A novel access to indole-3-substituted dihydrocoumarins in artificial sweetener saccharin based functional ionic liquids†
Atul
Kumar
,
Promod
Kumar
,
Vishwa Deepak
Tripathi
and
Suman
Srivastava
Medicinal and Process Chemistry Division, Central Drug Research Institute, CSIR, Lucknow, India. E-mail: dratulsax@gmail.com; Fax: +91-522-26234051; Tel: 91-522-2612411
First published on 18th September 2012
Abstract
An efficient new multicomponent protocol for the C-3 functionalization of indoles with dihydrocoumarin has been developed using an artificial sweetener (saccharin)-based functional ionic liquid (imidazolium saccharinate). The reaction consists of the Knoevenagel coupling of salicylaldehyde and Meldrum's acid followed by a Michael type reaction with indole, resulting in concomitant lactonization via decarboxylative elimination to yield indole-3-dihydro-coumarins in good to excellent yields at room temperature.
The indole ring system is the architectural core of the most widely distributed heterocycles found in nature.1 The substituted indole moiety has become a fascinating molecular scaffold in drug discovery, because of its presence in numerous drugs, medicinal leads and biologically active natural products.2 The essential amino acid tryptophan, the neurotransmitter serotonin and several important alkaloids and biological agents, like CK-636 and CK-666,3 are indole derivatives in which the third position is substituted. Furthermore, indoles substituted with heterocyclic rings at the 3-position, such as Meridianin A,4 Primprinine,5 Labradorin,6 Martefragin A7 and Almazole C8 (Fig. 1), have exhibited important biological activity.
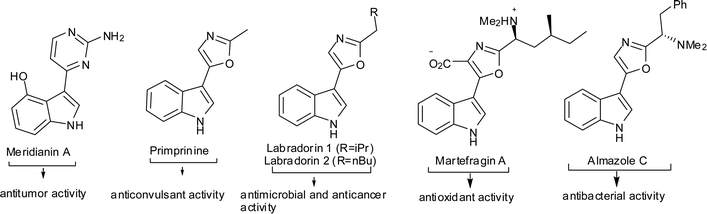 | ||
| Fig. 1 Representative biologically active molecules that possess a 3-substituted indole moiety. | ||
In recent years, environmentally benign synthesis has gained great importance for sustainability and this has resulted in an increased use of green protocols in organic synthesis. Ionic liquids (ILs) have been recently revealed to have dual properties as solvents and catalysts.9 The introduction of functional ionic liquids has added further interest in this area.10
In continuation of our work on the green synthesis of bioactive natural and drug like molecules,11 we have developed a novel synthetic strategy for the synthesis of indole-3-substituted dihydrocoumarins using the functional ionic liquid imidazolium saccharinate. To the best of our knowledge there is no direct method reported for the synthesis of indole-3-subsituted dihydrocoumarins.
Indoles and dihydrocoumarins are two intensely studied classes of compounds in drug discovery, therefore the synthesis of bifunctional compounds incorporating both of these moieties is desirable. The construction of a C-3-substituted indole ring has been previously carried out via hydroindolation of indoles with alkynes12 and Friedel–Crafts alkylation.13
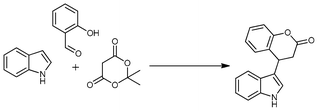
Jean-Yves Laronze et al. have utilized these three components (indole, subsituted aldehyde and Meldrum's acid) for the synthesis of indole-2-pyrrolidones14 and β-substituted tryptophan esters15 using a multistep process. However, they have reported zero percent yield with salicylaldehyde, indole and Meldrum's acid (Scheme 1). Here, we wish to report the synthesis of indole-3-substituted dihydrocoumarins using indole, salicylaldehyde and Meldrum's acid in the presence of functional ionic liquid imidazolium saccharinate via a multicomponent protocol (Scheme 1).
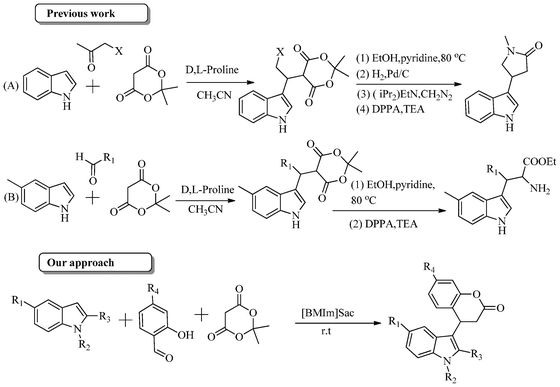 | ||
| Scheme 1 Previous and present work with indole, Meldrum's acid and carbonyl compounds. | ||
It is worth mentioning that, during the course of the reaction, at first two C–C bonds are formed followed by concomitant lactonization. For the optimization of the reaction conditions, we first took the condensation of indole, salicylaldehyde and Meldrum's acid as a model reaction and applied the reaction in organic solvents such as dichloromethane, chloroform and acetonitrile using triethylamine and potassium carbonate as a catalyst, which resulted in the formation of bis-indole as the sole product. Raising the reaction temperature to 60 °C, formation of the side product bis-indole took place in a short time. When imidazole was used as a catalyst (in acetonitrile) we obtained the desired product, indole-3-substituted dihydrocoumarin, in 60% yield in 30 h (Table 1). We observed that imidazole as a catalyst drives the reaction towards the desired product in low to moderate yields. To further optimize the reaction, we synthesized some imidazole based functional ionic liquids (FIL).
|
|
||||||
|---|---|---|---|---|---|---|
| Entry | Solvent | Catalyst | T/°C | Time (h) | Yieldd (%) | |
| a Reaction conditions: indole (1.0 mmol), Meldrum's acid (1.0 mmol), salicylaldehyde (1.0 mmol), solvent/ionic liquid (2.0 mL) and catalyst (10 mol %), rt. b Catalytic amount. c 5 equiv. d Isolated yield. | ||||||
| 1 | CH2Cl2 | TEAb | rt | 25 | 0 | |
| 2 | CHCl3 | TEA | rt | 25 | 0 | |
| 3 | CH3CN | TEA | rt | 25 | 0 | |
| 4 | CH3CN | imidazolea | rt | 30 | 60 | |
| 5 | CH3CN | K2CO3c | rt | 25 | 0 | |
| 6 | [C4MIM]Sac | None | rt | 8 | 93 | |
| 7 | [C2MIM]Sac | None | rt | 8 | 86 | |
| 8 | [C5MIM]Sac | None | rt | 8 | 87 | |
| 9 | [bmim][OH] | None | rt | 8 | 60 | |
| 10 | [bmim][Br] | None | rt | 8 | 50 | |
One of the attractive features of functional ionic liquids in synthesis and catalysis is that both the cationic and anionic components can be modified, so that a liquid can be customized for specific applications. In our previously reported work,16 we functionalized the cationic part of ILs and in continuation here we have functionalized the anionic part of the IL with saccharin, an artificial sweetening agent.
We have synthesized 1-methyl-3-(n-alkyl) imidazolium saccharinate ([CnMIM]Sac) from the sodium salt of saccharin with 1-methyl-3-(n-alkyl) imidazolium bromide in acetone (Scheme 2).
![Representative procedure for synthesis of [CnMIM]Sac.](/image/article/2012/RA/c2ra21284f/c2ra21284f-s2.gif) | ||
| Scheme 2 Representative procedure for synthesis of [CnMIM]Sac. | ||
We synthesized 1-methyl-3-(n-butyl) imidazolium saccharinate ([C4MIM]Sac), 1-methyl-3-(n-ethyl) imidazolium saccharinate([C2MIM]Sac), 1-methyl-3-(n-pentyl) imidazolium saccharinate([C5MIM]Sac) by using the above protocol.
It is interesting to report that the ionic liquid [C4MIM]Sac gave the best results at room temperature (Table 1). Raising the reaction temperature to 60 °C resulted in lower yields. We observed similar results with [C5MIM]Sac and [C2MIM]Sac. We also synthesized 1-methyl-3-(n-butyl) imidazolium bromide and 1-methyl-3-(n-butyl) imidazolium hydroxide, which gave moderate yields. In the present study functional ionic liquids work as catalysts as well as reaction media without using any additional catalyst or solvent. It has been recognized that hydrogen bonding plays an important role in the behaviour and catalytic activity of ionic liquids. The catalytic efficiency of most ionic liquids is strongly influenced by the synergetic effects between the C-2 hydrogen of the bmim and the counter anion of the functional ionic liquid.17
This optimized methodology was then applied to several indole derivatives on a 1 mmol scale (Table 2). The corresponding indole-3-substituted dihydrocoumarin products were efficiently synthesised with the reaction of 5-methoxy, 7-ethyl, 2-phenyl, 2-methyl, N-methyl, N-ethyl, N-allyl and N-butyl indoles with salicylaldehyde or 4-methoxy salicylaldehyde and Meldrum's acid at room temperature using [C4MIM]Sac. The reaction with electron-deficient 5-bromo-1H-indole was also successful, giving the corresponding product in high yield (85%). The structures of the indole-3-substituted dihydrocoumarins were confirmed by elemental analysis, 1H NMR, 13C NMR and mass spectroscopy.
| a Reaction conditions: indole derivative (1.0 mmol), Meldrum's acid (1.0 mmol), salicylaldehyde derivative (1.0 mmol), ionic liquid (2.0 mL), rt, 8–10 h. b Isolated yield. | ||
|---|---|---|

|
The proposed mechanism is given in Scheme 3. The reaction involves the Knoevenagel coupling of salicylaldehyde and Meldrum's acid, which undergoes a Michael type reaction with the indole followed by concomitant lactonization via decarboxylative elimination. It was also observed that the ionic liquid showed good reusability in at least four subsequent reactions under the same reaction conditions without any considerable loss in the reaction yield (Table 3).
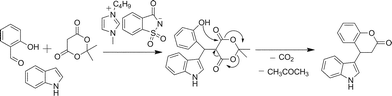 | ||
| Scheme 3 Reaction mechanism for the formation of indole-3-substituted dihydrocoumarins. | ||
| Run | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| a Reaction conditions: Indole (1.0 mmol), Meldrum's acid (1.0 mmol), salicylaldehyde (1.0 mmol), ionic liquid (2.0 mL), rt, 8 h. b Isolated yield. | ||||
| Yieldb (%) | 93 | 91 | 87 | 85 |
Conclusions
In conclusion, we have developed an efficient new multicomponent reaction for the synthesis of indole-3-substituted dihydrocoumarin derivatives using an artificial sweetener (saccharin)-based functional ionic liquid ([C4MIM]Sac)-mediated synthesis (FILMs) at room temperature. The advantages of this protocol are good yields, reusable medium, operational simplicity. The applicability of this strategy for the synthesis of other coumarin heterocycles is currently under investigation.Acknowledgements
Authors P. K., V. D. and S. S. thank CSIR-UGC New Delhi for the award of a senior research fellowship. The authors also acknowledge SAIF-CDRI for providing the spectral and analytical data. The CDRI communication no. is 8305.References
- (a) K. Huguchi and T. Kawasaki, Nat. Prod. Rep., 2007, 24, 843 RSC; (b) S. E. O'Connor and J. J. Maresh, Nat. Prod. Rep., 2006, 23, 532 RSC; (c) T. Kawasaki and K. Huguchi, Nat. Prod. Rep., 2005, 22, 761 RSC; (d) M. Somei and F. Yamada, Nat. Prod. Rep., 2004, 21, 278 RSC.
- (a) B. E. Evans, K. E. Rittle, M. G. Bock, R. M. DiPardo, R. M. Freidinger, W. L. Whitter, G. F. Lundell, D. F. Veber and P. S. Anderson, J. Med. Chem., 1988, 31, 2235 CrossRef CAS; (b) D. A. Horton, G. T. Bourne and M. L. Smythe, Chem. Rev., 2003, 103, 893 CrossRef CAS.
- (a) M. Gompel, M. Leost, E. B. D. Joffe, L. Puricelli, L. H. Franco, J. Palermo and L. Meijer, Bioorg. Med. Chem. Lett., 2004, 14, 1703 CrossRef CAS; (b) M. A. A. Radwan and M. El-Sherbiny, Bioorg. Med. Chem., 2007, 14, 1206 CrossRef.
- S. Takahashi, T. Mathsunase, C. Hasegawa, H. Saito, D. Fujita, F. Kiwchi and Y. Tsuda, Chem. Pharm. Bull., 1998, 46, 1527 CrossRef CAS.
- S. R. Naik, J. Harindran and A. B. Varde, J. Biotechnol., 2001, 88, 1 CrossRef CAS.
- G. R. Pettit, J. C. Knight, D. L. Herald, R. Davenport, R. K. Pettit, B. E. Tucker and J. M. Schmidt, J. Nat. Prod., 2002, 65, 1793 CrossRef CAS.
- A. Nishida, M. Fuwa, Y. Fujikawa, E. Nakahata, A. Furuno and M. Nakagawa, Tetrahedron Lett., 1998, 39, 5983 CrossRef CAS.
- (a) G. Guella, I. Mancini, I. N'Diaye and F. Pietra, Helv. Chim. Acta, 1994, 77, 1999 CrossRef CAS; (b) I. N'Diaye, G. Guella, I. Mancini and F. Pietra, Tetrahedron Lett., 1996, 37, 3049 CrossRef CAS.
- (a) P. Wasserscheid and W. Keim, Angew. Chem., Int. Ed., 2000, 39, 3772 CrossRef CAS; (b) M. J. Earle and K. R. Seddon, Pure Appl. Chem., 2000, 72, 1391 CrossRef CAS; (c) C. M. Gordon, Appl. Catal., A, 2001, 222, 101 CrossRef CAS.
- (a) T. Jiang, X. Ma, Y. Zhou, S. Liang, J. Zhang and B. Han, Green Chem., 2008, 10, 465 RSC; (b) D. Li, F. Shi, J. Peng, S. Guo and Y. Deng, J. Org. Chem., 2004, 69, 3582 CrossRef CAS; (c) G. Tao, L. He, W. Liu, L. Xu, W. Xiong, T. Wang and Y. Kou, Green Chem., 2006, 8, 639 RSC.
- (a) A. Kumar, V. D. Tripathi and P. Kumar, Green Chem., 2011, 13, 51 RSC; (b) A. Kumar and S. Sharma, Green Chem., 2011, 13, 2017 RSC.
- (a) D. Xia, Y. Wang, Z. Du, Q.-Y. Zheng and C. Wang, Org. Lett., 2012, 14, 588 CrossRef CAS; (b) C. Nevado and A. M. Echavarren, Synthesis, 2005, 167 CAS; (c) S. F. Kirsch, Synthesis, 2008, 3183 CrossRef CAS; (d) T. Kitamura, Eur. J. Org. Chem., 2009, 1111 CrossRef CAS; (e) M. Beller, J. Seayad, A. Tillack and H. Jiao, Angew. Chem., Int. Ed., 2004, 43, 3368 CrossRef CAS.
- (a) X. Liu, L. Lin and X. Feng, Acc. Chem. Res., 2011, 44, 574 CrossRef CAS; (b) M. Bandini, A. Melloni, S. Tommasi and A. Umani-Ronchi, Synlett, 2005, 1199 CrossRef CAS.
- L. Jeannin, T. Nagy, E. Vassileva, J. Sapi and J.-Y. Laronze, Tetrahedron Lett., 1995, 36, 2057 CrossRef CAS.
- M. Boisbrun, L. Jeannin, L. Toupet and J.-Y. Laronze, Eur. J. Org. Chem., 2000, 3051 CrossRef CAS.
- A. Kumar, G. Gupta and S. Srivastava, Green Chem., 2011, 13, 2459 RSC.
- (a) A. R. Gholap, K. Venkatesan, T. Daniel, R. J. Lahoti and K. V. Srinivasan, Green Chem., 2003, 5, 693 RSC; (b) X. Fu, Z. Zhang, C. Li, L. Wang, H. Ji, Y. Yang, T. Zou and G. Gao, Catal. Commun., 2009, 10, 665 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available. See DOI: 10.1039/c2ra21284f |
| This journal is © The Royal Society of Chemistry 2012 |