Efficient disentanglement of boron nitride nanotubes using water-soluble polysaccharides for protein immobilization†
Zhenghong
Gao
a,
Chunyi
Zhi
b,
Yoshio
Bando
b,
Dmitri
Golberg
b,
Makoto
Komiyama
c and
Takeshi
Serizawa
*d
aDepartment of Advanced Interdisciplinary Studies, The University of Tokyo, 4-6-1 Komaba, Meguro-ku, Tokyo, 153-8904, Japan
bInternational Center for Materials Nanoarchitectonics (MANA), National Institute for Materials Science (NIMS), Namiki 1-1, Tsukuba, Ibaraki, 305-0044, Japan
cResearch Center for Advanced Science and Technology (RCAST), The University of Tokyo, 4-6-1 Komaba, Meguro-ku, Tokyo, 153-8904, Japan
dDepartment of Organic and Polymeric Materials, Tokyo Institute of Technology, 2-12-1-H121 Ookayama, Meguro-ku, Tokyo, 152-8550, Japan. E-mail: serizawa@polymer.titech.ac.jp
First published on 11th June 2012
Abstract
We report that efficiently disentangled boron nitride nanotubes (BNNTs) can be obtained due to functionalization in an aqueous solution with a natural water-soluble polysaccharide, gum arabic (GA). An atomic force microscopy study showed excellent dispersion of GA-functionalized BNNTs in the aqueous phase. Fluorescent, ultraviolet, and infrared absorption spectroscopies revealed the strong interactions between GA and the sidewalls of BNNTs. Subsequently, several functional proteins were successfully immobilized onto the surfaces of GA-functionalized BNNTs via strong electrostatic interactions under suitable pH conditions.
1. Introduction
Over the last few decades, one-dimensional (1D) nanomaterials have attracted significant scientific attention because of their fantastic size- and geometry-related physiochemical properties1 in combination with their broad range of applications.2 On the other hand, it is realized that proteins at nanoscale materials' surfaces are vitally important for the establishment of high-performance nanobiosensors,3 nanobiochips,4 tissue engineering scaffolds,5 stem-cell expansion constructs,6 and drug delivery devices.7 It is therefore of particular interest to immobilize proteins onto a 1D nanotube or a nanowire. Such ensembles would be valuable for both improving our knowledge on proteins’ fundamental functions and enhancing possible device performance.8 However, the immobilization of functional proteins onto 1D nanostructures has been limited because of high-cost techniques, multiple step handling processes, and use of some harsh chemicals that may influence the activity of protein molecules.8Boron nitride nanotubes (BNNTs) are of increasing interest because of their peculiar shapes and unique physicochemical properties. In view of its molecular structure, BNNT is similar to carbon nanotubes (CNTs), and almost no changes in the tube's structural parameters are evident, except that B and N atoms alternatively substitute C atoms in a graphene-like network.9 However, the properties of BNNTs are dramatically distinctive from those of CNTs in many aspects.9 For example, the band-gap of BNNTs is independent of tube's chirality and geometry, while the corresponding properties of CNTs complicatedly correlate with those.10 The BNNT's resistance to oxidizing agents and robustness at high temperatures is also superior to those of CNTs. Because of these unique physiochemical properties, BNNTs are very promising nanomaterials in chemistry and physics, particularly for the development of new nanobiotechnologies.
In many cases, BNNTs factually exhibit superior performances over their C counterparts.10–12 For example, first, the highly thermostable nanoscale inner channels of BNNTs provide a perfect confined space for the controllable growth of semiconducting nanowires and the linear assembly of functional nanoparticles along the channels.13,14 Second, either B or N sites within BNNTs are available for selective chemical activation.15,16 These properties make BNNTs ideal candidates for use as cargo or vectors for massive loading of drugs or biomolecules on both their outer and inner sidewalls via diverse chemical methodologies. Moreover, a typical external multi-walled BNNT diameter ranges from several nanometers to around 80 nm.17 Such a polydispersed size of the material could increase the chance of immobilization since the nanostructures may select the nanotube of suitable diameter for making an ensemble.18
However, until now, many prospective applications of BNNTs are still hampered because of several issues. First, similar to their C analogs, BNNTs are insoluble in standard solvents, such as milli-Q and general buffers, but a good solubility is a basic requirement for the applications.19 Second, the sidewalls of pristine BNNTs are chemically inert, and they must primarily be activated for the following functionalizations in various useful bio-devices.20 Third, the poor biocompatibility of BNNTs is also needed to be improved for applying in vivo.21
In the case of CNTs, the sidewall functionalization with various biomolecules and biopolymers has paved an efficient pathway for solving the corresponding issues.22 However, the sidewall functionalization of BNNTs with similar techniques has just recently drawn attention. In our previous investigations, we have reported that peptides,23 single-strand DNA24 and nucleotides25,26 can work for the sidewall functionalization of multi-walled BNNTs, leading to excellent dispersions in aqueous solutions. However, these pathways are closely related to the organic syntheses, which are usually expensive, time-consuming, and extremely toxic. To facilitate and broaden the prospective applications of BNNTs in a more practical approach, new pathways for lower-cost, more efficient, and biocompatible sidewall functionalization are highly desired.
Natural polysaccharides are the most abundant biopolymers in nature. They are reasonably good candidates for functionalizing BNNTs because of their cheapness, good water solubility, and well-recognized biocompatibility. Among various natural polysaccharides, gum arabic (GA), which is found in exudates of Arabic Senegal and Acacia Seyal trees, is an abundant and renewable polysaccharide with highly branched complex molecular properties.27 Although properties of GA have been widely investigated, the precise chemical structure of GA is still not fully understood. The latest research has suggested that GA is composed of main three units (Scheme S1, ESI†).27 One major unit is a highly branched polysaccharide, which takes about 90 wt%. Another is high molecular weight arabinogalachan–protein complexes taking about 10 wt% of the total. The rest of the composition is around 1 wt% of the total. It is known that the major functional properties of GA are correlated with the arabinogalactan–protein complexes. The two kinds of molecular models for arabinogalactan–protein complexes have been proposed and well-established: the “wattle-blossom” and the “twisted hairy rope”.27 Very recently, the use of GA has started to be extended into the fields of nanoscience and nanotechnology. For example, GA can be capped onto the surface of inorganic quantum dots28 or magnetic nanoparticles,29 giving these nanoparticles excellent solubility and biocompatibility. It has also been reported as a suitable dispersant for the individual stabilization of single-walled CNTs in aqueous solutions.30
In this contribution, taking many of the outstanding advantages of GA into account, we performed the first trial to disentangle multi-walled BNNTs in the presence of GA in an aqueous solution. Meanwhile, we used dextran (DT), dextran sulfate (DS), and two starch compositions, such as amylose (AL) and amylopectin (AP), as references (Scheme S2, ESI†). The interactions between GA and BNNTs were investigated using fluorescence, Fourier transform-infrared (FT-IR), and ultraviolet-visible (UV-Vis) absorption spectroscopies. Subsequently, several model proteins with different biological functions, usually used in modern biotechnologies, were immobilized onto the GA-functionalized BNNT surfaces by utilizing the electrostatic interactions as a basic driving force by controlling pHs (Scheme 1). The functionalization of BNNT sidewalls with GA allows efficient disentanglement of GA-functionalized BNNTs. GA could increase the number of surface charges and the categories of functionalities of BNNTs, which is helpful for hierarchical functionalization and subsequent applications. GA-functionalized BNNTs offer great opportunities for studying the physiochemical properties of individual disentangled BNNTs. Meanwhile, considering the rapid advancements in the band-gap engineering of BNNTs,10–12 such BNNTs are promising for the construction of BNNT-based electronics and optics. On the other hand, the successful immobilization of proteins onto the surface of GA-functionalized BNNT is an important initial step towards the development of BNNT-based bio-devices, which are particularly meaningful for many bio-related technological applications.
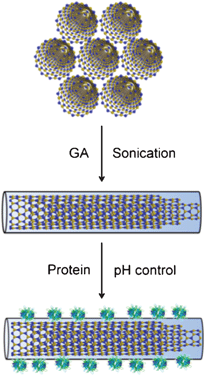 | ||
| Scheme 1 Schematic representation of disentanglement of multi-walled BNNTs via functionalization with GA for proteins immobilization. | ||
2. Experimental section
2.1. Materials
GA was purchased from Flaku Chemical Industries. Ltd. DT was purchased from the Wako Pure Chemical Industries. Ltd. DS was purchased from MPBiomedicals. AL and AP were purchased from Sigma-Adrich. Streptavidin (SAv) was obtained from Thermo Scientific. Bovine serum albumin (BSA), lysozyme (Lyz) and immunoglobulin G (IgG) were purchased from Sigma-Adrich. All these materials were used as received without further purification. High purity multi-walled BNNTs were synthesized by a carbon-free chemical vapor deposition technique,11 using boron and metal oxides as the reactants at around 1500 °C. Synthesized BNNTs had nearly perfect crystalline structures, lengths up to 10 μm and external diameters from several nanometers to 80 nm. The purity was up to 90 wt%. The outer sidewalls and inner channels were ultimately clean.2.2. Functionalization of BNNTs with GA
To disperse and functionalize BNNTs using GA, 1.5 mg of pristine BNNTs was mixed with 3 mL of water solution of GA with a series of concentrations, 0.02 mg mL−1, 0.05 mg mL−1, 0.15 mg mL−1, and 0.30 mg mL−1, respectively. The mixtures were then sonicated for 3 h by a Branson Advanced Sonifier Model 250 AA (output power: 20 W) with a water cooling system (controlling temperature: 20 ± 2 °C), followed by centrifugation at 2000 rpm for 25 min to remove any insoluble materials. The supernatant was finally collected and stored at room temperature for further characterizations.2.3. Characterizations
Atomic force microscopy (AFM) images were obtained with a SPM-9600 (Shimadzu) in a tapping mode using standard silicon cantilevers. For observing the morphologies of GA-functionalized BNNTs and the statistic analysis of the heights or lengths distributions, the AFM samples were prepared by spin-coating 10 μL of the resulting dispersion at 600 rpm on a freshly cleaved mica substrate, followed by drying at ambient atmosphere.The FT-IR spectra were recorded using a Perkin-Elmer Spectrometer, 10 times for each sample, and were Fourier-transformed at a resolution of 4 cm−1. All spectra were corrected with a baseline supported by Perkin-Elmer software. All stock solutions were drop-casted onto a gold film substrate after drying completely under ambient conditions for the measurements. GA-functionalized BNNTs solution was purified by using a centrifugation-filter (Millipore Ireland Ltd. Amicon ultra-2 mL, 100 K centrifugal filter; regenerated cellulose 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 MWCO) under 3500 rpm for 30 min at 25 °C against milli-Q water for 4 to 6 runs for removal of free GA. The pristine BNNTs and GA powder were used to measure the FT-IR spectra of the original BNNTs and pure GA, respectively.
000 MWCO) under 3500 rpm for 30 min at 25 °C against milli-Q water for 4 to 6 runs for removal of free GA. The pristine BNNTs and GA powder were used to measure the FT-IR spectra of the original BNNTs and pure GA, respectively.
The fluorescence was measured with a JASCO FP-6500 spectrofluorometer by excitation at 214 nm with a scan rate of 100 nm s−1. The measurement temperature was controlled at 25 °C. The UV-Vis spectra were recorded by a JASCO V-670 spectrophotometer at the ambient temperature. For fluorescence and UV-Vis spectra measurements, a GA-functionalized BNNTs solution was purified using a procedure similar to that for the FT-IR measurements. The original BNNT samples were similarly prepared by the same dispersing procedure without GA for recording reference spectra.
2.4. Immobilization of proteins on GA-functionalized BNNTs
To immobilize proteins onto the GA-functionalized BNNT surface, BNNTs were firstly functionalized by GA. Then, purified GA-functionalized BNNTs were drop-casted on the top of a clean silicon substrate, followed by complete drying at ambient environment and thorough washing with milli-Q water. In a typical experimental run, 40 μL of a purified GA-functionalized BNNT solution was dropped on a clean Si substrate, followed by complete drying under an ambient atmosphere. Then, to remove any free GA, 100 μL of milli-Q water was dropped on the substrate. After incubation for 5 min, all milli-Q water was removed by absorption with a filter paper. The processes were repeated 4 to 6 times. Continuously, 40 μL of 200 μg mL−1 protein solutions of SAv, BSA, Lyz, and IgG in Britton Robinson buffer with pH 3–4, the values which are lower than the isoelectric points of these proteins, were respectively drop-casted on the top of the sample prepared by the above-mentioned method, followed by incubation at room temperature for 2 h for full interaction. Not all the solution was evaporated after the incubation. Then, any free proteins were washed away from the sample surface. After drying at room temperature, the samples were examined by AFM. Control experiments using only GA-functionalized BNNTs were in parallel performed to confirm the fact of successful protein immobilization.3. Results and discussion
3.1. Functionalization of BNNTs with polysaccharides
The potential of GA for dispersing BNNTs in water was compared with DT, DS, AL and AP at the same concentration of 0.15 mg mL−1. The absorbance at 500 nm, the wavelength which corresponds to the amount of dispersed BNNTs in dispersion, was recorded for the full comparison. The typical UV-Vis absorption spectra of as-prepared BNNTs dispersions with and without GA are presented in Fig. 1a. The absorbance over the whole measured wavelength range of the dispersion with GA was increased in comparison with that without GA, suggesting that GA can work efficiently for dispersing BNNTs in water. As shown in Fig. 1b and Fig. S1 (ESI†), GA was the best polysaccharide among GA, DT, DS, AL and AP for dispersing BNNTs in water. The potentials of DT, DS, AL and AP were almost the same. On the other hand, the absorbance at 500 nm rapidly increased with increasing the concentration of GA from 0 to 0.15 mg mL−1, suggesting that the amount of dispersed BNNTs increased in the dispersion (Fig. 2). At a concentration of 0.15 mg mL−1 GA, the dispersed BNNTs reached the maximum concentration of 22 μg mL−1 calculated using an extinction coefficient of 5.3 mL mg−1 at 500 nm.31 The amount of dispersed BNNTs did not increase further if we continued to increase the GA concentration up to 0.30 mg mL−1. These observations suggest that GA can be used as an efficient dispersant for BNNTs in water.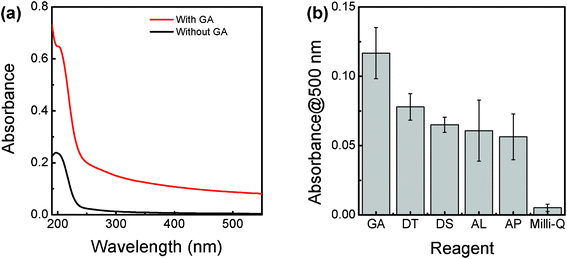 | ||
| Fig. 1 (a) UV-Vis absorption spectra of the as-prepared BNNT dispersions with and without GA; (b) comparative potential of GA, DT, DS, AL and AP for the disentanglement of BNNTs in an aqueous solution. All the concentrations are 0.15 mg mL−1. Absorbance at 500 nm was recorded for the comparison. | ||
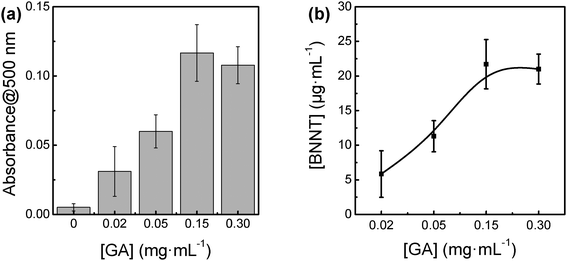 | ||
| Fig. 2 (a) Dependence of the amount of BNNTs on the concentration of GA, and (b) plots of BNNT amounts as a function of the concentration of GA. | ||
3.2. Morphologies and structures of GA-functionalized BNNTs
The resultant dispersion was slightly turbid (Fig. 3a), indicating the presence of a number of BNNTs. The morphologies of GA-functionalized BNNTs were observed by using AFM in the tapping mode. As shown in Fig. 3b, BNNTs were excellently dispersed on a freshly cleaved mica surface. However, the similar dispersion could not be observed using original BNNTs without GA after the same experimental process, suggesting that BNNTs were indeed functionalized by GA. The heights and lengths of GA-functionalized BNNTs were statistically analyzed from the AFM images. Fig. 3c and 3d‡ show that the heights were in a range from several nanometers to 80 nm, which were slightly greater than those for pristine BNNTs,11 also indicating that BNNTs were successfully functionalized by GA. The lengths were up to 7 μm, which is a little shorter than those for pristine BNNTs,11 implying that BNNTs were slightly shortened during the sonication process.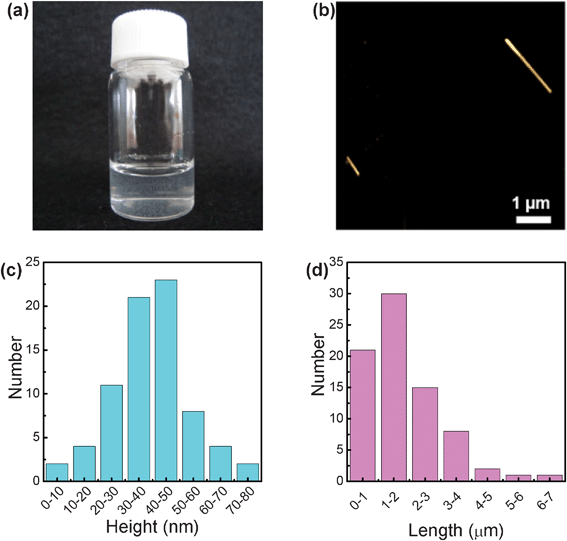 | ||
| Fig. 3 (a) Dispersion of GA-functionalized BNNTs; (b) AFM image of GA-functionalized BNNTs on the mica surface; (c) statistic distribution of heights and (d) lengths of GA-functionalized BNNTs. | ||
We assumed that arabinogalactan–protein complexes contained in GA were critical for the functionalization, while referring to previous studies on the molecular structure of GA.32 The presence of arabinogalactan–protein complexes in GA is an apparent difference of GA from other polysaccharides used in this study. The arabinogalactan–protein complexes in GA could play the key role for the high potential of GA for dispersing BNNTs in water. The “wattle-blossom” structural model of arabinogalactan–protein complexes has been widely accepted because it can be applied for perfectly explaining the behavior of GA on oil–water interfaces.32 The sidewalls of BNNTs are superhydrophobic, therefore, it was imagined here that the “wattle-blossom” structural model is also appropriate for explaining the mechanism of the regarded functionalization. The hydrophobic part of the arabinogalactan–proteins complex is soft and can adjust its low-energy conformation for matching the physical geometry of BNNTs via strong hydrophobic interactions. In the meantime, the hydrophilic part of GA is exposed for interacting with water molecules. Thus, the strong steric forces among GA molecule chains absorbed on BNNT sidewalls can prevent BNNTs from aggregation. It thereby endows an excellent dispersion of BNNTs.
FT-IR is a potential technique for gaining an insight into the assumed mechanism. As shown in Fig. 4, the peaks corresponding to the original BNNTs appeared at 771 cm−1 and 1346 cm−1, which were assigned to respective E1u (TO) and E1u (LO) modes (TO: transverse optic; LO: longitudinal optic), while they appeared at 791 cm−1 and 1361 cm−1 for the GA-functionalized BNNTs. The broad peak corresponding to the stretching vibration mode of –NH of arabinogalactan–protein complexes appeared at ∼3328 cm−1, while it appeared at ∼3312 cm−1 for the GA-functionalized BNNTs.33 The other changes in the peaks (ν(C–OH): 1024 cm−1; δ(C–H): 1416 cm−1 and β(N–H): 1560 cm−1) corresponding to GA were also found. These obvious changes in the FT-IR absorption spectra additionally verified the presence of interactions between the arabinogalactan–protein complexes of GA and the hydrophobic BNNT surfaces.
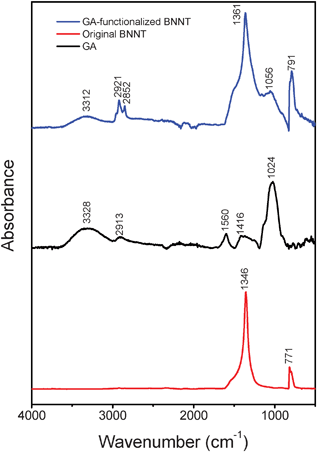 | ||
| Fig. 4 Comparative FT-IR spectra of purified GA-functionalized BNNTs, pure GA and the original BNNTs. | ||
It had been indicated that the arabinogalactan–protein complexes of GA could be degraded (primary structure breakdown) prior to carbohydrate components of GA by treating GA at the mild temperature (60 °C–100 °C) for less than 24 h.27 In order to further point out the importance of arabinogalactan–protein complexes of GA for the observed functionalization, GA was treated here by heating at 80 °C for 12 and 24 h to degrade arabinogalactan–protein complexes in GA. Then, resultant GA was applied for the BNNTs' functionalization. The amount of BNNTs functionalized with thermo-treated GA in solution was very much reduced (Fig. 5), as expected, further confirming that the arabinogalactan–protein complexes play an important role for interacting with the BNNT sidewalls.
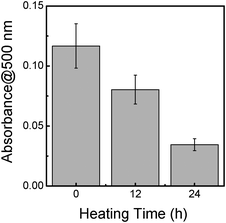 | ||
| Fig. 5 The effect of the heating treatment of GA on the potential for the disentanglement of BNNTs in an aqueous solution. Absorbance at 500 nm was recorded for the comparison. | ||
3.3. Optical properties of GA-functionalized BNNTs
The optical properties of BNNTs might be different from pristine BNNTs after successful functionalization with GA. Fig. 6 compares the fluorescence spectra of original BNNTs, GA-functionalized BNNTs, and pure GA. Interestingly, significant changes were observed for peaks assigned to BNNTs. The broad peak at ∼290 nm, which should be attributed to Frenkel type excitonic transitions of defect centers,34 became sharper, well-resolved, and its maximum slightly shifted from 290 to 286 nm. This change in fluorescence spectra of BNNTs suggested that GA-functionalized BNNTs were excellently disentangled according to the fact that the excitonic fluorescence peak is more resolved for an individual object.34 The peak appeared at 330 nm in the GA-functionalized BNNTs, while it did not appear for the originally bundled BNNTs. This peak is probably attributed to some impurity centers of BNNTs34 and/or the proteins complexes in GA. It implies that GA molecules were covered on the sidewalls of BNNTs, leading to the excellent disentanglement of BNNTs in an aqueous solution. It is noteworthy that the sharper profile and the appearance of the peak both suggest the presence of effectively disentangled BNNTs in the aqueous solution. These changes in the relative peak positions and shapes further confirmed that BNNTs were functionalized by GA.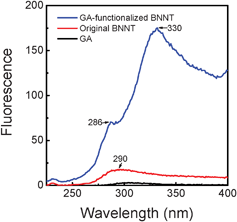 | ||
| Fig. 6 Comparative fluorescence spectra of GA, original BNNTs and purified GA-functionalized BNNTs. | ||
UV-Vis absorption spectra were acquired to further explore the effect of GA on the electronic structure of BNNTs (Fig. 7). After removing free GA from the dispersion, a notable red shift of the UV-Vis absorption peak for the BNNTs was observed. A sharp peak assigned to the band-gap transition of BNNTs appeared at 200 nm for the original BNNTs, while it appeared at 205 nm for the GA-functionalized BNNTs. This shift evidenced that the electrical structure of BNNTs was significantly affected by the strong hydrophobic interactions between the BNNTs and GA, thereby resulting into the band-gap reduction. This observation indicated that GA on the BNNT surface may behave as a dopant. The same doping effects have also been observed previously in other organic molecule-functionalized BNNT systems.19,35 In addition, the apparent mountain-like absorption peak appeared at around 280 nm for the GA-functionalized BNNTs. The peak may originate from the arabinogalactan–protein complexes of GA absorbed on BNNTs, suggesting that GA is indeed covered over the hydrophobic sidewalls of BNNTs. It is important to note that the absorption peak at around 280 nm might be a result of the Van Hove singularities of BNNTs,34 which could be related to the emission at 330 nm.
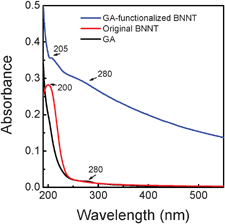 | ||
| Fig. 7 Comparative UV-Vis absorption spectra of GA, original BNNTs and purified GA-functionalized BNNTs. | ||
3.4. Immobilization of proteins on GA-functionalized BNNTs
Functional biomolecules such as proteins, DNA and RNA immobilized on the surface of nanostructured solid-state materials could exhibit superior biochemical properties (such as enzymatic activity) because of the large area, highly active surfaces, or interfaces of nanostructured materials.36,37 The immobilization of proteins onto 1D nanostructures is useful for developing highly sensitive chemical or biological detection systems, which can be utilized in therapy and nanobiotechnology.38–40 It has been reported that GA contains a large number of negative charges when the pH of the chemical environment is in the range 2.2–6.0.41 It is also noted that many proteins are positively charged if the pH is lower than their isoelectric points. Therefore, it was anticipated that those proteins can be immobilized onto the GA-functionalized BNNT surfaces under appropriate pH conditions via the strong electrostatic interactions between negatively charged GA and positively charged proteins. It is meaningful in two aspects. First, the immobilization of functional proteins onto tube surface endows specific biological functions to BNNTs, such as molecular recognition (e.g. antigen–antibody interactions), which is useful for the construction of bio-devices. Second, BNNTs are promising for functioning as a transporter for signals or masses in biological systems. Because the BNNT channels are very similar to those of biological microtubules found in living bodies (made by linear polymerization of tubulin proteins),42,43 BNNTs would be efficient artificial channels for signals and mass transport in biological systems. Understanding the interactions between proteins and the BNNTs is definitely beneficial to exploring the biological potential of BNNTs.For the immobilization of proteins onto GA-functionalized BNNTs, the pHs of model protein solutions of SAv, BSA, Lyz, and IgG were adjusted to pH 3 or 4 (Table 1) using a universal Britton Robinson buffer. The pHs of the proteins solutions were controlled to be lower than the isoelectric points of these proteins (SAv: 6.8–7.5;44 BSA: 4.8–5.4;45 Lyz: 10.7–11.0;46 IgG: 5.0–8.5.47), allowing the positively charged proteins to be attracted electrostatically and interact with the negatively charged GA-functionalized BNNT surfaces, thus leading to the required immobilization. AFM was employed for imaging the proteins immobilized on the surface of GA-functionalized BNNTs. Fig. 8a and 8b show AFM images of GA-functionalized BNNTs without proteins; the surfaces were rather smooth. As shown in Fig. 8c–e, it is clear that many protein particles locate on GA-functionalized BNNT surfaces, whereas the background of the sample is much cleaner. The same results can also be further seen in the 3D AFM images, as shown in Fig. S2 (ESI†). The height profile of these proteins on GA-functionalized BNNTs is further roughly monitored by using AFM (Fig. S3, ESI†). The sizes of SAv and BSA on GA-functionalized BNNTs are averaged as 8.2 ± 0.8 nm and 6.8 ± 1.2 nm (Table 1), respectively. These sizes are close to those for original SAv48 of 4.2 × 5.6 × 5.6 nm3 and BSA49 of 4.0 × 4.0 × 14.0 nm3 used in these experiments (Table 1), implying a thin layer coverage of proteins molecules on GA-functionalized BNNTs. In the cases of Lyz and IgG, the sizes of these proteins on GA-functionalized BNNTs are averaged as 12.9 ± 1.3 nm and 32.2 ± 3.2 nm (Table 1), respectively. These sizes are larger than those for original Lyz50 of 4.5 × 3.5 × 3.5 nm3 and IgG38 of 14.5 × 8.5 × 4.0 nm3. This difference may primarily result from the changes of protein conformations induced by the large surface curvature of BNNTs. On the other hand, the densities of Lyz and IgG on BNNTs are pretty high, which resulted in difficulties and errors during AFM measurements. These investigations strongly support the fact that the proteins were successfully immobilized onto the surface of GA-functionalized BNNTs. The presented approach is more efficient compared with the previous reports, in which immobilization of proteins onto BNNTs had been achieved by means of functionalizing BNNT using 1-pyrenebutyric acid N-hydroxysuccinimide ester (PAHE) in solution. Using the former approach, it took at least 24 h to stir the mixture of proteins and PAHE-functionalized BNNTs for the immobilization.17 Furthermore, in the present study, almost all proteins have closely been arrayed along with the longitudinal direction of GA-functionalized BNNTs, confirming that GA-functionalized BNNTs are indeed efficiently workable for proteins immobilization. Although the precise biological activities of those proteins immobilized on GA-functionalized BNNTs are needed to be carefully investigated in the future, the present approach is envisaged to be the significant breakthrough aiming at promoting BNNT applications in nanotechnologies.
 | ||
| Fig. 8 (a, b) AFM images of GA-functionalized BNNTs; (c) SAv (d) BSA (d) Lyz and (e) IgG on GA-functionalized BNNTs. | ||
| Protein | Molecular weight (kDa) | Isoelectric point | Size (nm3) | Immobilization pH | AFM height (nm) |
|---|---|---|---|---|---|
| SAv44,48 | 52.8 | 6.8–7.5 | 4.2 × 5.6 × 5.6 | 4.0 | 8.2 ± 0.8 |
| BSA45,49 | 66.8 | 4.8–5.9 | 4.0 × 4.0 × 14.0 | 3.0 | 6.8 ± 1.2 |
| Lyz46,50 | 14.7 | 10.7–11.0 | 4.5 × 3.5 × 3.5 | 4.0 | 12.9 ± 1.3 |
| IgG47,38 | 150 | 5.0–8.5 | 14.5 × 8.5 × 4.0 | 4.0 | 32.2 ± 3.2 |
4. Conclusions
We have illustrated herein that the facile disentanglement of BNNTs from their bundles can be achieved by functionalizing BNNTs with a water-soluble natural polysaccharide of GA. AFM observations indicated the excellent dispersion of GA-functionalized BNNTs in an aqueous solution. FT-IR and fluorescence spectra investigations suggested that strong interactions took place between GA and BNNTs. UV-Vis absorption spectra revealed a red shift for the maximum absorption peak, implying that the electronic structure of BNNT was modified through GA doping. Subsequently, several typical model proteins were successfully immobilized onto the GA-functionalized BNNT surfaces. AFM height investigations showed that these proteins maintained their compact molecular structures on the GA-functionalized BNNT surfaces. We have also proved that various proteins with the same surface charged states can be immobilized onto GA-functionalized BNNTs. The present study not only provides a novel, low-cost pathway for the isolation of efficiently disentangled BNNTs in an aqueous solution, but also serves as an extremely important initial step towards BNNT-based bio-devices, such as prospective biosensors and gene delivery systems. We are also going to further extend the present methodology to the suspension of protein-immobilized BNNTs in aqueous physiological solutions to address novel potential applications in bio-related fields.Acknowledgements
We are grateful to Dr T. Sawada (Univ. of Tokyo) for helpful discussions. This study was supported in part by the Grant-in-Aid for Scientific Research (21105605) and the Grant-in-Aid for Scientific Research (23310082). C.Z., Y.B. and D.G acknowledge the World Premier International (WPI) Center for Materials Nanoarchitectonics (MANA) tenable at the National Institute for Materials Science (NIMS) for a financial support of BNNT synthetic work.References
- Y. N. Xia, P. D. Yang, Y. G. Sun, Y. Y. Wu, B. Mayers, B. Gates, Y. D. Yin, F. Kim and H. Q. Yan, Adv. Mater., 2003, 15, 353 CrossRef CAS.
- N. A. Kotov, Science, 2010, 330, 188 CrossRef CAS.
- G. F. Zheng, F. Patolsky, Y. Cui, W. U. Wang and C. M. Lieber, Nat. Biotechnol., 2005, 23, 1294 CrossRef CAS.
- H. Chen, C. Jiang, C. Yu, S. Zhang, B. Liu and J. Kong, Biosens. Bioelectron., 2009, 24, 3399 CrossRef CAS.
- D. W. Hutmacher and S. Cool, J. Cell. Mol. Med., 2007, 11, 654 CrossRef CAS.
- E. Engvall and P. Perlmann, Immunochemistry, 1971, 8, 871 CrossRef CAS.
- Q. Ying, J. Nichols, I. Chambers and A. Smith, Cell, 2003, 115, 281 CrossRef CAS.
- K. K. Jain, Drug Discovery Today, 2005, 10, 1435 CrossRef CAS.
- N. G. Chopra, R. G. Luyken, K. Cherrey, V. H. Crespi, M. H. Cohen, S. G. Louie and A. Zettl, Science, 1995, 269, 966 CAS.
- D. Golberg, Y. Bando, C. Tang and C. Zhi, Adv. Mater., 2007, 19, 2413 CrossRef CAS.
- D. Golberg, Y. Bando, Y. Huang, T. Terao, M. Mitome, C. Tang and C. Zhi, ACS Nano, 2010, 4, 2979 CrossRef CAS.
- C. Zhi, Y. Bando, C. Tang and D. Golberg, Mater. Sci. Eng., R, 2010, 70, 92 CrossRef.
- W. Mickelson, S. Aloni, W. Han, J. Cumings and A. Zettl, Science, 2003, 300, 467 CrossRef CAS.
- Z. Shen, L. He, E. Wu, Y. Fan, J. He, H. Cheng, D. Li and H. Ye, J. Mater. Res., 2002, 17, 2761 CrossRef CAS.
- C. Zhi, Y. Bando, C. Tang, S. Honda, K. Sato, H. Kuwahara and D. Golberg, Angew. Chem., Int. Ed., 2005, 44, 7932 CrossRef CAS.
- C. Zhi, Y. Bando, T. Terao, C. Tang, H. Kuwahara and D. Golberg, Chem.–Asian J., 2009, 4, 1536 CrossRef CAS.
- C. Zhi, Y. Bando, C. Tang and D. Golberg, J. Am. Chem. Soc., 2005, 127, 17144 CrossRef CAS.
- F. Balavoine, P. Schultz, C. Richard, V. Mallouh, T. W. Ebbesen and C. Mioskowski, Angew. Chem., Int. Ed., 1999, 38, 1912 CrossRef CAS.
- C. Zhi, Y. Bando, C. Tang, R. Xie, T. Sekiguchi and D. Golberg, J. Am. Chem. Soc., 2005, 127, 15996 CrossRef CAS.
- X. Chen, P. Wu, M. Rousseas, D. Okawa, Z. Gartner, A. Zettl and C. R. Bertozzi, J. Am. Chem. Soc., 2009, 131, 890 CrossRef CAS.
- L. Horváth, A. Magrez, D. Golberg, C. Zhi, Y. Bando, R. Smajda, E. Horváth, L. Forró and B. Schwaller, ACS Nano, 2011, 5, 3800 CrossRef.
- E. Katz and I. Willner, ChemPhysChem, 2004, 5, 1084 CrossRef CAS.
- Z. Gao, C. Zhi, Y. Bando, D. Golberg and T. Serizawa, J. Am. Chem. Soc., 2010, 132, 4976 CrossRef CAS.
- C. Zhi, Y. Bando, W. Wang, C. Tang, H. Kuwahara and D. Golberg, Chem.–Asian J., 2007, 2, 1581 CrossRef CAS.
- Z. Gao, C. Zhi, Y. Bando, D. Golberg and T. Serizawa, ACS Appl. Mater. Interfaces, 2011, 3, 627 CAS.
- Z. Gao, T. Sawada, C. Zhi, Y. Bando, D. Golberg and T. Serizawa, Soft Matter, 2011, 7, 8753 RSC.
- A. M. Lslam, G. O. Phillipsl, A. Sljivo, M. J. Snowden and P. A. Williamsl, Food Hydrocolloids, 1997, 11, 493 CrossRef.
- C. Wu and D. Chen, Gold Bull., 2010, 43, 234 CrossRef CAS.
- L. Batalhaa, A. Hussaina and A. C. A. Roquea, J. Mol. Recognit., 2010, 23, 462 CrossRef.
- R. Bandyopadhyaya, E. Nativ-Roth, O. Regev and R. Yerushalmi-Rozen, Nano Lett., 2002, 2, 25 CrossRef CAS.
- V. Raffa, G. Ciofani and A. Cuschieri, Nanotechnology, 2009, 20, 075104 CrossRef CAS.
- M. L. Jayme, D. E. Dunstan and M. L. Gee, Food Hydrocolloids, 1999, 13, 459 CrossRef CAS.
- H. Espinosa-Andrews, O. Sandoval-Castilla, H. Vazquez-Torres, E. J. Vernon-Carter and C. Lobato-Calleros, Carbohydr. Polym., 2010, 79, 541 CrossRef CAS.
- P. Jaffrennou, J. Barjon, J. S. Lauret, A. Maguer, D. Golberg, B. Attal-Trétout, F. Ducastelle and A. Loiseau, Phys. Status Solidi B, 2007, 244, 4147 CrossRef CAS.
- Q. Huang, A. S. D. Sandanayaka, Y. Bando, C. Zhi, R. Ma, G. Shen, D. Golberg, J. Zhao, Y. Araki, O. Ito and L. Gao, Adv. Mater., 2007, 19, 934 CrossRef CAS.
- W. Huang, S. Taylor, K. Fu, Y. Lin, D. Zhang, T. W. Hanks, A. W. Rao and Y. Sun, Nano Lett., 2002, 2, 311 CrossRef CAS.
- J. Zhang, F. Zhang, H. Yang, X. Huang, H. Liu, J. Zhang and S. Guo, Langmuir, 2010, 26, 6083 CrossRef CAS.
- K. Lee, S. Park, C. A. Mirkin, J. C. Smith and M. Mrksich, Science, 2002, 295, 1702 CrossRef CAS.
- C. Fan, K. Kurabayashi and E. Meyhöfer, Nano Lett., 2006, 6, 2763 CrossRef CAS.
- G. Singh, S. Pillai, A. Arpanaei and P. Kingshott, Adv. Mater., 2011, 23, 1519 CrossRef CAS.
- T. Vinayahan, P. A. Williams and G. O. Phillips, Biomacromolecules, 2010, 11, 3367 CrossRef CAS.
- R. E. Stephens and K. T. Edds, Physiol. Rev., 1976, 56, 709 CAS.
- K. H. Downing and E. Nogales, Curr. Opin. Cell Biol., 1998, 10, 16 CrossRef CAS.
- M. J. Linman, H. Yu, X. Chen and Q. Cheng, ACS Appl. Mater. Interfaces, 2009, 1, 1755 CAS.
- P. Theodore, Adv. Protein Chem., 1985, 37, 161 CrossRef.
- J. Lu, Y. Wan and Z. Cui, Ind. Eng. Chem. Res., 2005, 44, 7610 CrossRef CAS.
- A. G. Norden, L. M. Fulcher and F. V. Flynn, Clin. Chim. Acta, 1987, 166, 307 CrossRef CAS.
- V. C. Yang and T. T. Ngo, Biosensors and Their Applications, NewYork, Kluwer/Plenum, 2000, 35 Search PubMed.
- S. Tsuneda, K. Saito, T. Sugo and K. Makuuchi, Radiat. Phys. Chem., 1995, 46, 239 CrossRef CAS.
- A. A. Vertegel, R. W. Siegel and J. S. Dordick, Langmuir, 2004, 20, 6800 CrossRef CAS.
Footnotes |
| † Electronic Supplementary Information (ESI) available: Schemes S1, S2 and Fig. S1–S3. See DOI: 10.1039/c2ra20765f/ |
| ‡ The difference in AFM size of BNNTs appearing in Fig. 3 and Fig. 8 originates from the AFM tip-broading effect which is generally caused by the non-perfect shape of the AMP tip and a notable dispersion in nanoobject sizes. |
| This journal is © The Royal Society of Chemistry 2012 |
