Hydrogenation of TiO2 nanosheets with exposed {001} facets for enhanced photocatalytc activity†
Wang
Wei
,
Ni
Yaru
,
Lu
Chunhua
* and
Xu
Zhongzi
State Key Laboratory of Materials-Orient Chemical Engineering, College of Materials Science and Engineering, Nanjing University of Technology, Nanjing, 210009, PR China. E-mail: lchnjut@163.com; Fax: +86-25-83587220; Tel: +86-25-83587252
First published on 24th July 2012
Abstract
Hydrogenated {001}-facets-dominated anatase TiO2 nanosheets have O–H bonds and O− on their surface. The high reactive {001} facets were maintained by the formation of Ti–H bonds. A large number of Ti3+ ions and oxygen vacancies were produced by hydrogenation, resulting in improved light absorption and enhanced photocatalytic activity.
TiO2, the most popular wide-band-gap photocatalysts, which can use the UV part of solar light to degrade organic pollutants and produce hydrogen from water, have attracted world-wide attention in the last two decades.1,2 The photoactivity performance of TiO2 is mainly dependent on its morphology, crystal structure, light absorption, and surface chemical environment. The {001} facets of anatase TiO2 have been considered to have higher photocatalytic activity than the traditional {101} facets because of their higher surface energy and the 100% unsaturated Ti5c atoms located on the {001} facets.3,4 To obtain highly reactive TiO2, most works have been focused on the controllable growth of {001}-facets-dominated TiO2. Liu et al. prepared {001}-facets-dominated TiO2 microspheres, resulting in high photocatalytic acitivity.5 Xie et al. successfully synthesized hollow TiO2 boxes with a high percentage of exposed {001} facets.6 However, systematic studies on the location position and effect of F, the most commonly used agent to control the exposure of the {001} facets, are scarce. On the other hand, it is also very important to enhance the visible light, even infrared light, absorption of TiO2. Recently, Chen et al. prepared hydrogenated TiO2 nanocrystals to introduce a surface disorder structure, extending the light absorption to the near infrared range (∼1200 nm).7 Wang et al. reported that the light absorption and water splitting ability of TiO2 nanowire arrays were significantly enhanced by hydrogenation.8 To date, there is no systematic experimental study on the hydrogenation of {001}-facets-dominated anatase TiO2 nanosheets, especially those that are F-modified (surface adsorption and inner substitution), to further improve their photocatalytic activity. Systematic studies on the nature of hydrogenation treatment and its correlation with the highly reactive {001} facets are very interesting topics.
The hydrothermal method is very effective in controlling the growth of crystal particles with certain morphologies. Herein, we report the hydrogenation of F-modified anatase TiO2 nanosheets with a high percentage of exposed {001} facets by annealing the fine shaped pristine hydrothermal product under a high pressure hydrogen atmosphere. The photocatalysts were characterized by UV-visble diffuse reflectance spectroscopy, transmission electron microscopy (TEM), X-ray diffraction (XRD), Raman spectroscopy, electron paramagnetic resonance (EPR), and X-ray photoelectron spectroscopy (XPS). We show that TiO2–H has Ti–H and O–H chemical bonds and O− species on its surface, and Ti3+ and an oxygen vacancy located inside the crystal structure. The {001} facets of TiO2–H are maintained by the formation of Ti–H chemical bonds, resulting in the change from Ti5c to Ti6c. The light absorption of TiO2–H is extended to the infrared range, and its photocatalytic activity is highly enhanced.
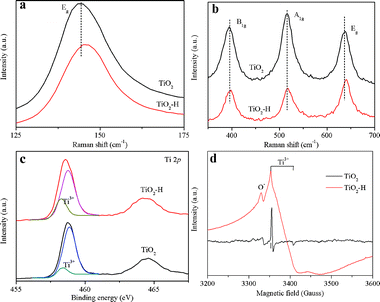
The TEM images of TiO2 (Fig. 1a) and TiO2–H (Fig. 1b) reveal that both of the photocatalysts are square sheets in shape. After hydrogenation, the particles of TiO2–H are mutually bonded to each other slightly, and the exposed {001} facets are maintained. The HRTEM (Fig. 1c and d) images of TiO2 further depict that the thickness of TiO2 is ca. 20 nm and prove the exposure of the {001} facets.9 Besides the TEM analysis, the XRD patterns (Fig. 1e) also prove the anatase phase of TiO2 and TiO2–H (JCPDS No. 21-1272). More importantly, the (101) diffraction peak of TiO2–H gives a slight shift toward a higher diffraction angle, indicating a smaller interplanar crystal spacing (Fig. S1, ESI†). This result demonstrates that structural changes have occured in TiO2 during the hydrogenation process. The UV-visible diffuse reflectance spectra show that {001}-facets-dominated TiO2–H exhibits a high and broad light absorption band toward the infrared range (Fig. 1f). This result is in good agreement with the color change of the photocatalysts from white TiO2 to dark blue TiO2 (inset of Fig. 1f). The XPS valence band spectra (Fig. S2, ESI†) depict that TiO2 and TiO2–H have a nearly overlapped band edge below the Fermi energy, which confirms that hydrogenation has a negligible effect on the valence band position of TiO2–H.8
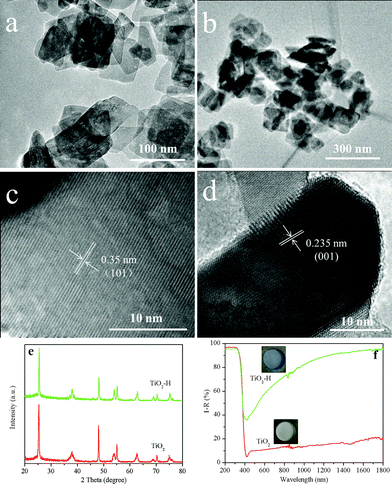 | ||
| Fig. 1 TEM images of TiO2 (a), TiO2–H (b) and high resolution TEM images of TiO2 correlating to the (101) (c) and (001) (d) interplanar crystal spacing. XRD patterns (e) and UV-visible diffuse reflectance spectra (f) of TiO2 and TiO2–H. | ||
Fig. 2a and b compares the Raman spectra differences of TiO2 and TiO2–H. Two features can be revealed by comparing the spectra: the first feature is the weakening of all the diffraction peaks at 144 cm−1, 396 cm−1, 515 cm−1 and 636 cm−1 in TiO2–H. Second, the two Eg modes at 144 cm−1 and 636 cm−1 of TiO2–H are shifted to a higher frequency. These two features demonstrate the increase in the number of oxygen vacancies in the lattice structure of TiO2–H.9,10 According to the XRD analysis, the shift of the diffraction peak to the higher angle indicates the reduction of the interplanar distance. This can be ascribed to the escape of O atoms from the crystal lattice, which often results in Ti3+.11Fig. 2c shows the Ti 2p core level XPS spectra of TiO2 and TiO2–H. The two peaks centered at ∼458.5 eV and ∼464.4 eV correspond to the characteristic Ti 2p3/2 and Ti 2p1/2 peaks of Ti4+.7,12 The peak of TiO2–H shows a shift towards the lower binding energy, indicating that Ti atoms in TiO2 and TiO2–H have different chemical environments. More importantly, a detectable shoulder, which can be ascribed to Ti3+,9 appears in the low-energy range in both of the samples. Regarding the origin of Ti3+ centers in both of the samples, low temperature EPR analysis (Fig. 2d) is conducted to give a systematic study. It is well known that HF will serve as the morphology control agent during the preparation of pristine {001}-facets-dominated TiO2. Thus the surface of TiO2 is adsorbed by the F atoms. However, TiO2 gives a strong EPR signal and the calculated g values are g⊥ = 1.992 and g∥ = 1.962, which are the typical g values for a paramagnetic Ti3+ center.13 This type of Ti3+ signal can be ascribed to originate from the substitution of O2− ions by the F− ions.14 The phenomenon shows that the F atoms not only serve as morphology control agents but also modify the inner structure of TiO2. No other signal, such as O− or O2−, is detected, indicating the Ti3+ are located in the bulk of the TiO2 because surface Ti3+ will adsorb O2.13 Compared to TiO2, TiO2–H gives a very different and stronger EPR signal which can be ascribed to the Ti3+ and O−.15 The stronger Ti3+ signal is in good agreement with the XPS analysis. The broadened Ti3+ signal of TiO2–H may result from the different coordinations of Ti3+. In TiO2, the Ti3+ is produced by F substitution, which induces the chemical bond Ti–F–Ti.14 In contrast, there will be Ti–□–Ti chemical bond in TiO2–H as a result of the escape of the O and F atoms. The oxygen atoms in TiO2 will interact with the H atoms, which will also produce one kind of Ti3+. This result can also be obtained from the O 1s XPS spectra (Fig. S3, ESI†). The signal centered at 529.8 eV and 531.3 eV are the typical signals of Ti–O–Ti and surface OH species, respectively.16 The O 1s spectra illustrate that TiO2–H has much more surface OH species, which are produced by the O and H interaction, than TiO2. Comparing to TiO2, the F atoms in TiO2–H are almost removed (Fig. S4, ESI†). The F atoms may escape from the surface and inner structure of TiO2–H because of the similar chemical properties of O and F. Thus, more oxygen vacancies are formed and the surface Ti–F bonds are broken.
Fig. 3a compares the photocatalytic activity of TiO2, TiO2–H, and P25 in decomposing MB under UV-visible light irradiation. The photocatalysis efficiency of TiO2–H is much higher than that of TiO2 and P25. The photocatalytic activity (Fig. S5, ESI†) of TiO2–H decreased when the photocatalysts were irradiated only by visible light, which may be ascribed to the decrease of the light intensity. More importantly, the visible light photocatalytic activity of TiO2 is higher than that of P25. As discussed before, this phenomenon may result from the existence of the Ti3+ center created by F substitution. We also tested the photocatalytic activity of the photocatalysts under UV (Fig. S6, ESI†) and visible light (Fig. S7, ESI†) irradiation in decomposing phenol. The results are very similar to those of MB degradation. The formation of ·OH (Fig. 3b), which is considered to be very favorable for photocatalysis reactions, in the photocatalytic reaction system was also examined upon UV light irradiation. The PL intensity of 2-hydroxyterephthalic acid, which was formed by the interaction of terephthalic acid with the generated ·OH, in the three reaction systems are very different. TiO2–H gives a much stronger photo-oxidation capability than P25, while TiO2 shows the weakest photo-oxidation capability. This depicts that TiO2–H is a very effective photocatalyst which can be used in a wide range of applications.
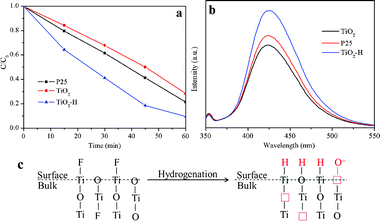 | ||
| Fig. 3 Photocatalytic decomposition of MB (a) and ·OH generation measurement (b) of TiO2 and TiO2–H under UV-visible light irradiation. Schematic illustration (c) of the hydrogenation effect on the structural change in TiO2 and TiO2–H. | ||
To get a deep insight on the hydrogenation effect on the structure change of TiO2, the schematic illustration is shown in Fig. 3c. The {001}-facets-dominated TiO2 is predominantly exposed with the unsaturated Ti5c and O2c atoms. As calculated, the H atoms will chemically bond with the O atoms, resulting in the formation of Ti3+ centers.17 On the other hand, the hydrogenation effect removes almost all of the F atoms on the surface of the TiO2. The Ti5c atoms, which were originally bonded with the F atoms, are now unsaturated again. After the H atoms reached an interaction equilibrium with the O atoms, the Ti5c atoms are very favorable for H absorption.16 As a result, the (001) surface of TiO2–H is now packed with Ti6c atoms, which may be the reason for the retention of the {001} facets even though the F atoms are removed from the surface of TiO2–H. On the other hand, the removal of the O and bulk F atoms induces the formation of oxygen vacancies and Ti3+, resulting in the band gap narrowing and the surface O− adsorption of TiO2–H.
In summary, we have hydrogenated the F-modified, both surface adsorption and inner substitution, anatase TiO2 nanosheets with a high percentage of the {001} facets. The hydrogenated {001} facets dominated TiO2–H shows higher photocatalytic activity than P25 and pristine TiO2 under UV-visible and visible light irradiation. Based on a systematic analysis, the surface of TiO2 is fully covered with the Ti–H and O–H chemical bonds. There are also a lot of Ti3+ and oxygen vacancies that induce the adsorption of O− species on the surface of TiO2–H, resulting in a significant band-gap narrowing. The Ti5c atoms on the {001} faces are fully coordinated by the formation of Ti–H chemical bonds. As a result, a high percentage of {001} facets is kept after the hydrogenation treatment. We believe the high-performance TiO2–H with exposed {001} facets can be used in other fields, such as self-cleaning and solar cells. This work gives new insight into the development of TiO2 photocatalysts with a high percentage of highly reactive facets and enhanced light absorption ability.
Acknowledgements
This work was supported by the Innovation Foundation for Graduate Students of Jiangsu Province China (CXLX11_0346) and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).References
- A. Fujishima and K. Honda, Nature, 1972, 238, 37 CrossRef CAS.
- A. Fujishima, X. Zhang and D. A. Tryk, Surf. Sci. Rep., 2008, 63, 515 CrossRef CAS.
- H. G. Yang, C. H. Sun, S. Z. Qiao, J. Zou, G. Liu, S. C. Smith, H. M. Cheng and G. Q. Lu, Nature, 2008, 453, 638 CrossRef CAS.
- J. Pan, G. Liu, G. Q. M. Lu and H.-M. Cheng, Angew. Chem., Int. Ed., 2011, 50, 2133 CrossRef CAS.
- S. Liu, J. Yu and M. Jaroniec, J. Am. Chem. Soc., 2010, 132, 11914–11916 CrossRef CAS.
- S. Xie, X. Han, Q. Kuang, J. Fu, L. Zhang, Z. Xie and L. Zheng, Chem. Commun., 2011, 47, 6722 RSC.
- X. Chen, L. Liu, P. Y. Yu and S. S. Mao, Science, 2011, 331, 746 CrossRef CAS.
- G. Wang, H. Wang, Y. Ling, Y. Tang, X. Yang, R. C. Fitzmorris, C. Wang, J. Z. Zhang and Y. Li, Nano Lett., 2011, 11, 3026 CrossRef CAS.
- G. Liu, H. G. Yang, X. Wang, L. Cheng, H. Lu, L. Wang, G. Q. Lu and H.-M. Cheng, J. Phys. Chem. C, 2009, 113, 21784 CAS.
- X. Pan and X. Ma, J. Solid State Chem., 2004, 177, 4098 CrossRef CAS.
- J.-Y. Shin, J. H. Joo, D. Samuelis and J. Maier, Chem. Mater., 2012, 24, 543 CrossRef CAS.
- M. S. Lazarus and T. K. Sham, Chem. Phys. Lett., 1982, 92, 670 CrossRef CAS.
- F. Zuo, L. Wang, T. Wu, Z. Y. Zhang, D. Borchardt and P. Y. Feng, J. Am. Chem. Soc., 2010, 132, 11856 CrossRef CAS.
- A. M. Czoska, S. Livraghi, M. Chiesa, E. Giamello, S. Agnoli, G. Granozzi, E. Finazzi, C. D. Valentin and G. Pacchioni, J. Phys. Chem. C, 2008, 112, 8951 CAS.
- J. Strunk, W. C. Vining and A. T. Bell, J. Phys. Chem. C, 2010, 114, 16937 CAS.
- Z. Zheng, B. Huang, J. Lu, Z. Wang, X. Qin, X. Zhang, Y. Dai and M.-H. Whangbo, Chem. Commun., 2012, 48, 5733 RSC.
- H. Pan, Y.-W. Zhang, V. B. Shenoy and H. Gao, J. Phys. Chem. C, 2011, 115, 12224 CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: details of the synthesis and characterization of the photocatalysts, powder XRD data of the (101) peak, valence band XPS spectra, O 1s and F 1s XPS spectra, photocatalytic activity measurement of TiO2 and TiO2–H. See DOI: 10.1039/c2ra21049e/ |
| This journal is © The Royal Society of Chemistry 2012 |