Smooth, transparent and nonperfluorinated surfaces exhibiting unusual contact angle behavior toward organic liquids†
Chihiro
Urata
,
Dalton F.
Cheng
,
Benjamin
Masheder
and
Atsushi
Hozumi
*
National Institute of Advanced Industrial Science and Technology (AIST), 2266-98, Anagahora, Shimoshidami, Moriyama, Nagoya, Aichi 463-8560, Japan. E-mail: a.hozumi@aist.go.jp; Fax: +81-52-736-7406; Tel: +81-52-736-7388
First published on 3rd September 2012
Abstract
Smooth, transparent and nonperfluorinated surfaces exhibiting unusual contact angle (CA) behavior toward various organic liquids have been prepared using a simple sol–gel solution containing decyltriethoxysilane (DTES) and tetramethoxysilane (TMOS). The insertion of co-condensed silica units derived from TMOS as a spacer between the neighboring alkyl (decyl) chains allows them to rotate freely and provide a surface with liquid-like properties. The resulting surfaces exhibit negligible CA hysteresis for probe liquids, allowing the droplets to move easily and roll off when only slightly tilted, without relying on conventional surface roughening and perfluorination.
The development of novel liquid-repellent surfaces inspired by examples from nature, including the lotus leaf, the water strider's leg, pitcher plant, etc., has attracted considerable attention across many scientific disciplines with interests ranging from fundamental research to practical applications.1 Although the creation of functional biomimetic surfaces effective for repelling water have been widely researched, the challenge of creating easily dewettable oleophobic/superoleophobic surfaces still remains. However, more important than the static contact angle (CA) is the ability of small volume droplets (∼3–5 μL) to easily roll across and off the surface when the tilt angle (TA) is low (<10°). Until now, there has only been a limited number of papers describing the preparation of oleophobic/superoleophobic surfaces demonstrating such low TAs for organic liquids.2 Such functional surfaces have been commonly prepared by combining the influence of textured surfaces with that of perfluorinated organic compound films of, in particular, perfluoroalkylsilanes2a–c,f or perfluorinated fluids.2d The reasoning behind their use is that the surface energies of perfluoroalkyl groups are extremely low and therefore allow only weak interaction between the surface and the liquid.3 This can be compared with the hydrophobic, but oleophilic nature of alkyl group containing surfaces, best demonstrated by the secreted plant wax on lotus leaves which famously display exceptional dewettability toward water but conversely, alkane liquids such as n-hexadecane completely spread on the surface.2a,4 Thus, most studies related to this field are unsurprisingly focused on the effects of perfluoroalkyl groups rather than alkyl groups. However, the chemical and physical effects of perfluorinated organic compounds on human health and the environment have lately been questioned, increasing the demand for alternative easily dewet surfaces against organic liquids.5
Vapor- and liquid-phase reactions of alkyl-terminated organosilanes (RnSiX4-n; usually X = Cl, OR, H) to hydrophilic Si (SiSiO2) surfaces under carefully controlled conditions are fairly well understood and highly-ordered monolayers have been successfully prepared.6 Although such monolayer-covered SiSiO2 surfaces are highly hydrophobic, alkane liquids tend to spread and pin to the surfaces. The result is a relatively large CA hysteresis (difference between the advancing (θA) and receding (θR) CAs) for moving droplets of n-hexadecane and consequently poor surface dewettability due to an affinity between the alkyl groups and alkane liquids. This is a major reason why alkyl-terminated smooth/flat surfaces showing extraordinary dewetting behaviour towards alkane liquids are rarely reported.6f,7 Our group and McCarthy's group have described how the use of branched-7b–e or ring-shaped7i organosilane monolayers and ultrathin layers of low-molecular-weight inert silicones2e,g,7e,f,j are effective for fabricating low CA hysteresis surfaces toward both water/alkane liquids. Such probe liquids have been shown to move very easily on these surfaces and roll off when only slightly tilted, regardless of the magnitude of CAs. A common feature among these surfaces is that the surface tethered groups are flexible and liquid-like, and that droplets in contact with them experience very low energy barriers between metastable states, leading to the formation of low CA hysteresis surfaces.7 However, the applicability of such surfaces is limited because of an incompatibility between the functional groups of organosilanes and the substrates.7e,f,j,8 In addition, substrate surface smoothness is required because these monomeric layers are generally very thin (∼3 nm). Moreover, such processes generally require relatively high temperatures (∼150 °C).
Co-hydrolysis and co-condensation of organosilanes and tetraalkoxysilanes have been found to produce sol–gel hybrid films with unique film nanostructures and mechanical/surface properties.9 Shimojima et al. reported that sol–gel solutions containing alkylalkoxysilanes (e.g. CnH2n+1Si(OEt)3; n = 6–18) and tetraethoxysilane (TEOS) successfully formed transparent multilayered self-assembled hybrid films composed of alternating alkyl and siloxane layers with nanometer-scale interval distances between layers (surface dewettability was not described.).10 Tang et al. also demonstrated the fabrication of a similar type of hybrid films using a mixture of octyltriethoxysilane and tetramethoxysilane (TMOS).11 The produced films showed hydrophobic properties (oleophobicity was not studied) and were effective as an antifouling coating. Additionally, incorporation of the organic functional groups into the siloxane network was found to produce a more flexible and less fragile surface.
We began to study these sol–gel methods in an attempt to fabricate new liquid-like surfaces, because it was envisioned that controlling the composition of binary mixtures of alkyltrialkoxysilane and tetraalkoxysilane could reduce the packing of alkyl chains. It was predicted that this would contribute to an enhancement of the dewetting properties of the surface by increasing the ability of functional groups to move and become liquid-like (Scheme 1), similar to the surfaces on which packing density was simply reduced either through shortening the reaction time7b or the use of specific molecular structures (branched7c,d,g or ring-shaped7i molecules). Upon creation of these surfaces, we found that the CA hysteresis for various organic liquids became almost negligible and that these droplets moved very easily on the surface, rolling off at low TAs (e.g., ∼3.4° for 5 μL of n-hexadecane) without pinning. We report here a simple and reproducible technique that enables the large area formation of extremely smooth, transparent and nonperfluorinated oleophobic surfaces exhibiting unusual dynamic CA behavior toward various organic liquids.
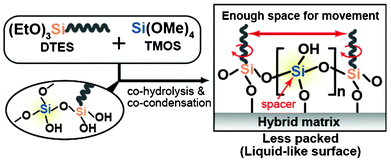 | ||
| Scheme 1 Conceptual scheme of this study. | ||
Our hybrid films containing decylsilyl groups (C10-hybrid films) were prepared using a conventional co-hydrolysis and co-condensation method according to the previous reports.10 Briefly, precursor solutions were prepared by mixing DTES and TMOS in an ethanol/hydrochloric acid solution for 24 h at room temperature (25 ± 2 °C). Typical molar ratio of precursor solutions were 1 DTES![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2–10 TMOS
2–10 TMOS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 EtOH
50 EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 H2O
22 H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 × 10−4 HCl.12 In these experimental conditions, complete hydrolysis of both alkoxysilanes10,13 were observed by nuclear magnetic resonance (NMR) spectra (data not shown). The obtained precursor solutions were spincoated (500 rpm for 5 s and 1000 rpm for 10 s) onto UV/ozone-cleaned glass, Si, or polymer ((polycarbonate (PC) and poly(methyl methacrylate) (PMMA)) substrates (24 × 48 mm2) at room temperature and under relative humidity of 40 ± 5%. All samples were dried in air at room temperature for more than 24 h. As a control, a decylsilyl-terminated monolayer (C10-monolayer) was prepared using a conventional chemical vapor deposition (CVD) method7g(see the Electronic Supplementary Information (ESI)† for detailed experimental conditions). The static (θS), θA, and θR CAs of water and n-hexadecane on C10-monolayer-covered glass surface were 99°/109°/86° and 20°/21°/8°, respectively, and the ellipsometric thickness of the monolayer was determined to be about 0.9 nm. These results are almost identical to those reported previously6d (Table S1; ESI†).
4 × 10−4 HCl.12 In these experimental conditions, complete hydrolysis of both alkoxysilanes10,13 were observed by nuclear magnetic resonance (NMR) spectra (data not shown). The obtained precursor solutions were spincoated (500 rpm for 5 s and 1000 rpm for 10 s) onto UV/ozone-cleaned glass, Si, or polymer ((polycarbonate (PC) and poly(methyl methacrylate) (PMMA)) substrates (24 × 48 mm2) at room temperature and under relative humidity of 40 ± 5%. All samples were dried in air at room temperature for more than 24 h. As a control, a decylsilyl-terminated monolayer (C10-monolayer) was prepared using a conventional chemical vapor deposition (CVD) method7g(see the Electronic Supplementary Information (ESI)† for detailed experimental conditions). The static (θS), θA, and θR CAs of water and n-hexadecane on C10-monolayer-covered glass surface were 99°/109°/86° and 20°/21°/8°, respectively, and the ellipsometric thickness of the monolayer was determined to be about 0.9 nm. These results are almost identical to those reported previously6d (Table S1; ESI†).
As shown in Fig. 1a, our C10-hybrid films were optically transparent: this was found to be independent of the RT/D ratios. X-ray diffraction (XRD) patterns (Fig. S1†) revealed that the films prepared at RT/D ratios between 2 and 4 exhibited one sharp diffraction peak at approximately 2θ = 3.3° (d = 3.3 nm) which disappeared after the thermal treatment at 540 °C for 24 h. This clearly indicates that the resulting films possessed well-ordered lamellar structures in which each layer is oriented parallel to the substrate.9a,10 In contrast, such characteristic diffraction peaks were undetectable when the RT/D ratios were above 5 (Fig. S1; ESI†). At these ratios, the self-assembly of co-condensed species into lamellar structures was considered to be prohibited by the large quantity of SiO2 spacer unit2.10 The total thicknesses of our films were estimated to be in the range of 0.63 to 1.2 μm using a stylus profiler. Hardness of the hybrid films was estimated by instrumented indentation microscope14 to be in the range of 50 and 560 MPa which is similar to that reported by Shimojima et al.,10e and was found to increase with an increase in the RT/D ratio. The hybrid film prepared at RT/D ratio 10 was not damaged by a simple scratch test using polypropylene tweezers, while it was scratched by stainless steel tweezers. Scanning electron microscopy (SEM) observation (Fig. S2; ESI†) revealed the film surface to be featureless and smooth over a large area (100 × 100 μm2). Root-mean-square roughness was calculated to be in the range of only 0.2–2.4 nm over an area of 3 × 3 μm2 using atomic force microscopy (AFM) for RT/D = 3–8. Hence, it could be concluded that any changes to the dynamic dewettability of the C10-hybrid film surfaces was not influenced by surface morphology and was determined only by the surface chemical and physical properties.
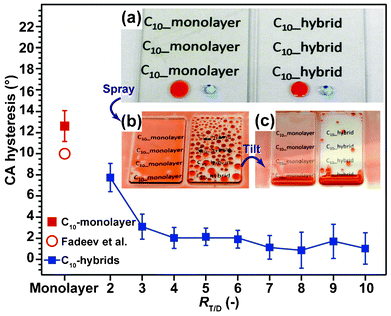 | ||
| Fig. 1 CA hysteresis of a C10-monolayer, monolayer surface by Fadeev et al.6f and C10-hybrid films for n-hexadecane. Data represents the average value from three independent experiments (n = 3). (Inset images: (a) Red-colored n-hexadecane and blue-colored water droplets (5 μL) were placed on each sample. (b) Appearance of films following spraying of a mist of red-colored n-hexadecane on the films and (c) after tilting the films at 40°. The left images in the inset show C10-monolayer and right images shows the C10-hybrid (RT/D = 4). | ||
All C10-hybrid films prepared over the full range of RT/D ratios were hydrophobic and their dynamic CAs for water (109°/100°, RT/D = 4) were almost identical to those of C10-monolayer films (Fig. S3 and Table S1; ESI†). However, only our C10-hybrid film surfaces showed unusual dynamic CAs behavior toward organic liquids. For example, the large CA hysteresis of n-hexadecane on a C10-monolayer-covered glass surface (Δθ = 13°) caused a mist of red-colored (Sudan III) n-hexadecane to fully spread across and strongly adhered to the surface (Fig. 1b and c, Movie S1; ESI†). In contrast, n-hexadecane droplets quickly rolled across and off our C10-hybrid film surface without pinning (Fig. 1b and c, Movie S1; ESI†) when the RT/D ratios were over 4. Separate measurement revealed that the CA hysteresis of n-hexadecane was less than 2° and even very small droplets of n-hexadecane (5 μL) fully dewet from the surface at a TA of only 3.4°. This value is almost equivalent to those of the best nonperfluorinated lyophobic SiSiO2 surfaces so far described2e,g,6f,7a–d,j (which are reported to be around 1–3°) and even less than those of conventional perfluorinated flat surfaces (e.g., Teflon AF1600, CA hysteresis is about 5°).15 In addition to n-hexadecane, excellent dynamic dewetting behavior was similarly observed for other organic liquids, such as diiodomethane, food oils (oleic acid and soybean oil), toluene, p-xylene, turpentine oil, n-dodecane, n-decane, and ethanol (Table 1 and Fig. S3; ESI†). Moreover, the dynamic dewetting behaviour still remained intact even after immersion of the hybrid films in n-hexane (Δθwater = 10° and Δθn-hexadecane = 1°) and acetone (Δθwater = 8° and Δθn-hexadecane = 2°) for 24 h. Our process was also applicable not only to glass slide and Si substrates but also thermally unstable polymer substrates (Table S2 and Fig. S4), since this process enables co-hydrolysis and co-condensation at room temperature.
| Probe liquid | Surface tension (dyn cm−1) | θ A/θR (°/°) | Δθa (°) | TA (°) |
|---|---|---|---|---|
| a CA hysteresis. b At 20 °C (from reference 17a and 17b). c At 20 °C (from reference 17a and 17b). d At 25 °C (from reference 17c). | ||||
| Water | 72.8b | 109/100 | 9 | 40 |
| 109/86 | 23 | 90 | ||
| Diiodomethane | 50.8b | 73/71 | 2 | 6.2 |
| 65/59 | 6 | 27 | ||
| Oleic acid | 32.8c | 52/47 | 5 | 9.0 |
| 42/27 | 15 | 57 | ||
| Soybean oil | 31.3d | 52/47 | 5 | 11 |
| 45/27 | 18 | 54 | ||
| Toluene | 28.4b | 37/36 | 1 | 2.5 |
| 23/14 | 9 | 26 | ||
| p-Xylene | 28.3b | 39/37 | 2 | 3.2 |
| 25/14 | 11 | 18 | ||
| n-Hexadecane | 27.5b | 36/34 | 2 | 3.4 |
| 21/8 | 13 | 21 | ||
| Turpentine oil | 27.0d | 31/31 | 0 | 2.7 |
| 16/<5 | --- | --- | ||
| n-Dodecane | 25.4b | 27/25 | 2 | 2.5 |
| 7/<5 | --- | --- | ||
| n-Decane | 23.8b | 21/20 | 1 | 2.4 |
| <5/<5 | --- | --- | ||
| Ethanol | 22.1b | 29/25 | 4 | 8.8 |
| <5/<5 | --- | --- | ||
The marked difference in the final dynamic oleophobicity observed between the C10-monolayer- and C10-hybrid-covered samples was most likely due to the difference in the mobility of the alkyl chains, which must be attributed to the distances between the neighboring alkyl chains. This was confirmed by infrared (IR) transmission spectroscopy (in this case, Si wafers were used as substrates) because it is well known that the CH2 stretching vibration of alkyl groups is sensitive to the mobility of the chains.16a The CH2 asymmetric (νas) vibration of C10-hybrid (RT/D = 4)-covered surface was recorded at 2925.0 cm−1, which is higher than the relatively immobile solid-like alkyl chains reported previously for the monolayer film (νas: about 2920–2915 cm−1).16 Increasing the TMOS concentration caused the absorption band to gradually shift to higher wavenumbers (Fig. S5; ESI†). This increased surface chain mobility was further evidenced by X-ray photoelectron spectroscopy (XPS). The carbon (C) concentration of our hybrid films began to decrease steadily with an increase in RT/D ratio, reaching a plateau at an RT/D of around 5 (Fig. S6; ESI†). The actual C concentration of the C10-hybrid-(RT/D = 4) covered surface was smaller than that of the C10-monolayer-covered surface (40 and 47 at.%, respectively) indicating a lower density of alkyl groups in the hybrid film surfaces. In addition, as described in the previous section, the d-spacing of our hybrid films remained virtually constant, even when the concentration of TMOS (silica) species in the matrix was doubled (from RT/D = 2 to RT/D = 4). Thus, it is most likely that TMOS was preferentially consumed in the growth of siloxane networks not in a vertical (out-of-plane) but in a horizontal (in-plain) direction. Consequently, the neighboring alkyl chains at the outermost surface can be considered to be separated by co-condensed silica species acting as spacer moieties (Scheme 1). These findings suggest that the alkyl chains at the surface are able to move freely, providing liquid-like surface properties as a consequence of the addition of TMOS as a molecular spacer. Additionally, as recently reported by the authors, the high affinity of probe organic liquids to alkyl chains is another factor for the enhancement of the chain mobility (we refer to this as solvent effects).2e,g In the present case, the organic liquids are expected to interact with alkyl chains and likely form a “blended interface” by taking advantage of their mutual miscibility, similar to alkane liquids/polydimethylsiloxane (PDMS) brush film interfaces, as previously reported.2e,g Such an effect may also contribute to imparting a more liquid-like character to the surface. We believe that these two simple parameters may help to form oleophobic surfaces showing unusual dynamic CA behavior towards various organic liquids.
Finally, by taking advantage of our hybrid film's excellent dynamic oleophobicity, two simple liquid transportation demonstrations were undertaken. C10-hybrid (RT/D = 4) and C10-monolayer covered glass tubes were prepared using dip coating and CVD methods, respectively. First, colored n-hexadecane was slowly introduced into the glass tubes from the top of vertically-oriented tubes, and allowed to flow down and out of the bottom (Fig. 2a and Movie S2; ESI†). Due to the excellent dynamic oleophobicity of the C10-hybrid coating, the colored n-hexadecane flowed quickly through the tube without staining the inside. In the case of the C10-monolayer, the tube inside became stained due to the poor dewettability. In a second experiment, two liquids, water and n-hexadecane, were added into the open end of closed C10-hybrid (RT/D = 4) coated glass tubes. When water was slowly and carefully added down the side of the tube, it became suspended and plugged in the tube, because of the large CA (magnitude) of water on the C10-hybrid coated surfaces (Fig. 2b and Movie S3; ESI†). In contrast, n-hexadecane smoothly and easily reached the bottom of the tube without either pinning or staining (Fig. 2c and Movie S4; ESI†). These demonstrations clearly show that controlling the CA behaviour of oleophobic surfaces have a useful potential application for the transportation of organic liquids within a tube or pipe and that large CAs may impede the flow of liquid in such applications.
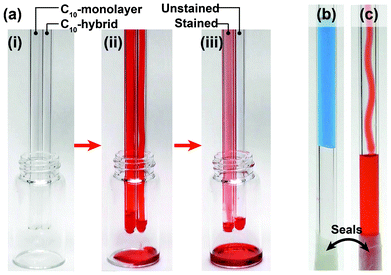 | ||
| Fig. 2 (a) Appearances of C10-monolayer and C10-hybrid (RT/D = 4) coated glass tubes (i) before, (ii) in the course of, and (iii) after flowing the red-colored n-hexadecane. Blue-colored water (b) became blocked in the tube, while red-colored n-hexadecane flowed smoothly through the C10-hybrid (RT/D = 4) coated glass tube (sealed at one end with paraffin film). | ||
Conclusions
In conclusion, the simple combination of alkyl-terminated trialkoxysilane (DTES) with tetraalkoxysilane (TMOS) was found to be effective in creating oleophobic surfaces showing unusual dynamic CA behavior toward various kinds of organic liquids. We hesitate to interpret this phenomenon at the present time, but we feel confident that the mobility of the surface tethered alkyl chains was enhanced both by simple addition of TMOS as a molecular spacer and solvent effects that had a significant influence on the interaction between liquid droplets and the surface. Furthermore, we would like to emphasize that this effect is not limited to DTES: the use of other organosilanes in place of the DTES precursor will be reported in detail in a future publication. Our one-pot coating system reported here is simple and reproducible for the preparation of transparent dynamically oleophobic surfaces over a large area. We hope that this work might provide a novel, facile alternative to conventional methods for the fabrication of superoleophobic surfaces, highlighting that neither surface roughening nor perfluorination is necessary. Unlike previously reported branched and cyclic alkyl containing coatings, there are no significant limitations for available substrate and coating method. Thus, this method would be ideal for practical applications.Acknowledgements
We would like to thank Prof. Kazuyuki Kuroda and Dr Ryutaro Wakabayashi of Waseda University for the permission of the use of SEM and NMR, and their technical assistance and Dr Tatsuo Kimura and Dr Tatsuya Miyajima of AIST for the measurement of XRD and hardness of hybrid films. This work was partially supported by Grant-in-Aid for Research (KAKENHI) on Activity Start-up (C.U.; No. 23850020) of JSPS.References
- (a) E. Ruiz-Hitzky, M. Darder, P. Aranda and K. Ariga, Adv. Mater., 2010, 22, 323 CrossRef CAS; (b) B. Bhushan, Philos. Trans. R. Soc. London, Ser. A, 2009, 367, 1445 CrossRef CAS; (c) K. Koch and W. Bartholott, Philos. Trans. R. Soc. London, Ser. A, 2009, 367, 1487 CrossRef CAS.
- (a) A. Tuteja, W. Choi, M. L. Ma, J. M. Mabry, S. A. Mazella, G. C. Rutledge, G. H. McKinley and R. E. Cohen, Science, 2007, 318, 1618 CrossRef CAS; (b) A. Tuteja, W. Choi, J. M. Mabry, G. H. McKinley and R. E. Cohen, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 18200 CrossRef CAS; (c) J. Zhang and S. Seeger, Angew. Chem., Int. Ed., 2011, 50, 6652 CrossRef CAS; (d) T.-S. Wong, S.-H. Kang, S. K. Y. Tang, E. J. Smythe, B. D. Hatton, A. Grinthal and J. Aizenberg, Nature, 2011, 477, 443 CrossRef CAS; (e) D. F. Cheng, C. Urata, M. Yagihashi and A. Hozumi, Angew. Chem. Int. Ed., 2012, 51, 2956 CAS; (f) X. Deng, L. Mammen, H.-J. Butt and D. Vollmer, Science, 2011, 335, 67 CrossRef; (g) D. F. Cheng, C. Urata, B. Masheder and A. Hozumi, J. Am. Chem. Soc., 2012, 134, 10191 CrossRef CAS.
- (a) E. F. Hare, E. G. Sharfrin and W. A. Zisman, J. Phys. Chem., 1954, 58, 236 CrossRef CAS; (b) W. A. Zisman, Contact Angle, Wettability, and Adhesion, ed. Fowkes, F. M., American Chemical Society, Washington, DC, 1964, vol.43 Search PubMed.
- A. Tuteja, W. Choi, G. McKinley, R. E. Cohen and M. F. Rubner, MRS Bull., 2011, 33, 752 CrossRef.
- (a) Y. Zushi, J. N. Hogarh and S. Masunaga, Clean Technol. Environ. Policy, 2011, 14, 9 CrossRef; (b) A. B. Lindstrom, M. J. Strynar and E. L. Libelo, Environ. Sci. Technol., 2011, 45, 7954 CrossRef CAS.
- (a) J. J. Sagiv, J. Am. Chem. Soc., 1980, 102, 92 CrossRef CAS; (b) S. R. Wasserman, Y.-T. Tao and G. M. Whitesides, Langmuir, 1989, 5, 1074 CrossRef CAS; (c) A. Ulman, Chem. Rev., 1996, 96, 1533 CrossRef CAS; (d) A. Hozumi, K. Ushiyama, H. Sugimura and O. Takai, Langmuir, 1999, 15, 7600 CrossRef CAS; (e) A. Y. Fadeev and T. J. McCarthy, J. Am. Chem. Soc., 1999, 121, 12184 CrossRef CAS; (f) A. Y. Fadeev and T. J. McCarthy, Langmuir, 2000, 16, 7268 CrossRef CAS.
- (a) W. Chen, A. Y. Fadeev, M. C. Hsieh, D. Öner, J. Youngblood and T. J. McCarthy, Langmuir, 1999, 15, 3395 CrossRef CAS; (b) A. Y. Fadeev and T. J. McCarthy, Langmuir, 1999, 15, 3759 CrossRef CAS; (c) A. Y. Fadeev and T. J. McCarthy, Langmuir, 1999, 15, 7238 CrossRef CAS; (d) C. M. Stafford, A. Y. Fadeev, T. P. Russell and T. J. McCarthy, Langmuir, 2001, 17, 6547 CrossRef CAS; (e) A. F. Fadeev and Y. V. Kazakevich, Langmuir, 2002, 18, 2665 CrossRef CAS; (f) Y. V. Kazakevich and A. F. Fadeev, Langmuir, 2002, 18, 3117 CrossRef CAS; (g) A. Hozumi and T. J. McCarthy, Langmuir, 2010, 26, 2567 CrossRef CAS; (h) J. W. Krumpfer and T. J. McCarthy, Faraday Discuss., 2010, 146, 103 RSC; (i) A. Hozumi, D. F. Cheng and M. Yagihashi, J. Colloid Interface Sci., 2011, 353, 582 CrossRef CAS; (j) J. W. Krumpfer and T. J. McCarthy, Langmuir, 2011, 27, 11514 CrossRef CAS.
- http://www.gelest.com/goods/pdf/couplingagents.pdf .
- (a) A. Shimojima and K. Kuroda, Chem. Rec., 2006, 6, 53 CrossRef CAS; (b) M. Pagliaro, R. Ciriminna and G. Palmisano, J. Mater. Chem., 2009, 19, 3116 RSC.
- (a) A. Shimojima, Y. Sugahara and K. Kuroda, J. Am. Chem. Soc., 1998, 120, 4528 CrossRef CAS; (b) A. Shimojima and K. Kuroda, Langmuir, 2002, 18, 1144 CrossRef CAS; (c) A. Shimojima, N. Umeda and K. Kuroda, Chem. Mater., 2001, 13, 3610 CrossRef CAS; (d) N. Umeda, A. Shimojima and K. Kuroda, J. Organomet. Chem., 2003, 686, 223 CrossRef CAS; (e) A. Shimojima, C.-W. Wu and K. Kuroda, J. Mater. Chem., 2007, 17, 658 RSC.
- Y. Tang, L. A. Finlay, G. L. Kowalke, A. E. Meyer, F. V. Bright, M. E. Callow, J. A. Callow, D. E. Wendt and M. R. Detty, Biofouling, 2005, 21, 59 CrossRef CAS.
- We prepared the hybrid films with the value of TMOS between 0 and 10. However, transparent and flat films were not obtained when the value is 0 and 1.
- S. A. Rodríguez and L. A Colón, Chem. Mater., 1999, 11, 754 CrossRef.
- (a) M. Sakai and N. Hakiri, J. Mater. Res., 2006, 21, 2298 CrossRef CAS; (b) N. Hakiri, A. Matsuda and M. Sakai, J. Mater. Res., 2009, 24, 1950 CrossRef CAS.
- H. Tavana, D. Jehnichen, K. Grundke, M. L. Hair and A. W. Neumann, Adv. Colloid Interface Sci., 2007, 134, 236 CrossRef.
- (a) R. A. Vaia, R. K. Teukolsky and E. P. Giannelis, Chem. Mater., 1994, 6, 1017 CrossRef CAS; (b) K. Kojio, A. Takahara and T. Kajiyama, Langmuir, 2000, 16, 9314 CrossRef CAS; (c) J.-S. Park, A. C. Smith and T. R. Lee, Langmuir, 2004, 20, 5829 CrossRef CAS.
- (a) http://www.surface-tension.de. ; (b) L. D. A. Chumpitaz, L. F. Coutinho and J. A. Meirelles, J. Am. Oil Chem. Soc., 1999, 76, 379 CrossRef CAS; (c) http://www.etc-cte.ec.gc.ca/databases/Oilproperties/ .
Footnote |
| † Electronic Supplementary Information (ESI) available: CA data for C10-monolayer-covered surfaces reported in the literature, XRD patterns, SEM and AFM images, dynamic CAs and CA hysteresis of C10-monolayer- and C10-hybrid-covered samples, XPS data, IR spectra, and movies. See DOI: 10.1039/c2ra21360e |
| This journal is © The Royal Society of Chemistry 2012 |
