Preparation of 4,4′-(arylmethylene)-bis(3-methyl-1-phenyl-1H-pyrazol-5-ol)s over 1,3-disulfonic acid imidazolium tetrachloroaluminate as a novel catalyst
Ardeshir
Khazaei
*a,
Mohammad Ali
Zolfigol
*a,
Ahmad Reza
Moosavi-Zare
a,
Zhila
Asgari
a,
Mohsen
Shekouhy
b,
Abdolkarim
Zare
c and
Alireza
Hasaninejad
d
aFaculty of Chemistry, Bu-Ali Sina University, Hamedan, Iran 6517838683. E-mail: Khzaei_1326@yahoo.com; mzolfigol@yahoo.com; Fax: +988118257407; Tel: +988118282807
bDepartment of Applied Chemistry, Faculty of Science, Shiraz Branch, Islamic Azad University, P. O. Box 71993–5, Shiraz, Iran
cDepartment of Chemistry, Payame Noor University, PO BOX 19395-4697, Tehran, Iran
dDepartment of Chemistry, Faculty of Sciences, Persian Gulf University, Bushehr, 75169, Iran
First published on 13th July 2012
Abstract
In this work, a green, simple and efficient method for the synthesis of 4,4′-(arylmethylene)-bis(3-methyl-1-phenyl-1H-pyrazol-5-ol)s by the condensation of 1-phenyl-3-methylpyrazol-5-one with aromatic aldehydes using 1,3-disulfonic acid imidazolium tetrachloroaluminate {[Dsim]AlCl4} as a new, heterogeneous and reusable catalyst is reported.
Among the heterocyclic ring systems, pyrazolone derivatives have a wide range of unique biological activities. Some of the pyrazolone derivatives are included in many of the commercialized drugs for brain ischemia,1 and myocardial ischemia.2 Among them, bis(pyrazolyl)methanes (BPMs) such as 4,4′-(arylmethylene)-bis(3-methyl-1-phenyl-1H-pyrazol-5-ol)s have a broad spectrum of approved biological activity, being used as anti-inflammatory,3 antipyretic,4 gastric secretion stimulatory,5 antidepressant,6 antibacterial,7 and antifilarial agents.8 Moreover, these compounds have been applied as fungicides,9 pesticides,10 insecticides,11 dyestuffs,12 and chelating as well as extracting reagents for different metal ions.12 In spite of extensive application of 4,4′-(arylmethylene)-bis(3-methyl-1-phenyl-1H-pyrazol-5-ol)s, a few methods have been reported for their preparation. These methods include application of piperidine in ethanolic solution,13 the tandem Knoevenagel–Michael reaction in benzene solutions,14 application of sodium dodecyl sulfate in aqueous media,15 and electrocatalytic synthesis.16–18 Nevertheless, most of these methods suffer from limitations such as moderate yields, long reaction times, harsh reaction conditions, application of hazardous solvents and/or tedious workup procedures. Therefore, finding an efficient and capable protocol for the preparation of 4,4′-(arylmethylene)-bis(3-methyl-1-phenyl-1H-pyrazol-5-ol)s is very important.
Solvent-free reactions have been demonstrated to be as an efficient technique for various organic transformations instead of using harmful organic solvents. Solvent-free conditions often lead to a remarkable decrease in reaction times, increased yields, easier workup, matches with green chemistry protocols, and may enhance the regio- and stereoselectivity of reactions.19–21
Recently, we have introduced a new category of acidic ionic liquids, namely sulfonic acid functionalized imidazolium salts (SAFIS).22–27 These ILs have been successfully used as catalysts or reagents for the synthesis of bis(indolyl)methans,22N-sulfonyl imines,23 nitro aromatic compounds,24,25 1-amidoalkyl-2-naphthols,26 benzimidazoles27 and β-acetamido ketones.28 Among this category, 3-methyl-1-sulfonic acid imidazolium tetrachloroaluminate {[Msim]AlCl4}, another analogue of these salts (Fig. 1), has been produced as a solid and catalyzed the synthesis of 1-amidoalkyl-2-naphthols.26
![The structure of 3-methyl-1-sulfonic acid imidazolium tetrachloroaluminate {[Msim]AlCl4}.](/image/article/2012/RA/c2ra20988h/c2ra20988h-f1.gif) | ||
| Fig. 1 The structure of 3-methyl-1-sulfonic acid imidazolium tetrachloroaluminate {[Msim]AlCl4}. | ||
In continuation of our previous investigations involving the production and applications of acidic ILs and solid imidazolium salts in organic transformations, we report here 1,3-disulfonic acid imidazolium tetrachloroaluminate {[Dsim]AlCl4} (Fig. 2) as a novel analogue of SAFIS, which exhibits many interesting properties.†
![The structure and color of 1,3-disulfonic acid imidazolium tetrachloroaluminate {[Dsim]AlCl4}.](/image/article/2012/RA/c2ra20988h/c2ra20988h-f2.gif) | ||
| Fig. 2 The structure and color of 1,3-disulfonic acid imidazolium tetrachloroaluminate {[Dsim]AlCl4}. | ||
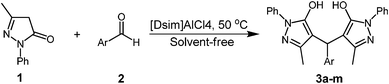
In this presented work, we wish to introduce 1,3-disulfonic acid imidazolium tetrachloroaluminate {[Dsim]AlCl4} as a new, efficient and heterogeneous catalyst for the facile and green synthesis of 4,4′-(arylmethylene)-bis(3-methyl-1-phenyl-1H-pyrazol-5-ol)s under solvent-free conditions (Scheme 1). This method is highly efficient and free from the aforesaid drawbacks.
![The preparation of 4,4′-(arylmethylene)-bis(3-methyl-1-phenyl-1H-pyrazol-5-ol)s by [Dsim]AlCl4.](/image/article/2012/RA/c2ra20988h/c2ra20988h-s1.gif) | ||
| Scheme 1 The preparation of 4,4′-(arylmethylene)-bis(3-methyl-1-phenyl-1H-pyrazol-5-ol)s by [Dsim]AlCl4. | ||
First of all, [Dsim]AlCl4 was synthesized by the reaction of [Dsim]Cl with AlCl3 (Scheme 2), and its structure was identified by IR, 1H NMR and 13C NMR and mass spectra.
![The preparation of 1,3-disulfonic acid imidazolium tetrachloroaluminate {[Dsim]AlCl4}.](/image/article/2012/RA/c2ra20988h/c2ra20988h-s2.gif) | ||
| Scheme 2 The preparation of 1,3-disulfonic acid imidazolium tetrachloroaluminate {[Dsim]AlCl4}. | ||
The IR spectrum of the catalyst shows one peak observed at 1315 cm−1 corresponding to the vibrational modes of N–SO2 bond, others at 629 cm−1 and 1200 cm−1 related to stretching vibrations of S–O and S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds, and a broad peak at 2950–3400 cm−1 related to the stretching vibration of O–H in the SO3H group. The 1H NMR spectrum of [Dsim]AlCl4 shows the unmistakeable acidic hydrogen (SO3H) peak at 14.37 ppm. Also, two peaks besides 14.37 ppm in the 1H NMR spectrum in Fig. 3, are related to two hydrogen groups of the imidazoliom ring. The 13C NMR spectrum of [Dsim]AlCl4 shows two peaks at 119.8 and 134.8 related to carbons of imidazolium ring. The IR, 1H NMR and 13C NMR spectra of [Dsim]AlCl4 are given in Fig. 3. The mass spectrum of the compound gave the correct molecular ion peak at 398.
O bonds, and a broad peak at 2950–3400 cm−1 related to the stretching vibration of O–H in the SO3H group. The 1H NMR spectrum of [Dsim]AlCl4 shows the unmistakeable acidic hydrogen (SO3H) peak at 14.37 ppm. Also, two peaks besides 14.37 ppm in the 1H NMR spectrum in Fig. 3, are related to two hydrogen groups of the imidazoliom ring. The 13C NMR spectrum of [Dsim]AlCl4 shows two peaks at 119.8 and 134.8 related to carbons of imidazolium ring. The IR, 1H NMR and 13C NMR spectra of [Dsim]AlCl4 are given in Fig. 3. The mass spectrum of the compound gave the correct molecular ion peak at 398.
![The IR, 1H NMR and 13C NMR spectra of 1,3-disulfonic acid imidazolium tetrachloroaluminate {[Dsim]AlCl4}.](/image/article/2012/RA/c2ra20988h/c2ra20988h-f3.gif) | ||
| Fig. 3 The IR, 1H NMR and 13C NMR spectra of 1,3-disulfonic acid imidazolium tetrachloroaluminate {[Dsim]AlCl4}. | ||
To optimize the reaction conditions, the solvent-free condensation of 3-methyl-1-phenyl-5-pyrazolone (1) (2 mmol) with 4-nitrobenzaldehyde (1 mmol), as model reaction using different amounts of [Dsim]AlCl4, [Msim]AlCl4, [Dsim]Cl and [Msim]Cl at range of 25–50 °C was examined (Table 1).
| Catalysts | Mol% of catalyst | Temperature/°C | Time/min | Yield (%)a |
|---|---|---|---|---|
| a Isolated yield. b 3-Methyl-1-sulfonic acid imidazolium tetrachloro aluminate. c 1,3-Disulfonic acid imidazolium chloride. d 3-Methyl-1-sulfonic acid imidazolium chlororide. | ||||
| — | — | 50 | 12 h | 15 |
| [Dsim]AlCl4 | 0.5 | 50 | 40 | 78 |
| [Dsim]AlCl4 | 1 | 50 | 40 | 91 |
| [Dsim]AlCl4 | 3 | 50 | 40 | 91 |
| [Dsim]AlCl4 | 1 | 25 | 40 | 35 |
| [Msim]AlCl4b | 1 | 50 | 40 | 82 |
| [Msim]AlCl4b | 3 | 50 | 40 | 90 |
| [Dsim]Clc | 3 | 50 | 40 | 36 |
| [Dsim]Clc | 5 | 50 | 40 | 42 |
| [Msim]Cld | 3 | 50 | 40 | 29 |
| [Msim]Cld | 5 | 50 | 40 | 34 |
The best results were obtained using 1 mol% of [Dsim]AlCl4 at 50 °C. Increasing the reaction time did not improve the results. This reaction was also examined at 50 °C in the absence of catalyst under solvent-free conditions in which the reaction did not noticeable progress even after a long reaction time (12 h).
To compare the efficiency of the solution versus solvent-free conditions, a mixture of 3-methyl-1-phenyl-5-pyrazolone (1) (2 mmol) with 4-nitrobenzaldehyde (1 mmol), as model reaction, in the presence of [Dsim]AlCl4 in some various solvents was heated in an oil-bath (50 °C) for 90 min. Low yields of the product were obtained, even after elongated reaction times. Using solvents such as CHCl3, EtOAc, EtOH, THF and CH3CN, the product was isolated in low yields (Table 2). Therefore, the solvent-free reaction is more efficient.
After optimization of the reaction conditions, to explore the efficiency and the scope of the presented protocol, 3-methyl-1-phenyl-5-pyrazolone was treated with structurally diverse aromatic aldehydes under the optimized reaction conditions in the presence of [Dsim]AlCl4 as catalyst. The corresponding results are depicted in Table 3.
|
|
||||
|---|---|---|---|---|
| Entry | Ar | Time | Yield (%)a | M.p./°C (lit.) |
| a Isolated yield. | ||||
| 3a | C6H5 | 60 | 86 | 168–170 (171–172)15 |
| 3b | 4-ClC6H4 | 50 | 91 | 213–215 (207–209)15 |
| 3c | 3-ClC6H4 | 45 | 89 | 150–152 (153–154)15 |
| 3d | 2-ClC6H4 | 45 | 88 | 235–236 (236–237)15 |
| 3e | 4-EtOC6H4 | 60 | 84 | 184–186(185–188)18 |
| 3f | 4-NO2C6H4 | 40 | 91 | 229–231(230–232)15 |
| 3g | 3-NO2C6H4 | 30 | 93 | 145–147 (149–150)15 |
| 3h | 2-NO2C6H4 | 30 | 90 | 221–223 (224–225)15 |
| 3i | 2-Thienyl | 60 | 81 | 189–190 (190–192)18 |
| 3j | 2-Furyl | 60 | 72 | 190–193 (189–191)18 |
| 3k | 2-Pyridyl | 60 | 74 | 228–231 (230–232)18 |
| 3l | 3-BrC6H4 | 30 | 90 | 173–175 (172–175)18 |
| 3m | 4-MeC6H4 | 45 | 87 | 201–203 (203–204)15 |
As Table 3 indicates, all aldehydes (including benzaldehyde and arylaldehydes bearing halogens, electron-withdrawing or electron-releasing substituents) were successfully reacted with 3-methyl-1-phenyl-5-pyrazolone to give the corresponding 4,4′-(arylmethylene)-bis(3-methyl-1-phenylpyrazol-5-ol) derivatives in good to excellent yields and in relatively short reaction times. The presented method was successfully applied for the condensation of 3-methyl-1-phenyl-5-pyrazolone with heteroaromatic aldehydes, although, acid-sensitive aldehydes (for example furfural) as well as basic aldehydes (for example 2-pyridinecarbaldehyde) decreased the reaction yields (Table 3, entry 3i and 3j). Interestingly, the condensation of 3-methyl-1-phenyl-5-pyrazolone (2 eq.) with terephthaldehyde (1 eq.) in the presence of [Dsim]AlCl4 at 50 °C under solvent-free conditions, afforded di-4,4′-(arylmethylene)-bis(3-methyl-1-phenylpyrazol-5-ol) 5 in 84% yield within 60 min (Scheme 3).
![The preparation of di-4,4′-(arylmethylene)-bis(3-methyl-1-phenylpyrazol-5-ol) using [Dsim]AlCl4 as catalyst.](/image/article/2012/RA/c2ra20988h/c2ra20988h-s3.gif) | ||
| Scheme 3 The preparation of di-4,4′-(arylmethylene)-bis(3-methyl-1-phenylpyrazol-5-ol) using [Dsim]AlCl4 as catalyst. | ||
In a plausible mechanism that is shown in Scheme 4, at first, 3-methyl-1-phenyl-5-pyrazolone I converts to II after tautomerisation. Then, II attacks to the carbonyl group of aldehyde that is activated by the [Dsim]AlCl4via hydrogen bonds and affords to intermediate III after removing one molecule of H2O. III acts as a Michael acceptor and is activated by [Dsim]AlCl4. In this step, another molecule of 3-methyl-1-phenyl-5-pyrazolone in II tautomer form, attacks to III to give Intermediate IV. Finally, IV converts to V after tautomerisation and aromatization as product.
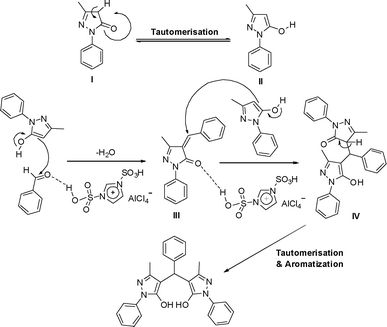 | ||
| Scheme 4 Plausible mechanism for the synthesis of 4,4′-(arylmethylene)-bis(3-methyl-1-phenyl-1H-pyrazol-5-ol)s. | ||
In another study, recyclability of the catalyst was examined upon the condensation of 3-methyl-1-phenyl-5-pyrazolone with 4-nitrobenzaldehyde. The reaction mixture was extracted by warm absolute ethanol and separated from the catalyst. Afterward the reused catalyst was employed for another reaction. We observed that the catalytic activity of the catalyst was restored within the limits of experimental error for five successive runs (Fig. 4).
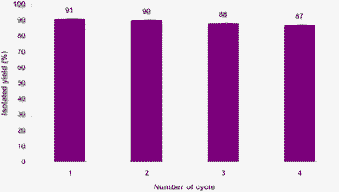 | ||
| Fig. 4 Recyclability of the catalyst for the synthesis of 4,4′-((4-nitrophenyl) methylene) bis(3-methyl-1-phenyl-1H-pyrazol-5-ol) (3f). | ||
In summary, we have reported the synthesis of 4,4′-(arylmethylene)-bis(3-methyl-1-phenylpyrazol-5-ols) in a green recyclable media. Promising points of the presented methodology are efficiency, generality, high yield, relatively short reaction time, low cost, cleaner reaction profile, ease of product isolation, simplicity, and finally compliance with green chemistry protocols.
References
- Comprehensive Heterocyclic Chemistry: Pyrazoles and their Benzo Derivatives, ed. J. Elguero, A. R. Katritzky and C. W. Rees, Pergamon Press, Oxford, 1984, vol. 5 Search PubMed.
- T. Kessler, T. Aybek, G. Neidhart, S. Dogan, D. Bremerich, D. Lischke and C. Byhahan, J. Cardiothorac. Vasc. Anesth., 2005, 19, 32 CrossRef.
- S. Sugiura, S. Ohno, O. Ohtani, K. Izumi, T. Kitamikado, H. Asai and K. Kato, J. Med. Chem., 1977, 20, 80 CrossRef CAS.
- L. C. Behr, R. Fusco and C. H. JarboeThe Chemistry of Heterocyclic Compounds, Pyrazoles, Pyrazolines, Pyrazolidines, Indazoles and Condensed Rings, ed. A. Weissberger, Interscience Publishers, New York, 1967 Search PubMed.
- C. E. Rosiere and M. I. Grossman, Science, 1951, 113, 651 CAS.
- D. M. Bailey, P. E. Hansen, A. G. Hlavac, E. R. Baizman, J. Pearl, A. F. Defelice and M. E. Feigenson, J. Med. Chem., 1985, 28, 256 CrossRef CAS.
- R. N. Mahajan, F. H. Havaldar and P. S. Fernandes, J. Ind. Chem. Soc., 1991, 68, 245 CAS.
- P. M. S. Chauhan, S. Singh and R. K. Chatterjee, Ind. J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 1993, 32, 858 Search PubMed.
- D. Singh and D. Singh, J. Ind. Chem. Soc., 1991, 68, 165 CAS.
- M. Londershausen, Pestic. Sci., 1996, 48, 269 CrossRef CAS.
- The Chemistry of Synthetic Dyes and Pigments, ed. H. A. Lubs, American Chemical Society, Washington D.C., 1970 Search PubMed.
- A. D. Garnovskii, A. I. Uraev and V. I. Minkin, ARKIVOC, 2004, iii, 29 Search PubMed.
- D. Singh and D. Singh, J. Chem. Eng. Data, 1984, 29, 355 CrossRef CAS.
- B. I. Buzykin and T. I. Lonshchakova, Bull. Acad. Sci. USSR, Div. Chem. Sci. (Engl. Transl.), 1971, 20, 2224 CrossRef.
- W. Wang, S.-X. Wang, X.-Y. Qin and J.-T. Li, Synth. Commun., 2005, 35, 1263 CrossRef CAS.
- M. N. Elinson, A. S. Dorofeev, R. F. Nasybullin and G. I. Nikishin, Synthesis, 2008, 1933 CrossRef CAS.
- A. Hasaninejad, A. Zare, M. Shekouhy, S. M. S. H. Ghattali and N. Golzar, J. Iran. Chem. Soc., 2011, 8, 411 CrossRef CAS.
- A. Hasaninejad, A. Zare, M. Shekouhy and N. Golzar, Org. Prep. Proced. Int., 2011, 43, 131 CrossRef CAS.
- K. Tanaka, Solvent-Free Organic Synthesis, Wiley- VCH, GmbH and KGaA, Weinheim, 2004 Search PubMed.
- G. H. Imanzadeh, A. Khalafi-Nezhad, A. Zare, A. Hasaninejad, A. R. Moosavi-Zare and A. J. Parhami, J. Iran. Chem. Soc., 2007, 4, 229 CrossRef CAS.
- A. Zare, A. Hasaninejad, A. Khalafi-Nezhad, A. R. Moosavi-Zare, A. Parhami and G. R. Nejabat, ARKIVOC, 2007, 1, 58 Search PubMed.
- M. A. Zolfigol, A. Khazaei, A. R. Moosavi-Zare and A. Zare, Org. Prep. Proced. Int., 2010, 42, 95 CrossRef CAS.
- M. A. Zolfigol, A. Khazaei, A. R. Moosavi-Zare and A. Zare, J. Iran. Chem. Soc., 2010, 7, 646 CrossRef CAS.
- A. Khazaei, M. A. Zolfigol, A. R. Moosavi-Zare and A. Zare, Scientia Iranica: Trans. C: Chem. Chem. Engin., 2010, 17, 31 CAS.
- M. A. Zolfigol, A. Khazaei, A. R. Moosavi-Zare, A. Zare, H. G. Kruger, Z. Asgari, V. Khakyzadeh and M. Kazem-Rostami, J. Org. Chem., 2012, 77, 3640 CrossRef CAS.
- M. A. Zolfigol, A. Khazaei, A. R. Moosavi-Zare, A. Zare and V. Khakyzadeh, Appl. Catal., A, 2011, 400, 70 CrossRef CAS.
- A. Khazaei, M. A. Zolfigol, A. R. Moosavi-Zare, A. Zare, E. Ghaemi, V. Khakyzadeh, Z. Asgari and A. Hasaninejad, Scientia Iranica: Trans. C: Chem. Chem. Engin., 2011, 18, 1365 CAS.
- A. Zare, T. Hekmat-Zadeh, S. Mirzaei-Monfared, M. Merajoddin, H. Torabi-Monfared, M. A. Zolfigol, A. R. Moosavi-Zare, E. Rostami, M. Mokhlesi, F. Derakhshan-Panah, S. Porbahi and S. Balandeh, S. Afr. J. Chem., 2012, 65, 63 CAS.
Footnote |
† Preparation of 1: A round-bottomed flask (50 mL) was charged with 1,3-disulfonic acid imidazolium chloride (1.323 g, 5mmol), and then AlCl3 (0.6667 g, 5 mmol) was added over a period of 5 min at 50 °C. Afterward, the reaction mixture was stirred for 30 min at 50 °C to give [Dsim]AlCl4 as a white powder in 98% yield, 1.95 g. White powder, mp 395 °C (dec.); IR (KBr) 629, δ (ppm) 7.66 (s, 2H), 9.06 (s, 1H), 14.37 (s, 1H); 13C NMR (75 MHz, DMSO-d6): δ (ppm) 119.8, 134.8; MS: m/z = 399 (M+ + 1), 398 (M+. General procedure for the synthesis of 4,4′-(arylmethylene)-bis(3-methyl-1-phenyl-1H-pyrazol-5-ol)s: A mixture of 1-phenyl-3-methylpyrazol-5-one (1.74 g, 10 mmol), aldehyde (5 mmol) and [Dsim]AlCl4 (0.0199 g, 1 mol%) was added to a test tube, and stirred at 50 °C. After completion of the reaction, as monitored by TLC, the reaction mixture was cooled to room temperature, extracted with warm absolute ethanol (20 mL) and filtered to separate the catalyst. The solid residue (crude product) was recrystallized by adding water (2 mL) to the filtrate to give the pure product.The recovered catalyst was washed with EtOAc (2 × 20 mL), dried and reused for the preparation of 4,4′-(arylmethylene)-bis(3-methyl-1-phenyl-1H-pyrazol-5-ol)s according to the mentioned procedure. The catalyst was recovered and reused for four times without any significant changes in the yield and the reaction time. Note: The 1-phenyl-3-methylpyrazol-5-one/aldehyde molar ratio in the synthesis of compound 5 was 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Scheme 3). Compound 3a: Pale yellow solid (from 3 1 (Scheme 3). Compound 3a: Pale yellow solid (from 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 EtOH–H2O), mp. 168–170 °C (lit.27 171–172 °C); 1H NMR (DMSO-d6): δ 2.33 (s, 6H, 2CH3), 4.88 (s, 1H, CH), 7.07 (m, 1H), 7.18 (m, 6H), 7.44 (t, 4H), 7.58 (d, 4H); 13C NMR DMSO-d6): δ 12.0, 33.5, 121.4, 126.2, 126.8, 127.6, 128.2, 129.1, 137.3, 142.9, 146.1. Compound 3e: White solid (from EtOH), mp. 185–188 °C; 1H NMR (DMSO-d6): δ 1.06–1.08 (t, J = 7 Hz, 3H, CH3), 2.34 (s, 6H, 2CH3), 3.44–3.48 (q, J = 7 Hz, 2H, CH2), 5.01 (s, 1H, CH) 7.25–7.29 (m, 4H), 7.38–7.46 (m, 6H), 7.71–7.73 (d, J = 8 Hz, 4H), 13.94 (s, 1H, OH); 13C NMR (DMSO-d6): δ 12.4, 19.4, 33.7, 56.9, 121.5, 122.4, 126.5, 127.3, 129.8, 130.8, 131.2, 146.0, 147.2; MS (m/z): 480 (M+). Compound 3f: Pale yellow solid (from EtOH), mp. 229–231 °C (lit.27 230–232 °C); 1H NMR (DMSO-d6): δ 2.35 (s, 6H, 2CH3), 5.13 (s, 1H, CH), 7.25–7.27 (m, 2H, Arm H), 7.43–7.46 (t, J = 7 Hz, 4H), 7.51–7.53 (d, J = 8 Hz, 2H), 7.70–7.72 (d, J = 8 Hz, 4H), 8.16–8.18 (d, J = 8 Hz, 2H), 12.64 (s, 1H, OH), 13.86 (s, 1H, OH); 13C NMR (DMSO-d6): δ 12.5, 19.4, 34.0, 56.9, 121.5, 124.2, 126.6, 129.5, 129.8, 146.8, 147.1, 151.2. Compound 5: Pale yellow solid (from EtOH), mp. 193–196 °C (dec.); 1H-NMR (DMSO-d6): δ 2.16 (s, 12H, 4CH3), 4.71 (s, 2H, CH), 7.00–7.03 (t, J = 7.5 Hz, 4H), 7.06 (s, 4H), 7.17–7.21 (t, J = 8.0 Hz, 8H), 7.55–7.57 (d, J = 8.0 Hz, 8H), 13.40 (s, 4H, OH); 13C NMR (DMSO-d6): δ 12.2, 33.8, 120.6, 121.5, 125.9, 127.5, 129.0, 131.1, 137.0, 139.2, 146.7; MS (m/z): 448 (M+−C20H18N4O2 431, 354). 1 EtOH–H2O), mp. 168–170 °C (lit.27 171–172 °C); 1H NMR (DMSO-d6): δ 2.33 (s, 6H, 2CH3), 4.88 (s, 1H, CH), 7.07 (m, 1H), 7.18 (m, 6H), 7.44 (t, 4H), 7.58 (d, 4H); 13C NMR DMSO-d6): δ 12.0, 33.5, 121.4, 126.2, 126.8, 127.6, 128.2, 129.1, 137.3, 142.9, 146.1. Compound 3e: White solid (from EtOH), mp. 185–188 °C; 1H NMR (DMSO-d6): δ 1.06–1.08 (t, J = 7 Hz, 3H, CH3), 2.34 (s, 6H, 2CH3), 3.44–3.48 (q, J = 7 Hz, 2H, CH2), 5.01 (s, 1H, CH) 7.25–7.29 (m, 4H), 7.38–7.46 (m, 6H), 7.71–7.73 (d, J = 8 Hz, 4H), 13.94 (s, 1H, OH); 13C NMR (DMSO-d6): δ 12.4, 19.4, 33.7, 56.9, 121.5, 122.4, 126.5, 127.3, 129.8, 130.8, 131.2, 146.0, 147.2; MS (m/z): 480 (M+). Compound 3f: Pale yellow solid (from EtOH), mp. 229–231 °C (lit.27 230–232 °C); 1H NMR (DMSO-d6): δ 2.35 (s, 6H, 2CH3), 5.13 (s, 1H, CH), 7.25–7.27 (m, 2H, Arm H), 7.43–7.46 (t, J = 7 Hz, 4H), 7.51–7.53 (d, J = 8 Hz, 2H), 7.70–7.72 (d, J = 8 Hz, 4H), 8.16–8.18 (d, J = 8 Hz, 2H), 12.64 (s, 1H, OH), 13.86 (s, 1H, OH); 13C NMR (DMSO-d6): δ 12.5, 19.4, 34.0, 56.9, 121.5, 124.2, 126.6, 129.5, 129.8, 146.8, 147.1, 151.2. Compound 5: Pale yellow solid (from EtOH), mp. 193–196 °C (dec.); 1H-NMR (DMSO-d6): δ 2.16 (s, 12H, 4CH3), 4.71 (s, 2H, CH), 7.00–7.03 (t, J = 7.5 Hz, 4H), 7.06 (s, 4H), 7.17–7.21 (t, J = 8.0 Hz, 8H), 7.55–7.57 (d, J = 8.0 Hz, 8H), 13.40 (s, 4H, OH); 13C NMR (DMSO-d6): δ 12.2, 33.8, 120.6, 121.5, 125.9, 127.5, 129.0, 131.1, 137.0, 139.2, 146.7; MS (m/z): 448 (M+−C20H18N4O2 431, 354). |
| This journal is © The Royal Society of Chemistry 2012 |