Improving the stability of an organic battery with an ionic liquid-based polymer electrolyte†
Jae-Kwang
Kim
*a,
Aleksandar
Matic
a,
Jou-Hyeon
Ahn
b and
Per
Jacobsson
*a
aDepartment of Applied Physics, Chalmers University of Technology, 412 96 Göteborg, Sweden. E-mail: jaekwang@chalmers.se; pjacob@chalmers.se
bDepartment of Chemical & Biological Engineering and Engineering Research Institute, Gyeongsang National University, 900, Gajwa-dong, Jinju 660-701, Republic of Korea
First published on 17th September 2012
Abstract
A gel polymer electrolyte based on the ionic liquid {N-butyl-N-methyl-pyrrolidiniumbis(trifluoromethanesulfonyl)imide (Py14TFSI) and lithium bis(trifluoromethanesulfony)imide (LiTFSI)} is shown to prevent the dissolution from an organic electrode. The composite cell shows high energy efficiency although poor cycle stability is exhibited at very high current density (10 C-rate).
The market for rechargeable batteries has grown rapidly with the development of mobile electronic devices and more lately the development of hybrid and electric vehicles.1 In the near future the market for large scale energy storage system is also expected. In parallel with the main stream development of cheaper and more efficient batteries several niche markets develop; for example flexible batteries for bendable devices and flexible display equipment.2 Several research groups have tried to improve the flexibility of inorganic electrode materials by the addition of carbon and polymer substrates, however, with limited success.3 Organic materials have also been used for flexible batteries such as the 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) based materials. TEMPO is a well-known, stable organic radical with reversible oxidation and reduction behavior in aprotic solvents. The nitroxide radical moiety of TEMPO is oxidized to an oxoammonium cation during charging.4 The reversible electrochemical reaction occurred rapidly with the anion of the electrolyte. This rather remarkable property of the TEMPO radical and its polymer has attracted the attention of research groups developing flexible batteries and the most studied TEMPO-based electrode material is poly (2,2,6,6-tetramethylpiperidinyloxy-4-vinylmethacrylate) (PTMA) (Fig. S1†).
In a working battery TEMPO-based organic electrodes suffer from dissolution into the organic solvent based electrolytes. The dissolution induces self-discharge and eventually a short circuit of the battery.5 Different strategies have been launched in order to make more stable electrodes using crosslinking, brush and mesocellular carbon etc.6 So far the progress is small and the procedure often introduces other problems; e.g. low mechanical strength, and low energy density. In addition, liquid based gel polymer electrolytes have been applied to improve the flexibility; however they do not prevent the electrode dissolution problem.7
In this study, we propose a new strategy to prevent dissolution of organic active materials in flexible batteries; an ionic liquid based electrolyte immobilized in an electrospun polymer matrix, forming an ion conducting gel. Ionic liquid electrolytes (hereafter, ILE) have a high intrinsic ionic conductivity at room temperature, high thermal stability and exhibit a good electrochemical stability in the range up to 4.0–5.2 V.8 However, it has to be noted that at least at room temperature a high viscosity limits ionic mobility and therefore also ionic conductivity.8 The ILE selected for the flexible organic battery in this work is; N-butyl-N-methyl-pyrrolidiniumbis(trifluoromethanesulfonyl)imide (Py14TFSI) complexed with lithium bis(trifluoromethanesulfony)imide (LiTFSI). Py14TFSI has attracted interest for applications in lithium batteries as it shows a high cathodic decomposition potential (compared to non-cyclic and unsaturated cyclic quaternary ammonium ILs), a high ionic conductivity, and an ability to form a solid electrolyte interface (SEI) thus preventing undesired reactions with the electrodes.9,10
Two electrolytes, a conventional liquid electrolyte of 1 M LiPF6 in EC/DMC (1:1,v/v) and an ionic liquid electrolyte of 1 M LiTFSI in Py14TFSI, were prepared and PTMA was added under argon atmosphere. Fig. 1 shows the time evolution of the blends. PTMA is fully dissolved in the conventional liquid electrolyte (hereafter LE) after only 1 hour, however, does not dissolve in the ionic liquid electrolyte during observation. Moreover, after one year of storage in the ionic liquid, the PTMA electrode material was still stable. Thus, the ionic liquid electrolyte seems to overcome the dissolution problems of PTMA – at least during passive storage.
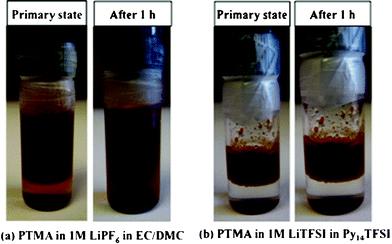 | ||
| Fig. 1 Photographs of the conventional electrolyte with PTMA (a) and the ionic liquid electrolyte with PTMA (b) directly after mixing and after 1 hour. | ||
The stability of PTMA towards the ionic liquid is further underlined by looking at the conductivities of the electrolytes before and after contact with the electrode material, see Fig. 2. The ionic conductivity of the LE has decreased significantly from the starting value whereas the conductivity of the ILE is virtually unchanged. We note before passing that the ILE shows a reasonable ion conductivity for high power battery applications (>10−4 S cm−1 was achieved at 10 °C).
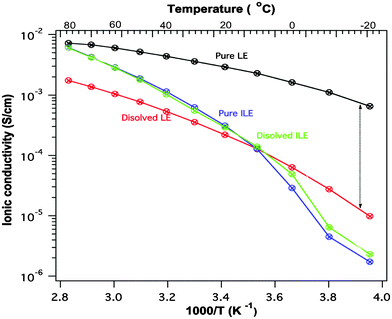 | ||
| Fig. 2 Ionic conductivity of the liquid electrolyte (LE) and the ionic liquid electrolyte (ILE) before and after one month storage over PTMA. | ||
When preparing the gel membrane a nano-fibrous poly(vinylidene fluoride-co-hexafluoropropylene) (PVdF-HFP) matrix, prepared by electrospinning (see Fig. S2) was soaked with the ILE (ILPE). The matrix is made up of a network of interlaid fibers with a diameter of <800 nm. The interlaying of the fibers provides mechanical strength to the membrane. The presence of interconnected micron-sized pores in the structure is arguably also an asset for a host matrix for the preparation of gel polymer electrolytes.11
Open-circuit-voltage (OCV) was investigated over 15 days for a self-discharge test (see Fig. 3) and both electrodes were charged to 90% SOC and a potential of ca 3.6 V. The OCV of PTMA in LE decreased in a continuous way from the initial value to 2.5 V. In contrast the OCV of PTMA in ILPE stabilized at 3.5 V after 5 days.
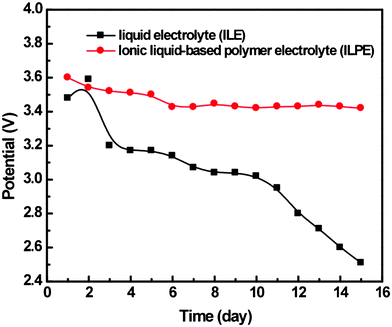 | ||
| Fig. 3 Open circuit voltage (OCV) of the liquid and ionic liquid polymer electrolyte based battery over fifteen days. | ||
Fig. 4 shows the electrochemical mechanism of PTMA with 1 M LiTFSI in Py14TFSI. The nitroxyl radical of TEMPO is oxidized to form a cation and joins an electrolyte anion (TFSI−) to form oxoammonium salt during the anodic stage and its reverse reaction occurs during the cathodic stage. PTMA has a theoretical cathode capacity of 111 mAh g−1.
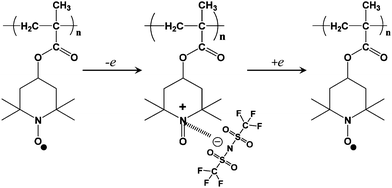 | ||
| Fig. 4 Electrochemical mechanism of PTMA with 1 M LiTFSI in Py14TFSI. | ||
Galvanostatic charge-discharge curves for cells composed of PTMA/ILPE/Li in the voltage window 3.0–4.0 V are shown in Fig. 5a. At 1 C (0.12 mA cm−2)rate the cell demonstrated a single charge-discharge plateau around 3.5 V reflecting a low and stable cell resistance with a slight increase of the discharge capacity over the first few cycles. For the 10 C-rate (1.2 mA cm−2) the voltage separation between the charge and discharge increased to ∼0.1 V as a result of increased cell resistance and the first discharge capacity has decreased to 33 mAh g−1. However, over the following cycles the discharge capacity increased. The irreversible capacity loss between the charge and discharge reaction is less than 3 mAh g−1 at both 1 and 10 C, and the columbic efficiency is almost 99% for the PTMA cell with ILPE.
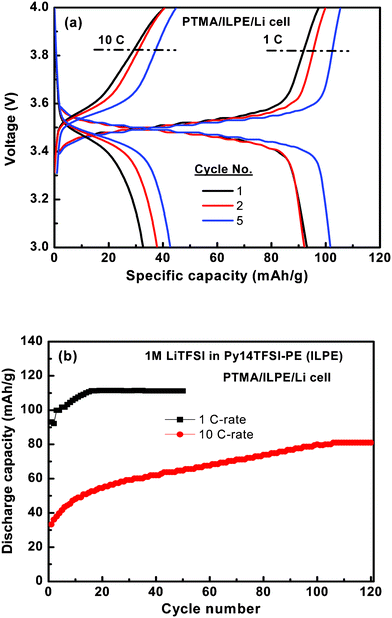 | ||
| Fig. 5 Electrochemical properties of the Py14TFSI based polymer electrolyte cell at 1 and 10 C-rate. | ||
The cycling performance of the PTMA cell with 1 M LiTFSI in the Py14TFSI-based polymer electrolyte at room temperature under 1 and 10 C-rates is presented in Fig. 5b. At 1 C-rate the cell shows a very stable cycling performance. From the 15th cycle and onward the cell cycles at a stable capacity of 110 mAh g−1 (99% is retained at the 50th cycle). However, according to reported values in the literature, the conventional liquid electrolytes exhibits a >0.13% capacity fade per cycle.12 For the 10 C-rate the initial discharge capacity of 33 mAh g−1 increased to 80 mAh g−1 over 100 cycles. Thus, after 100 cycles at 10 C-rate the energy efficiency is above 72% as compared to cycling at the 1 C-rate. The good electrochemical performance of the Py14TFSI-based polymer cell is promising and reflects a combination of thermal stability and electrochemical stability of the ionic liquid electrolyte together with excellent conduction properties.
In summary, we propose an ionic liquid based polymer electrolyte in order to prevent the instability of an organic radical polymer electrode based on PTMA without any additional process for electrode design. The ILPE also improves the flexibility of the battery. The Py14TFSI-based polymer electrolyte cell shows very good electrochemical properties at moderate C-rates. However, due to an intrinsic high viscosity of the ionic liquid the performance decreases at higher C-rate.
The present work was supported by STINT and NRF in a joint Korea-Sweden program, FORMAS, and the Chalmers Area of Advance – Energy.
References
- (a) B. Scrosati, J. Hassoun and Y.-K. Sun, Energy Environ. Sci., 2011, 4, 3287 RSC; (b) G. Jeong, Y.-U. Kim, H. Kim, Y.-J. Kim and H.-J. Sohn, Energy Environ. Sci., 2011, 4, 1986 RSC.
- B. Scrosati, Nat. Nanotechnol., 2007, 2, 598 CrossRef CAS.
- (a) N. J. Dudney, Electrochem. Soc. Interface fall, 2008, 44 CAS; (b) S.-W. Song, H. Choi, H. Y. Park, G. B. Park, K. C. Lee and H.-J. Lee, J. Power Sources, 2010, 195, 8275 CrossRef CAS; (c) J.-Z. Wang, S.-L. Chou, J. Chen, S.-Y. Chew, G.-X. Wang, K. Konstantinov, J. Wu, S.-X. Dou and H. K Liu, Electrochem. Commun., 2008, 10, 1781 CrossRef CAS.
- (a) K. Nakahara, S. Iwasa, M. Satoh, Y. Morioka, J. Iriyama, M. Suguro and E. Hasegawa, Chem. Phys. Lett., 2002, 359, 351 CrossRef CAS; (b) H. Nishide, S. Iwasa, Y.-J. Pu, T. Suga, K. Nakahara and M. Satoh, Electrochim. Acta, 2004, 50, 827 CrossRef CAS; (c) K. Oyaizu and H. Nishide, Adv. Mater., 2009, 21, 2339 CrossRef CAS.
- (a) T. Suga, H. Konishi and H. Nishide, Chem. Commun., 2007, 1730 RSC; (b) K. Nakahara, J. Iriyama, S. Iwasa, M. Suguro, M. Satoh and E. J. Cairns, J. Power Sources, 2007, 165, 398 CrossRef CAS.
- (a) T. Suga, H. Konishi and H. Nishide, Chem. Commun., 2007, 1730 RSC; (b) Y.-H. Wang, M.-K. Hung, C.-H. Lin, H.-C. Lin and J.-T. Lee, Chem. Commun., 2011, 47, 1249 RSC; (c) Y. Kim, C. Jo, J. Lee, C. W. Lee and S. Yoon, J. Mater. Chem., 2012, 22, 1453 RSC.
- J.-K. Kim, G. Cheruvally, J.-W. Choi, J.-H. Ahn, D.-S. Choi and C.-E. Song, J. Electrochem. Soc., 2007, 154, A839 CrossRef CAS.
- (a) M. Galiński, A. Lewanddowski and I. Stepniak, Electrochim. Acta, 2006, 51, 5567 CrossRef; (b) M. Armand, F. Endres, D. R. MacFarlane, H. Ohno and B. Scrosati, Nat. Mater., 2009, 8, 621 CrossRef CAS.
- (a) A. Lewandowski and A. Swiderska-Mocek, J. Power Sources, 2009, 194, 601 CrossRef CAS; (b) S. Randström, G. B. Appetecchi, C. Lagergren, A. Moreno and S. Passerini, Electrochim. Acta, 2007, 53, 1837 CrossRef.
- J.-K. Kim, P. Jacobsson and A. Matic, 12thInternational Symposium on Polymer Electrolytes, Italy, Padova, Aug. 29. – Sep. 3. 2010 Abatract p242 Search PubMed.
- (a) X. Li, G. Cheruvally, J.-K. Kim, J.-W. Choi, J.-H. Ahn, K.-W. Kim and H.-J. Ahn, J. Power Sources, 2007, 167, 491 CrossRef CAS; (b) F. Croce, M. L. Focarete, J. Hassoun, I. Meschini and B. Scrosati, Energy Environ. Sci., 2011, 4, 921 RSC; (c) S. Cavaliere, S. Subianto, I. Savych, D. J. Jones and J. Rozière, Energy Environ. Sci., 2011, 4, 4761 RSC.
- (a) K. Nakahara, J. Iriyama, S. Iwasa, M. Suguro, M. Satoh and E. J. Cairns, J. Power Sources, 2007, 165, 398 CrossRef CAS; (b) J.-K. Kim, J.-H. Ahn, G. Cheruvally, G. S. Chauhan, J.-W. Choi, D.-S. Kim, H.-J. Ahn, S.-H. Lee and C.-E. Song, Met. Mater. Int., 2009, 15, 77 CrossRef CAS; (c) Y. Dai, Y. Zhang, L. Gao, G. Xu and J. Xie, J. Electrochem. Soc., 2011, 158, A291 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: Preparation of PTMA and SEM of ionic liquid-based polymer electrolyte. See DOI: 10.1039/c2ra21416d |
| This journal is © The Royal Society of Chemistry 2012 |
