Y. Bai, Studies on pervaporation characteristics of polyacrylonitrile–b-poly(ethylene glycol)–b-polyacrylonitrile block copolymer membrane for dehydration of aqueous acetone solutions, J. Membr. Sci., 2008, 311, 284–293 CrossRef CAS.
- A.K. Jha, L. Chen, R.D. Offeman and N. P. Balsara, Effect of nanoscale morphology on selective ethanol transport through block copolymer membranes, J. Membr. Sci., 2011, 373, 112–120 CrossRef CAS.
- M. G. Buonomenna, G. Golemme, C. M. Tone, M. P. De Santo, F. Ciuchi, E. Perrotta, B. Zappone, F. Galiano and A. Figoli, J. Membr. Sci., 2011, 385, 162–170 CrossRef.
- M. I. Loría-Bastarrachea and M. Aguilar-Vega, Synthesis and gas transport properties of rigid block copolyaramides, J. Membr. Sci., 2011, 369, 40–48 CrossRef.
- L. M. Robeson, Polymer blends in membrane transport processes, Ind. Eng. Chem. Res., 2010, 49, 11859–118965 CrossRef CAS.
- S. F. Querelle, L. Chen, M. A. Hillmyer and E. L. Cussler, Copolymer Derived Membranes for Enhanced Carbon Dioxide–Methane Separations, Ind. Eng. Chem. Res., 2010, 49, 12051–12059 CrossRef CAS.
- Y. Gu and T. P. Lodge, Synthesis and gas separation performance of triblock copolymer ion gels with a polymerized ionic liquid mid-block, Macromolecules, 2011, 44, 1732–1736 CrossRef CAS.
- R. D. Noble and D. L. Gin, Perspective on Ionic Liquids and Ionic Liquid Membranes, J. Membr. Sci., 2011, 369, 1–4 CrossRef CAS.
- I. Blume, I. Pinnau, US Patent 4, 963, 165 ( 1990) Search PubMed.
- N.P. Patel and R.J. Spontak, Mesoblends of polyether block copolymers with Poly(ethylene glycol), Macromolecules, 2004, 37, 1394–1402 CrossRef CAS.
- B. Bae, K. Miyatake, M. Uchida, Y. Sakiyama, T. Okanishi and M. Matanabe, Sulfonated poly(arylene ether sulfone ketone) multiblock copolymers with highly sulfonated blocks. Long-term fuel cell operation and post-test analyses, ACS Appl. Mater. Interfaces, 2011, 3, 2786–93 CAS.
- H.S. Lee, A. Roy, O. Lane, S. Dunn and J.E. McGrath, Hydrophilic–hydrophobic multiblock copolymers based on poly(arylene ether sulfone) via low-temperature coupling reactions for proton exchange membrane fuel cells, Polymer, 2008, 49, 715–723 CrossRef CAS.
- W. Yave, A. Car, S. S. Funari, S. P. Nunes and K.-V. Peinemann, CO2-philic polymer membrane with high separation performance, Macromolecules, 2010, 43, 326–333 CrossRef CAS.
- K.H. Hsieh, C.C. Tsai and D.M. Chang, Vapor and gas permeability of polyurethane membranes. Part II. Effect of functional group, J. Membr. Sci., 1991, 56, 279–287 CrossRef CAS.
- M. A. Osman, V. Mittal, M. Morbidelli and U. W. Suter, Polyurethane Adhesive Nanocomposites as Gas Permeation Barrier, Macromolecules, 2003, 36, 9851–9858 CrossRef CAS.
- H. Li, B.D. Freeman and O.M. Ekiner, Gas permeation properties of poly(urethane-urea)s containing different polyethers, J. Membr. Sci., 2011, 369, 49–58 CrossRef CAS.
- D. Gomes, K.-V. Peinemann, S.P. Nunes, W. Kujawski and J. Kozakiewicz, Gas transport properties of segmented poly(ether siloxane urethane urea) membranes, J. Membr. Sci., 2006, 281, 747–753 CrossRef CAS.
- M. Sadeghi, M. A. Semsarzadeh, M. Barikani and M. P. Chenar, Gas separation properties of polyether-based polyurethane–silica nanocomposite membranes, J. Membr. Sci., 2011, 376, 188–195 CrossRef CAS.
- M. Kawakami, H. Iwanaga, Y. Hara, M. Iwamoto and S. Kagawa, Gas permeabilities of cellulose nitrate/poly(ethylene glycol) blend membranes, J. Appl. Polym. Sci., 1982, 27, 2387–2393 CrossRef CAS.
- S. J. Metz, M. H. V. Mulder and M. Wessling, Gas-permeation properties of poly(ethylene oxide) poly(butylene terephthalate) block polymers, Macromolecules, 2004, 37, 4590–4597 CrossRef CAS.
- V. Barbi, S. S. Funari, R. Gehrke, N. Scharnagl and N. Stribeck, SAXS and the Gas Transport in Polyether-block-polyamide Copolymer Membranes, Macromolecules, 2003, 36, 749–758 CrossRef CAS.
- S. Sridhar, R. Suryamurali, B. Smitha and T. M. Aminabhavi, Development of crosslinked poly(ether-block-amide) membrane for CO2/CH4 separation, Colloids Surf., A, 2007, 297, 267–274 CrossRef CAS.
- S. B. Hamouda, Q. T. Nguyen, D. Langevin, C. Chappey and S. Roudesli, Polyamide 12-polytetramethyleneoxide block copolymer membranes with silver nanoparticles – Synthesis and water permeation properties, React. Funct. Polym., 2007, 67, 893–904 CrossRef.
- S. Sridhar, T.M. Aminabhavi, S.J. Mayor and M. Ramakrishna, Permeation of Carbon Dioxide and Methane Gases through Novel Silver-Incorporated Thin Film Composite Pebax Membranes, Ind. Eng. Chem. Res., 2007, 46, 8144–8151 CrossRef CAS.
- A. Car, C. Stropnik, W. Yave and K.-V. Peinemann, PEG modified poly(amide-b-ethylene oxide) membranes for CO2 separation, J. Membr. Sci., 2008, 307, 88–95 CrossRef CAS.
- A. Car, C. Stropnik, W. Yave and K.-V. Peinemann, Pebax®/polyethylene glycol blend thin film composite membranes for CO2 separation: Performance with mixed gases, Sep. Purif. Technol., 2008, 62, 110–117 CrossRef CAS.
- H. Sijbesma, K. Nymeijer, R. van Marwijk, R. Heijboer, J. Potreck and M. Wessling, Flue gas dehydration using polymer membranes, J. Membr. Sci., 2008, 313, 263–267 CrossRef CAS.
- J. S. Louie, I. Pinnau and M. Reinhard, Gas and liquid permeation properties of modified interfacial composite reverse osmosis membranes, J. Membr. Sci., 2008, 325, 793–800 CrossRef CAS.
- W. Yave, A. Car, K.-V. Peinemann, M.Q. Shaikh, K. Rätzke and F. Faupel, Gas permeability and free volume in poly(amide-b-ethylene oxide)/polyethylene glycol blend membranes, J. Membr. Sci., 2009, 339, 177–183 CrossRef CAS.
- W. Yave, A. Car and K.-V. Peinemann, Nanostructured membrane materials designed for carbon dioxide separation, J. Membr. Sci., 2010, 350, 124–129 CrossRef CAS.
- S.R. Reijerkerk, M.H. Knoef, K. Nijmeijer and M. Wessling, Poly(ethylene glycol) and poly(dimethyl siloxane) into efficient CO2 gas separation membranes, J. Membr. Sci., 2010, 352, 126–135 CrossRef CAS.
- S. Shishatskiy, J.R. Pauls, S.P. Nunes and K.-V. Peinemann, Quaternary Ammonium Membrane Materials for CO2 Separation, J. Membr. Sci., 2010, 359, 44–53 CrossRef CAS.
- R.S. Murali, S. Sridhar, T. Sankarshana and Y.V.L. Ravikumar, Gas permeation behavior of Pebax-1657 nanocomposite membrane incorporated with multiwalled carbon nanotubes, Ind. Eng. Chem. Res., 2010, 49, 6530–6538 CrossRef CAS.
- N.L. Le, Y. Wang and T.-S. Chung, Pebax/POSS mixed matrix membranes for ethanol recovery from aqueous solutions via pervaporation, J. Membr. Sci., 2011, 379, 174–183 CrossRef CAS.
- Y. Wang, J. Ren and M. Deng, Ultrathin solid polymer electrolyte PEI/Pebax2533/AgBF4 composite membrane for propylene/propane separation, Sep. Purif. Technol., 2011, 77, 46–52 CrossRef CAS.
- K. Friess, V. Hynek, M. Šípek, W.M. Kujawski, O. Vopička, M. Zgažar and M.W. Kujawski, Permeation and sorption properties of poly(ether-block-amide) membranes filled by two types of zeolites, Sep. Purif. Technol., 2011, 80, 418 CrossRef CAS.
- M.A. Ardestani, A.A. Babaluo, M. Peyravi, M.K. Razavi Aghjeh and E. Jannatdoost, Fabrication of PEBA/ceramic nanocomposite membranes in gas sweetening, Desalination, 2010, 250, 1140–1143 CrossRef CAS.
- T. Sabu, P.M. Visakh, D. Jasma and M.S. Nikolic, Handbook of Engineering and Speciality thermoplastics, Polyethers and Polyesters. Cap. 10, 2011 Search PubMed.
- A. Car, C. Stropnik, W. Yave and K.-V. Peinemann, Tailor-made Polymeric Membranes based on Segmented Block Copolymers for CO2 Separation, Adv. Funct. Mater., 2008, 18, 2815–2823 CrossRef CAS.
- W. Yave, A. Car, J. Wind and K.-V. Peinemann, Nanometric Thin Film Membranes Manufactured on Square Meter Scale: Ultrathin Films for CO2 Capture, Nanotechnology, 2010, 21, 395301–395308 CrossRef PubMed.
- W. Yave, H. Huth, A. Car and C. Schick, Peculiarity of a CO2-philic block copolymer confined in thin films with constrained thickness: “a super membrane for CO2-capture”, Energy Environ. Sci., 2011, 4, 4656–4661 CAS.
- J.W. Simmons, US Patent 6,860,920, B2, 2005 Search PubMed.
- S.R. Reijerkerk, A. Arun, R.J. Gaymans, K. Nijmeijer and M. Wessling, Tuning of mass transport properties of multi-block copolymers for CO2 capture applications, J. Membr. Sci., 2010, 359, 54–63 CrossRef CAS.
- S.R. Reijerkerk, A.C. Ijzer, K. Nijmeijer, A. Arun, R.J. Gaymans and M. Wessling, Sub-ambient temperature CO2 and light gas permeation trough segmented block copolymers with tailored soft phase, ACS Appl. Mater. Interfaces, 2010, 2, 551–560 CAS.
- D. Husken, T. Visser, M. Wessling and R.J. Gaymans, CO2 permeation properties of poly(ethylene oxide)-based segmented block copolymers, J. Membr. Sci., 2010, 346, 194–201 CrossRef CAS.
- W. Yave, A. Szymczyk, N. Yave and Z. Roslaniec, Design, synthesis, characterization and optimization of PTT-b-PEO copolymers: A new membrane material for CO2 separation, J. Membr. Sci., 2010, 362, 407–416 CrossRef CAS.
- D.M. Muñoz, E.M. Maya, J. de Abajo, J.G. de la Campa and A.E. Lozano, Thermal treatment of poly(ethylene oxide)-segmented copolyimide based membranes: An effective way to improve the gas separation properties, J. Membr. Sci., 2008, 323, 53–59 CrossRef.
- S.R. Reijerkerk, M. Wessling and K. Nijmeijer, Pushing the limits of block copolymer membranes for CO2 separation, J. Membr. Sci., 2011, 378, 479–484 CrossRef CAS.
- J.G. Wijmans and R.W. Baker, The solution–diffusion model: a review, J. Membr. Sci., 1997, 107, 1–21 CrossRef.
- S.H. Kim, M.J. Misner, T. Xu, M. Kimura and T.P. Russell, Highly oriented and ordered arrays from block copolymers via solvent evaporation, Adv. Mater., 2004, 16, 226–230 CrossRef CAS.
- B. Xue, X. Li, L. Gao, M. Gao, Y. Wang and L. Jiang, CO2-selective free-standing membrane by self-assembly of a UV-crosslinkable diblock copolymer, J. Mater. Chem., 2012, 22, 10918–10923 RSC.
- B. Zhao, H. Hu, A. Yu, D. Perea and R.C. Haddon, Synthesis and characterization of water soluble single-walled carbon nanotube graft copolymers, J. Am. Chem. Soc., 2005, 127, 8197–8203 CrossRef CAS PubMed.
- J.K. Holt, H.G. Park, Y. Wang, M. Stadermann, A.B. Artyukhin, C.P. Grigoropoulos, A. Noy and O. Bakajin, Fast Mass Transport Through Sub-2 nm Carbon Nanotubes, Science, 2006, 312, 1034–1037 CrossRef CAS PubMed.
- A. Haryono and W.H. Binder, Controlled Arrangement of Nanoparticle Arrays in Block-Copolymer Domains, Small, 2006, 2, 600–611 CrossRef CAS PubMed.
- S.-W. Yeh, K.-H. Wei and Y.-S. Sun, U-S. Jeng, K.S. Liang, : Morphological Transformation of PS-b-PEO Diblock Copolymer by Selectively Dispersed Colloidal CdS Quantum Dots, Macromolecules, 2003, 36, 7903–7907 CrossRef CAS.
- T. Xu, N. Zhao, F. Ren, R. Hourani, M.T. Lee, J.Y. Shu, S. Mao and B.A. Helms, Subnanometer Porous Thin Films by the Co-assembly of Nanotube Subunits and Block Copolymers, ACSNano, 2011, 5, 1376–1384 CrossRef CAS PubMed.
- G. Srinivas, D.E. Discher and M.L. Klein, Key roles for chain flexibility in block copolymer membranes that contain pores or make tubes, Nano Lett., 2005, 5, 2343–2349 CrossRef CAS PubMed.
- P.R.L. Malenfant, J. Wan, S.T. Taylor and M. Manoharan, Self-assembly of an organic–inorganic block copolymer for nano-ordered ceramics, Nat. Nanotechnol., 2007, 2, 43–46 CrossRef CAS PubMed.
- W. Yave, S. Shishatskiy, V. Abetz, S. Matson, E. Litvinova, V. Khotimskiy and K.-V. Peinemann, A novel poly(4-methyl-2-pentyne)/TiO2 hybrid nanocomposite membrane for natural gas conditioning: butane/methane separation, Macromol. Chem. Phys., 2007, 208, 2412–2418 CrossRef CAS.
- C.H. Lau and T.-S. Chung, Liquid like poly(ethylene glycol) supported in the organic-inorganic matrix for CO2 removal, Macromolecules, 2011, 44, 6057–6066 CrossRef CAS.
- A. Yu Alentiev and Yu. P Yampolskii, Free volume model and trade-off relations of gas permeability and selectivity in glassy polymers, J. Membr. Sci., 2000, 165, 201–216 CrossRef.
- M. M. Dal-Cin, A. Kumar and L. Layton, Revisiting the experimental and theoretical upper bounds of light pure gas selectivity–permeability for polymeric membranes, J. Membr. Sci., 2008, 323, 299–308 CrossRef CAS.
- B. D. Freeman, Basis of permeability/selectivity tradeoff relations in polymeric gas separation membranes, Macromolecules, 1999, 32, 375–380 CrossRef CAS.
- (a) L. M. Robeson, B.D. Freeman, D.R. Paul and B.W. Rowe, Permeability Correlation: Correlation of Gas Pairs to Predict Kinetic Diameters for Gas Transport in Polymers, J. Membr. Sci., 2009, 341, 178–185 CrossRef CAS; (b) B.W. Rowe, L.M. Robeson, B.D. Freeman and D.R. Paul, Influence of temperature on the upper bound: Theoretical considerations and comparison with experimental results, J. Membr. Sci., 2010, 360, 58–69 CrossRef CAS.
- J. Caro, Are MOF membranes better in gas separation than those made of zeolites?, Current Opinion in Chemical Engineering, 2011, 1, 77–83 CrossRef CAS.
- A. F. Ismail and L. I. B. David, A review on the latest development of carbon membranes for gas separation, J. Membr. Sci., 2001, 193, 1–18 CrossRef CAS.
- S. Kusakabe, S. Morooka, Gas Separation with Carbon Membranes, in Carbon Alloys. Novel Concepts to Develop Carbon Science and Technology, ed. E. Yasuda, M. Inagaki, K. Kaneko, M. Endo, A. Oya, Y. Tanabe, Elsevier, Oxford, 2003,p. 469 Search PubMed.
- P. J. Williams, W. J. Koros, Gas separation by carbon membranes; in Advanced Membrane Technology and Applications, ed. N. N. Li, A. G. Fane, W. S. W. Ho and T. Matsuura, John Wiley & Sons, Hoboken, 2008, p. 599 Search PubMed.
- A. F. Ismail in Preparation of carbon membranes for gas separation; in Comprehensive Membrane Science and Engineering, Vol. I, Basic aspects of Membrane Science and Engineering, ed. E. Drioli, L. Giorno, Elsevier, Kidlington, 2010, p. 275 Search PubMed.
- W. N. W. Salleh, A. F. Ismail, T. Matsuura and M. S. Abdullah, Precursor Selection and Process Conditions in the Preparation of Carbon Membrane for Gas Separation: A Review, Sep. Purif. Rev., 2011, 40, 261–311 CrossRef CAS.
- A. F. Ismail, D. Rana, T. Matsuura, H. C. Foley, Carbon-based Membranes for Separation Processes, Springer, New York, 2011 Search PubMed.
- K. M. Steel and W. J. Koros, Investigation of porosity of carbon materials and related effects on gas separation properties, Carbon, 2003, 41, 253–266 CrossRef CAS.
- P. Kowalczyk, P. A. Gauden, A. P. Terzyk and S. Furmaniak, Microscopic model of carbonaceous nanoporous molecular sieves–anomalous transport in molecularly confined spaces, Phys. Chem. Chem. Phys., 2010, 12, 11351–11361 RSC.
- Y. J. Fu, K. S. Liao, C. C. Hu, K. R. Lee and J. Y. Lai, Development and characterization of micropores in carbon molecular sieve membrane for gas separation, Microporous Mesoporous Mater., 2011, 143, 78–86 CrossRef CAS.
- V. C. Geiszler and W. J. Koros, Effect of polyimide pyrolysis conditions on carbon molecular sieve membrane properties, Ind. Eng. Chem. Res., 1996, 35, 2999–3003 CrossRef CAS.
- A. C. Lua and J. Su, Effects of carbonization on pore evolution and gas permeation properties of carbon membranes from Kapton polyimide, Carbon, 2006, 44, 2964–2972 CrossRef CAS.
- J. E. Koresh and A. Soffer, Molecular sieve carbon permselective membrane. Part 1. Presentation of a new device for gas mixture separation, Sep. Sci. Technol., 1983, 18, 723–734 CrossRef CAS.
- M. Kiyono, P. J. Williams, W. J. Koros, S Pat. Appl. US20110100211A1 Search PubMed.
- M. Kiyono, P. J. Williams and W. J. Koros, Effect of pyrolysis atmosphere on separation performance of carbon molecular sieve membranes, J. Membr. Sci., 2010, 359, 2–10 CrossRef CAS.
- M. Kiyono, P. J. Williams and W. J. Koros, Effect of polymer precursors on carbon molecular sieve structure and separation performance properties, Carbon, 2010, 48, 4432–4441 CrossRef CAS.
- M. Kiyono, P. J. Williams and W. J. Koros, Generalization of effect of oxygen exposure on formation and performance of carbon molecular sieve membranes, Carbon, 2010, 48, 4442–4449 CrossRef CAS.
- C. W. Jones and W. J. Koros, Carbon molecular sieve gas separation membranes, Part 1: Ultramicroporous carbon membranes produced from a polyimide precursor, Carbon, 1994, 32, 1419–1425 CrossRef CAS.
- C. W. Jones and W. J. Koros, Carbon molecular sieve gas separation membranes, Part 2: Effects of organic contamination on ultramicroporous carbon membranes, Carbon, 1994, 32, 1427–1432 CrossRef CAS.
- E. P. Favvas, E. P. Kouvelos, G. E. Romanos, G. I Pilatos, A. Ch. Mitropoulos and N. K. Kannellopoulos, Characterization of highly selective microporous carbon hollow fiber membranes prepared from a commercial co-polyimide precursor, J. Porous Mater., 2008, 15, 625–633 CrossRef CAS.
- L. Xu, M. Rungta and W. J. Koros, Matrimid® derived carbon molecular sieve hollow fiber membranes for ethylene/ethane separation, J. Membr. Sci., 2011, 380, 138–147 CrossRef CAS.
- K.-I. Okamoto, S. Kawamura, M. Yoshino, H. Kita, Y. Hirayama, N. Tanihara and Y. Kusuki, Olefin/Paraffin Separation through Carbonized Membranes Derived from an Asymmetric Polyimide Hollow Fiber Membrane, Ind. Eng. Chem. Res., 1999, 38, 4424–4432 CrossRef CAS.
- M. Yoshimune and K. Haraya, Flexible carbon hollow fiber membranes derived from sulfonated poly (phenylene oxide), Sep. Purif. Technol., 2010, 75, 193–197 CrossRef CAS.
- M. Nurul Islam, W. Zhou, T. Honda, K. Tanaka and K.-I. Okamoto, Preparation and gas separation performance of flexible pyrolytic membranes by low-temperature pyrolysis of sulfonated polyimides, J. Membr. Sci., 2005, 261, 17–26 CrossRef.
- Y. K. Kim, H. B. Park and Y. M. Lee, Carbon molecular sieve membranes derived from thermally labile polymer containing blend polymers and their gas separation properties, J. Membr. Sci., 2004, 243, 9–17 CrossRef CAS.
- H. J. Lee, M. Yoshimune, H. Suda and K. Haraya, Gas permeation properties of poly(2,6-dimethyl-1,4-phenylene oxide) (PPO) derived carbon membranes prepared on a tubular ceramic support, J. Membr. Sci., 2006, 279, 372–379 CrossRef CAS.
- H. J. Lee, H. Suda, K. Haraya and S. H. Moon, Gas permeation properties of carbon molecular sieving membranes derived from the polymer blend of polyphenylene oxide (PPO)/polyvinylpyrrolidone (PVP), J. Membr. Sci., 2007, 296, 139–146 CrossRef CAS.
- W. N. W. Salleh and A. F. Ismail, Carbon hollow fiber membranes derived from PEI/PVP for gas separation, Sep. Purif. Technol., 2011, 80, 541–548 CrossRef CAS.
- A. K. Itta, H.-H. Tseng and M.-Y. Wey, Fabrication and characterization of PPO/PVP blend carbon molecular sieve membranes for H2/N2 and H2/CH4 separation, J. Membr. Sci., 2011, 372, 387–395 CrossRef CAS.
- X. He, J. A. Lie, E. Sheridan and M.-B. Hägg, Preparation and Characterization of Hollow Fiber Carbon Membranes from Cellulose Acetate Precursors, Ind. Eng. Chem. Res., 2011, 50, 2080–2087 CrossRef CAS.
- X. He and M.-B. Hägg, Optimization of Carbonization Process for Preparation of High Performance Hollow Fiber Carbon Membranes, Ind. Eng. Chem. Res., 2011, 50, 8065–8072 CrossRef CAS.
- H. B. Park, I. Y. Suh and Y. M. Lee, Novel Pyrolytic Carbon Membranes Containing Silica: Preparation and Characterization, Chem. Mater., 2002, 14, 3034–3046 CrossRef CAS.
- H. B. Park and Y. M. Lee, Pyrolytic carbon–silica membrane: a promising membrane material for improved gas separation, J. Membr. Sci., 2003, 213, 263–272 CrossRef CAS.
- H. B. Park, C. H. Jung, Y. K. Kim, S. Y. Nam, S. Y. Lee and Y. M. Lee, Pyrolytic carbon membranes containing silica derived from poly(imide siloxane): the effect of siloxane chain length on gas transport behavior and a study on the separation of mixed gases, J. Membr. Sci., 2004, 235, 87–98 CrossRef CAS.
- J. A. Lie and M.-B. Hägg, Carbon membranes from cellulose and metal loaded cellulose, Carbon, 2005, 43, 2600–2607 CrossRef CAS.
- M. L. Chng, Y. Xiao, T.-S. Chung, M. Toriida and S. Tamai, Enhanced propylene/propane separation by carbonaceous membrane derived from poly (aryl ether ketone)/2,6-bis(4-azidobenzylidene)-4-methyl-cyclohexanone interpenetrating network, Carbon, 2009, 47, 1857–1866 CrossRef CAS.
- B. T. Low and T.-S. Chung, Carbon molecular sieve membranes derived from pseudointerpenetrating polymer networks for gas separation, Carbon, 2011, 49, 2104–2112 CrossRef CAS.
- P. S. Tin, T. S. Chung and A. J. Hill, Advanced fabrication of carbon molecular sieve membranes by nonsolvent pretreatment of precursor polymers, Ind. Eng. Chem. Res., 2004, 43, 6476–6483 CrossRef CAS.
- A. Soffer, M. Azariah, A. Amar, H. Cohen, D. Golub, S. Saguee, et al., Method of improving the selectivity of carbon membranes by chemical carbon vapour deposition, US Pat. 5,695,818 ( 1997) Search PubMed.
- J. N. Barsema, N. F. A. van der Vegt, G. H. Koops and M. Wessling, Carbon molecular sieve membranes prepared from porous fibre precursor, J. Membr. Sci., 2002, 205, 239–246 CrossRef CAS.
- S. Lagorsse, F. D. Magalhães and A. Mendes, Carbon molecular sieve membranes. Sorption, kinetic and structural characterization, J. Membr. Sci., 2004, 241, 275–287 CrossRef CAS.
- J. Kunstmann, A. Noack, J. Rathenow, Germ. Pat. DE AZ10311950.7 Search PubMed.
- S. Lagorsse, A. Leite, F. D. Magalhães, N. Bischofberger, J. Rathenow and A. Mendes, Novel carbon molecular sieve honeycomb membrane module: Configuration and membrane characterization, Carbon, 2005, 43, 809–819 CrossRef CAS.
- A. Noack, J. Kunstmann, G. Frank, C. Gnabs, N. Bischofberger, A. Bán, US. Pat. 7,014,681B2 ( 2006) Search PubMed.
- Y. Kawabuchi, S. Kawano and I. Mochida, Molecular sieving selectivity of activated carbons and activated carbon fibers improved by chemical vapor deposition of benzene, Carbon, 1996, 34, 711–717 CrossRef CAS.
- Y. Kawabuchi, M. Kishino, S. Kawano, D. D. Whitehurst and I. Mochida, Carbon deposition from benzene and cyclohexane onto active carbon fiber to control its pore size, Langmuir, 1996, 12, 4281–4285 CrossRef CAS.
- Y. Kawabuchi, H. Oka, S. Kawano, I. Mochida and N. Yoshizawa, The modification of pore size in activated carbon fibers by chemical vapor deposition and its effect on molecular sieve selectivity, Carbon, 1998, 36, 377–382 CrossRef CAS.
- J. Hayashi, H. Mizuta, M. Yamamoto, K. Kusakabe and S. Morooka, Effects of carbonization on pore evolution and gas permeation properties of carbon molecular sieve membranes prepared from porous fibre precursor, J. Membr. Sci., 1997, 127, 243–251 CrossRef.
- J. Hayashi, M. Yamamoto, K. Kusakabe and S. Morooka, Effect of oxidation on gas permeation of carbon molecular sieving membranes base on BPDA-pp'ODA polyimide, Ind. Eng. Chem. Res., 1997, 36, 2134–2140 CrossRef CAS.
- K. Kusakabe, M. Yamamoto and S. Morooka, Gas Permeation and Micropore Structure of Carbon Molecular Sieving Membranes Modified by Oxidation, J. Membr. Sci., 1998, 149, 59–67 CrossRef CAS.
- H.-J. Lee, M. Yoshimune, H. Suda and K. Haraya, Effects of oxidation curing on the permeation performances of polyphenylene oxide-derived carbon membranes, Desalination, 2006, 193, 51–57 CrossRef CAS.
- H.-J. Lee, D.-P. Kim, H. Suda and K. Haraya, Gas permeation properties for the post-oxidized polyphenylene oxide (PPO) derived carbon membranes: Effect of the oxidation temperature, J. Membr. Sci., 2006, 282, 82–88 CrossRef CAS.
- H.-J. Lee, H. Suda and K. Haraya, Characterization of the post-oxidized carbon membranes derived from poly(2,4-dimethyl-1,4-phenylene oxide) and their gas permeation properties, Sep. Purif. Technol., 2008, 59, 190–196 CrossRef CAS.
- H.-H. Tseng and A. K. Itta, Modification of carbon molecular sieve membrane structure by self-assisted deposition carbon segment for gas separation, J. Membr. Sci., 2012, 389, 223–233 CrossRef CAS.
- H. Karkhanechi, H. Kazemian, H. Nazockdast, M. R. Mozdianfard and S. M. Bidoki, Fabrication of Homogenous Polymer-Zeolite Nanocomposites as Mixed-Matrix Membranes for Gas Separation, Chem. Eng. Technol., 2012, 35, 885–892 CrossRef CAS.
- C. Sanchez, B. Julián, P. Belleville and M. Popall, Applications of hybrid organic–inorganic nanocomposites, J. Mater. Chem., 2005, 15, 3559–3592 RSC.
- T. T. Moore, R. Mahajan and De Q. Vu, Hybrid membrane materials comprising organic polymers with rigid dispersed phases, AIChE J., 2004, 50, 311–321 CrossRef CAS.
- P.S. Goh, A.F. Ismail, S.M. Sanip, B.C. Ng and M. Aziz, Recent advances of inorganic fillers in mixed matrix membranes for gas separation, Sep. Purif. Technol., 2011, 81, 243–264 CrossRef CAS.
- S.A. Hashemifard, A.F. Ismail and T. Matsuura, Predictions of gas permeability in mixed matrix membranes using theoretical models, J. Membr. Sci., 2010, 347, 53–61 CrossRef CAS.
- H.H. Yong, H.C. Park, Y.S. Kang, J. Won and W. N. Kim, Zeolite-filled polyimide membrane containing 2,4,6-triaminopyrimidine, J. Membr. Sci., 2001, 188, 151–163 CrossRef CAS.
- R. Mahajan, R. Burns, M. Schaeffer and W. J. Koros, Challenges in forming successful mixed matrix membranes with rigid polymeric materials, J. Appl. Polym. Sci., 2002, 86, 881–890 CrossRef CAS.
- T. T. Moore and W. J. Koros, Non-ideal effects in organic–inorganic materials for gas separation membranes, J. Mol. Struct., 2005, 739, 87–98 CrossRef CAS.
- M.G. Buonomenna, G. Golemme, E. Sardella, P. Favia, F. Ciuchi, Effects of synthesis parameters on SAPO-34 morphology and adsorption properties for the CO2/CH4 separation, 10th International Conference on Materials Chemistry (MC10), Manchester, UK, 4–7 July 2011 Search PubMed.
- T. C. Merkel, Z. He, I. Pinnau, B. D. Freeman, P. Meakin and A. J. Hill, Sorption and transport in poly(2,2,-bis(trifluoro-methyl)-4,5-difluoro-1,3-dioxole-co-tetrafluoro-ethylene) containing nanoscale fumed silica, Macromolecules, 2003, 36, 8406–8414 CrossRef CAS.
- T. C. Merkel, Z. He, I. Pinnau, B. D. Freeman, P. Meakin and A. J. Hill, Effect of nanoparticles on gas sorption and transport in poly(1-trimethylsilyl-1-propyne), Macromolecules, 2003, 36, 6844–6855 CrossRef CAS.
- B. D. Freeman and I. Pinnau, Separation of gases using solubility-selective polymer, Trends Polym. Sci, 1997, 5, 167–173 CAS.
- M. C. Ferrari, M. Galizia, M. G. De Angelis and G. C. Sarti, Gas and vapor Transport in Mixed Matrix membranes based on amorphous Teflon AF1600 and AF2400 and fumed silica, Ind. Eng. Chem. Res., 2010, 49, 11920–11935 CrossRef CAS.
- M. C. Ferrari, M. Galizia, M. G. De Angelis and G. C. Sarti, Gas and vapor Transport in Mixed Matrix membranes based on amorphous Teflon AF1600 and AF2400 and fumed silica, Ind. Eng. Chem. Res., 2010, 49, 11920–11935 CrossRef CAS.
- J. Ahn, W.-J. Chung, I. Pinnau and M. D. Guiver, Polysulfone/silica nanoparticle mixed matrix membranes for gas separation, J. Membr. Sci., 2008, 314, 123–133 CrossRef CAS.
- J. Ahn, W.-J. Chung, I. Pinnau, J. Song, N. Du, G. P. Robertson and M. D. Guiver, Gas transport behavior of mixed matrix membranes composed of silica nanoparticles in a polymer of intrinsic microporosity (PIM-1), J. Membr. Sci., 2010, 346, 280–287 CrossRef CAS.
- T. Kono, Y. Hu, T. Masuda, K. Tanaka, R. D. Priestley and B. D. Freeman, Effect of Fumed Silica Nanoparticles on the Gas Permeation Properties of Substituted Polyacetylene Membranes, Polym. Bull., 2007, 58, 995–1003 CrossRef CAS.
- D. Gomes, S. P. Nunes and K. K. Peinemann, Membranes for gas separation based on poly(1-trimethylsilyl-1-propyne)-silica nanocomposites, J. Membr. Sci., 2005, 246, 13–25 CrossRef CAS.
- T. C. Merkel, B. D. Freeman, R. J. Spontak, Z. He, I. Pinnau, P. Meakin and A. J. Hill, Sorption transport and structural evidence for enhanced free volume in poly(4-methyl-2-penthyne)/fumed silica nancomposite membranes, Chem. Mater., 2003, 15, 109–123 CrossRef CAS.
- H. Kong, M. Radosz, B. Francis Towler and Y. Shen, Polymer–inorganic nanocomposite membranes for gas separation, Sep. Purif. Technol., 2007, 55, 281–291 CrossRef.
- Q. Ha, E. Marand, S. Dhingra, D. Fritsch, J. Wen and G. Wilkes, Poly(amide-imide)/TiO2 nano-composite gas separation membranes: Fabrication and characterization, J. Membr. Sci., 1997, 135, 65–79 CrossRef.
- A. B. Beltran, G. M. Nisola, E. Cho, E. E. D. Lee and W.-J. Chung, Organosilanemodified silica/polydimethylsiloxane mixed matrix membranes for enhanced propylene/nitrogen separation, Appl. Surf. Sci., 2011, 258, 337–345 CrossRef CAS.
- H. S. Kim, H. G. Kim, S. Y. Kim and S. S. Kim, PDMS silica composite membranes with silane coupling for propylene separation, J. Membr. Sci., 2009, 344, 211–218 CrossRef CAS.
- G. M. Nisola, A. B. Beltran, D. Sim, D. Lee, B. Jung and W. J. Chung, Dimethyl silane-modified silica in polydimethylsiloxane as gas pemeation mixed matrix membrane, J. Polym. Res., 2011, 18, 2415–2424 CrossRef CAS.
- J. C. Maxwell, A treatise on electricity and magnetism, Chapter IX, Dover Publications, New York, 1954 Search PubMed.
- Cussler. E.L, Membranes containing selective flakes, J. Membr. Sci., 1990, 52, 275–288 CrossRef.
- J. A. Sheffel and M. Tsapatsis, A semi-emprical approach for predicting the performance of mixed matrix membranes containing selective flakes, J. Membr. Sci., 2009, 326, 595–607 CrossRef CAS.
- R. Mahajan and W. J. Koros, Mixed matrix membranes materials with glassy polymers. Part 1, Polym. Eng. Sci., 2002, 42, 1420–1431 CAS.
- R. Mahajan and W. J. Koros, Factors controlling successful formation of mixed matrix gas separation materials, Ind. Eng. Chem. Res., 2000, 39, 2692–2696 CrossRef CAS.
- R. Mahajan and W. J. Koros, Mixed Matrix membrane materials with glassy polymers, Part 2, Polym. Eng. Sci., 2002, 42, 1432–1441 CAS.
- A. M. W. Hillock, S. J. Miller and W. J. Koros, Crosslinked mixed matrix membranes for the purification of natural gas: effects of sieve surface modification, J. Membr. Sci., 2008, 314, 193–199 CrossRef CAS.
- E. Karatay, H. Kalipcilar and L. Yilmaz, Preparation and performance assessment of binary and ternary PES-SAPO 34-HMA based gas separation membranes, J. Membr. Sci., 2010, 364, 75–81 CrossRef CAS.
- P. Jha and J. Douglas Way, Carbon dioxide selective mixed-matrix membranes formulation and characterization using rubbery substituted polyphosphazene, J. Membr. Sci., 2008, 324, 151–161 CrossRef CAS.
- Y.C. Hudiono, T. K. Carlise, J. E. Bara, Y. Zhang, D. L. Gin and R. D. Noble, A three-component mixed-matrix membrane with enhanced CO2 separation properties based on zeolites and ionic liquid materials, J. Membr. Sci., 2010, 350, 117–123 CrossRef CAS.
- M. A. Aroon, A. F. Ismail, T. Matsuura and M. M. Montazer-Rahmati, Performances studies of mixed matrix membranes for gas separation: a review, Sep. Purif. Technol., 2010, 75, 229–242 CrossRef CAS.
- B. Zornoza, C. Tellez, J. Coronas, J. Gascon and F. Kapteijn, Metal organic framework based mixed matrix membranes: an increasingly important field of research with a large application potential, Microporous Mesoporous Mater., 2012 DOI:10.1016/j.micromeso.2012.03.012.
- J. K. Ward and W. J. Koros, Crosslinkable mixed matrix membranes with surface modified molecular sieves for natural gas purification: II. Performance characterization under contaminated feed conditions, J. Membr. Sci., 2011, 377, 75–81 CrossRef CAS.
- R. T. Adams, J. Suk Lee, Tae-Hyun Bae, J. K. Ward, J. R. Johnson, C. W. Jones, S. Nair and W. J. Koros, CO2-CH4 permeation in high zeolite 4A loading mixed matrix membranes, J. Membr. Sci., 2011, 367, 197–203 CrossRef CAS.
- O. Ghaffari Nik, X. Yuan Chen and S. Kaliaguine, Amine-functionalized zeolite FAU/EMT-polyimide mixed matrix membranes for CO2/CH4 separation, J. Membr. Sci., 2011, 379, 468–478 CrossRef.
- C. Casado-Coterillo, J. Soto, M. T. Jimaré, S. Valencia, A. Corma, C. Té llez and J. Coronas, Preparation and characterization of ITQ-29/polysulfone mixed-matrix membranes for gas separation: Effect of zeolite composition and crystal size, Chem. Eng. Sci., 2012, 73, 116–122 CrossRef CAS.
- B. Zornoza, O. Esekhile, W. J. Koros, C. Téllez and J. Coronas, Hollow silicalite-1 Sphere polymer mixed matrix membranes for gas separation, Sep. Purif. Technol., 2011, 77, 137–145 CrossRef CAS.
- W.A. Rafizah and A.F. Ismail, Effect of carbon molecular sieve sizing with poly(vinyl pyrrolidone) K-15 on carbon molecular sieve-polysulfone mixed matrix membrane, J. Membr. Sci., 2008, 307, 53–61 CrossRef CAS.
- J. Gascon, U. Aktay, M. D. Hernandez-Alonso, G. P. M. van Klink and F. Kapteijn, Amino-Based Metal Organic Frameworks as Stable, Highly Active Basic Catalysts, J. Catal., 2009, 261, 75–87 CrossRef CAS.
- Z. Q. Wang and S. M. Cohen, Postsynthetic modification of metal–organic frameworks, Chem. Soc. Rev., 2009, 38, 1315–1329 RSC.
- Kyo Sung Park, Zheng Ni, A. P. Côté, J. Y. Choi, R. Huang, F. J. Uribe-Romo, H. K. Chae, M. O'Keeffe and O. M. Yaghi, Exceptional chemical and thermal stability of zeolitic imidazolate frameworks, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 10186–10191 CrossRef PubMed.
- T.-H. Bae, J. S. Lee, W. Qiu, W. J. Koros, C. W. Jones and S. Nair, A high performance gas-separation membrane containing submicrometer-sized metal–organic framework crystals, Angew. Chem., Int. Ed., 2010, 49, 9863–9866 CrossRef CAS PubMed.
- Ma. J. C. Ordoñez, K.J. Balkus, J. P. Ferraris and I. H. Musselman, Molecular sieving realized with ZIF-8/Matrimid mixed-matrix membranes, J. Membr. Sci., 2010, 361, 28–37 CrossRef.
- K. Diaz, M. López-Gonzalez, L. F. del Castillo and E. Riande, Effect of zeolitic imidazolate frameworks on the gas transport performance of ZIF8-poly(1,4-phenylene ether-ether-sulfone) hybrid membranes, J. Membr. Sci., 2011, 383, 206–213 CrossRef CAS.
- S. Kim, T. W. Pechar and E. Marand, Poly(imide siloxane) and carbon nanotube mixed matrix membranes for gas separations, Desalination, 2006, 192, 330–339 CrossRef CAS.
- http://en.wikipedia.org/wiki/Carbon_nanotube .
- D. S. Sholl and J. K. Johnson, Making high-flux membranes with carbon nanotubes, Science 19 May, 2006, 1003–1004 CAS.
- A. F. Ismail, N. H. Rahim, A. Mustafa, T. Matsuura, B. C. Ng, S. Abdullah and S. A. Hashemifard, Gas separation performance of polyethersulfone/multiwalled carbon nanotubes mixed matrix membranes, Sep. Purif. Technol., 2011, 80, 20–31 CrossRef CAS.
- L. Ge, Z. Zhu and V. Rudolph, Enhanced gas permeability by fabricating functionalized multi-walled carbon nanotubes and polyethersulfone nanocomposite membrane, Sep. Purif. Technol., 2011, 78, 76–82 CrossRef CAS.
- M. A. Aroon, A. F. Ismail, M. M. Montazer-Rahmati and T. Matsuura, Effect of chitosan as a functionalization agent on the performance and separation properties of polyimide/multiwalled carbon nanotubes mixed matrix flat sheet membranes, J. Membr. Sci., 2010, 364, 309–317 CrossRef CAS.
- R. Baker, Future directions of membrane gas separation technology, Ind. Eng. Chem. Res., 2002, 41, 1393–1411 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
