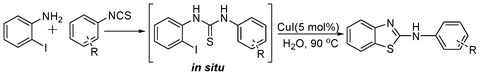
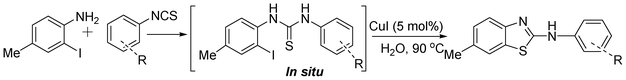
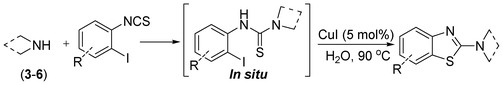
A one-pot strategy for the synthesis of 2-aminobenzothiazole in water by copper catalysis†
Nilufa
Khatun
a,
Latonglila
Jamir
ab,
Majji
Ganesh
a and
Bhisma K.
Patel
*a
aDepartment of Chemistry, Indian Institute of Technology Guwahati, 781 039, Assam, India
bSchool of Engineering and Technology and Management, Nagaland University, Dimapur - 797112, India. E-mail: patel@iitg.ernet.in; Fax: +91-3612690762
First published on 1st October 2012
Abstract
A straightforward, efficient and sustainable method for the synthesis of 2-aminobenzothiazoles has been achieved from in situ generated 2-halothioureas using copper(I) catalyst in water. The present study demonstrates that copper iodide (CuI) exhibits better efficiency in water towards intramolecular S-arylation compared to organic solvents. While (o-iodoaryl) thioureas are smoothly converted to the desired product in high yields with only catalytic quantity of CuI, addition of base and ligand are essential for bromo and chloro analogues. An unprecedented demethoxylation of o-methoxy-o-iodoarylthiourea and demethoxylation followed by a Friedel–Crafts type methylation (para to nitrogen) was observed in the aryl ring possessing the original methoxy group.
Introduction
‘On-water’ chemistry has gained tremendous attention as an alternative reaction medium in recent times to reduce the ever mounting concerns for environment sustainability. It has been observed that reactions are accelerated in water medium due to the hydrophobic effect of water which generates internal pressure and facilitates the association of reactants in solvent cavities during reaction processes.1 In addition, the use of water as the reaction medium for various types of organic transformations, including transition metal-catalysed coupling reactions, has marked advantages over reactions in organic solvents in terms of safety, ready availability, cost efficiency, non-toxicity and non-flammability, thus being very attractive from both the economical and environmental point of view.2 2-Aminobenzothiazoles can be traced to the late 1800s when Hoffmann (1887) first introduced the scaffold via cyclisation of the resultant intermediate obtained by the reaction of 2-aminothiophenol and phenyl isothiocyanate3 and later on Hugerschoff reported the cyclisation of arylthioureas with liquid bromine.4 Benzothiazoles, particularly 2-aminobenzothiazoles are currently considered as privileged scaffolds and have gained remarkable popularity in the fields of bioorganic and medicinal chemistry.5 Various pharmacophores bearing 2-aminobenzothiazole have revealed a broad spectrum of biological activities that encompass anti-inflammatory, anti-microbial, anti-tumour, neuroprotective, anti-convulsant, anti-diabetic and anti-epileptic activities.6Bioactive molecules possessing the 2-aminobenzothiazole core include Riluzole (I), a glutamate neurotransmitor,7 R116010 (II), a potent inhibitor of retinoic acid metabolism,6d a tRA-induced expression of CYP26B1 enhancer (III)6e and N-myristoyltransferase (Nmr) inhibitor (IV)5b,8 as shown in Fig. 1. Some other pharmacophores possessing the 2-aminobenzothiazole as the core unit include the antibacterial compound (V),9 the PPAR agonist (VI),10 the H3-receptor ligand (VII),11 and the nicotinic-acetylcholine-receptor ligand (VIII)12 as also shown in Fig. 1.
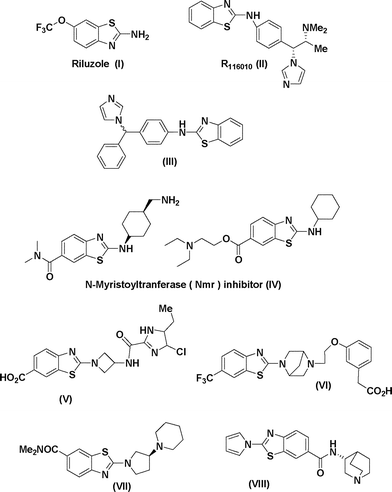 | ||
| Fig. 1 2-Aminobenzothiazoles containing bioactive molecules. | ||
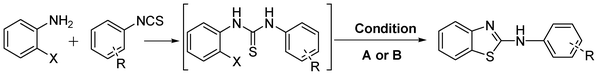
Due to their numerous applications, considerable effort has been made in the development of efficient strategies for the construction of 2-aminobenzothiazoles as shown in Scheme 1. Earlier reports of their synthesis involve the condensation of substituted anilines with isothiocyanates followed by oxidative cyclisation of the resultant thioureas (path A). For such conversions, various oxidising agents have been utilised such as molecular bromine,4 or its equivalents benzyltrimethylammonium tribromide,5b [Bmim]Br3,13 coupling of arylamines with preformed 2-halobenzothiazoles (path B),14 coupling of 2-aminobenzothiazoles with aryl halides (path C),15 desulfurative cyclisation of 2-mercaptoarylthiourea (path D),3,16 Fe(III)-catalysed cross-coupling reactions of 2-haloanilines with isothiocyanates (path E),17 palladium catalysed C–H activation and/or Pd or Cu catalysed intramolecular heteroarylation of 2-halobenzothioureas (path F).18 Some metal-free approaches for the synthesis of 2-aminobenzthiazole have also been reported recently at high temperature (path F).19
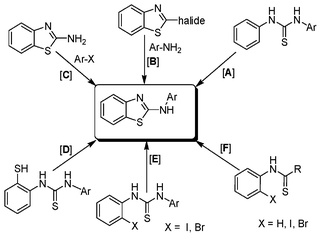 | ||
| Scheme 1 Available literature methods for the preparation of 2-aminobenzothiazoles. | ||
Although literature enumerates a considerable number of efficient and high yielding methods for the synthesis of 2-aminobenzothiazoles, some drawbacks in terms of their limited utility in large and industrial-scale preparation make them unsuitable. The use of highly toxic and corrosive reagents, organic solvents, additives, expensive catalysts, hazardous wastes, choice of specific ligands, difficulties associated with the removal of catalyst from the polar organic solvents, and drastic reaction condition demands a need to explore the use of inexpensive and more suitable alternatives for such coupling reactions. To overcome some of the drawbacks, a number of modified approaches have been made which involve the use of inexpensive metal catalysts,17 recyclable catalysts with ligand18f or under ligand-free condition18g or metal-free base-mediated condition.19 On the other hand, in recent times, organic reactions without the use of conventional organic solvents have covered a broad area of modern synthetic organic chemistry. As a result, many greener, novel, and environmentally sustainable solvents such as scCO2,20 fluorous media,21 ionic liquids,22 and water1h,23 have been studied broadly in organic synthesis. As a part of our efforts towards the development of expeditious metal-catalysed diverse methodologies for the synthesis of biologically active heterocycles,24 herein we wish to report a practical, greener way to 2-aminobenzothiazoles via a CuI catalysed/water-mediated intramolecular C–S coupling of 2-haloaryl thioureas. It is noteworthy to mention that though the copper-catalysed intramolecular coupling reaction is well established, the reported cases involve the use of organic solvents.17a,18c,d,e,19b Recently, Ding et al.17b have reported a novel protocol for the synthesis of 2-aminobenzothiazole via a FeCl3 (5 mol%) catalysed tandem reaction of 2-iodoaniline with isothiocyanate in water in the presence of a phase-transfer catalyst, base and ligand at 80 °C. Although the protocol is claimed to be green, it is strictly restricted to 2-iodoaryl thioureas and require additives such as phase-transfer reagent and ligand. Most recently, a highly efficient ‘on water’ methodology for the same transformation has been achieved by our group where crystalline CuO nanoparticles are used as the catalyst.18i
Results and discussion
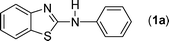
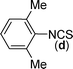
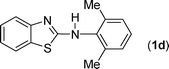
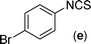
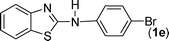
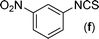
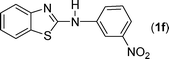
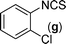
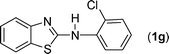
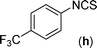
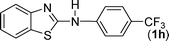
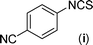
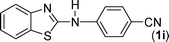
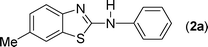
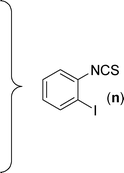
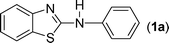
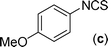
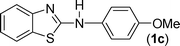
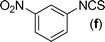
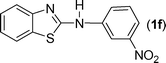
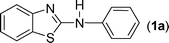
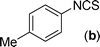
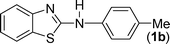
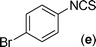
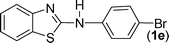
In our initial approach, the in situ generated thiourea obtained by the reaction of 2-iodoaniline (1) and phenyl isothiocyanate (a) in water was treated with CuI (5 mol%) at room temperature. No reaction was observed even after 24 h. However, to our delight, the same reaction when performed at 90 °C showed complete disappearance of thiourea within 13 h, resulting in the formation of a new spot in TLC having a higher Rf than the corresponding thiourea. The product upon isolation and characterisation was found to be N-phenylbenzo[d]thiazol-2-amine (1a) obtained in 85% yield. Although the catalyst loading could be reduced from 5 to 1 mol% without significantly affecting the yield, the reaction took a longer time (24 h). The reaction temperature also plays an important role in this intramolecular coupling reaction. While a decrease in the temperature failed to provide the desired yield, an increase in the temperature (110 °C) led to a number of side products and thereby a decrease in the overall yield. Thus, 5 mol% of the catalyst at a temperature of 90–100 °C was chosen as the condition for all subsequent reactions.After establishing the optimised conditions, we investigated the substrate scope for this methodology. It was found that functional groups such as methyl, methoxy, nitro, chloro, cyano groups when present in the phenyl ring exerted considerable effect on this coupling reaction. As can be seen from Table 1, for substrates containing electron donating groups, the reaction is faster giving better yield while the opposite effect was observed for substrates possessing electron-withdrawing groups. Initially, the reaction of 2-iodoaniline (1) with various substituted isothiocyanates (a–k) were examined under the present optimised conditions. The thioureas generated from the reaction of 2-iodoaniline (1) and aromatic isothiocyanates bearing electron-donating substituents p-Me (b), p-OMe (c) and 2,6-Me2 (d) (Table 1) showed most effective coupling to afford the corresponding benzothiazoles 1b, 1c and 1d, respectively, in good to excellent yields. On the other hand aromatic isothiocyanates bearing electron-withdrawing substituents such as p-Br (e), m-NO2 (f), o-Cl (g), p-CF3 (h), p-CN (i) groups exhibited slow reaction rates and the reaction took a longer time (14–33 h).
The thiourea derived from 1-naphthyl isothiocyanate (k) and 2-iodoaniline (1) gave the dehalogenative C–S coupled product (1k) in 79% yield. It may be noted here that the same thiourea when reacted with another thiophilic reagent 1,1′[ethane-1,2-diyl]dipyridinium bistribromide (EDPBT), a bromine equivalent, is reported to give the C–S coupled product obtained via intramolecular aromatic electrophilic substitution (Hugerschoff type product) and not the dehalogenative product as shown in Scheme 2.25 However, thioureas derived from 1-naphthyl isothiocyanate and 2-bromo- or 2-chloroanilines were completely inert when subjected to Cu-catalysed coupling reaction, demonstrating the differential thiophilicity and reactivity of bromine and Cu. Besides aryl isothiocyanates, alkyl isothiocyanates such as cyclohexyl isothiocyanates (j) also underwent reaction under the standard conditions giving corresponding products (1j) in lower yield (43%).
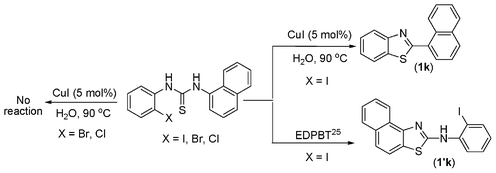 | ||
| Scheme 2 Differential reactivity of copper salt and bromine equivalent (EDPBT). | ||
The thiourea derived from 2-methoxyphenyl isothiocyanate (l) and 2-iodoaniline (1) gave almost an equimolar mixture of normal C–S coupled product (1l) along with demethoxylated benzothiazole (1l′) as shown in Scheme 3. While the manuscript was under review, another report on 2-methoxy as a leaving group appeared in the literature, thus further supporting our onservation.26
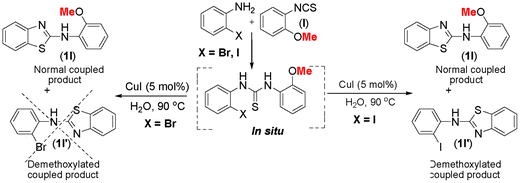 | ||
| Scheme 3 Demethoxylation of 2-methoxyarylthiourea. | ||
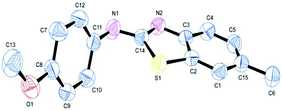
Such kind of result is unprecedented in the literature. To get further insight into the mechanism, the thiourea generated from 4-methyl-2-iodoaniline (2) and 2-methoxyphenyl isothiocyanate (l) was subjected to the present reaction conditions. Here, along with the usual coupled product (2l), a demethoxylation followed by a Friedel–Crafts type methylation (2l′) (para to nitrogen) was observed in the aryl ring bearing the original methoxy group as shown in Scheme 4. The structure of the product 2l′ has been unambiguously confirmed by XRD as shown in Fig. 2.
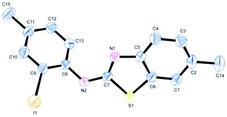 | ||
| Fig. 2 ORTEP illustration of product 2l′ (for clarity H atoms are omitted). | ||
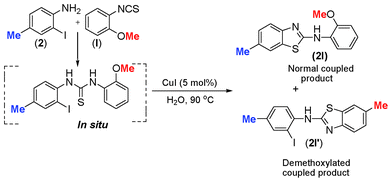 | ||
| Scheme 4 Demethoxylation followed by methylation in 1-(2-iodo-4-methylphenyl)-3-(2-methoxyphenyl)thioureas. | ||
However, 2-bromoaniline (7) derived thiourea, an analogue of 2-iodoaniline failed to provide the demethoxylated product but only normal coupled product (1l) was obtained (Scheme 3). The exact mechanism for the formation of this unprecedented demethoxylation product could not be ascertained.
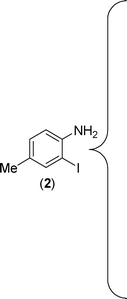
The substrate scope of this aqueous mediated reaction was further extended. 4-Methyl-2-iodoaniline (2) was treated with different isothiocyanates (a, c, e, f) and the resulting in situ generated thioureas were subjected to the present reaction conditions. It was found that substituted 2-iodoaniline derived thioureas showed better coupling efficiency than simple 2-iodoaniline (Table 2). The reactions were completed within 5–12 h furnishing the corresponding products in good to excellent yields. For example, reaction of 4-methyl-2-iodoaniline (2) with phenyl isothiocyanate (a) proceeded smoothly to give 87% yield of the product (2a) within 9 h. The structure of the product (2c) was confirmed by X-ray crystallography as shown in Fig. 3. Likewise, a variety of isothiocyanates (c, e and f) were reacted with 4-methyl-2-iodoaniline (2) to afford the corresponding thioureas in situ which gave the respective 2-aminobenzothiazoles (2c, 2e and 2f) in high yields when subjected to the present reaction conditions. The thiourea generated from 4-methyl-2-iodoaniline (2) and 2-methoxy-4-bromophenyl isothiocyanate (m) when subjected to the present reaction conditions yielded the normal C–S coupled product (2m) and no demethoxylated product was observed at all as was the case with o-methoxy substrates described earlier (Scheme 3 and 4).
 | ||
| Fig. 3 ORTEP illustration of product 2c (for clarity H atoms are omitted). | ||
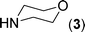
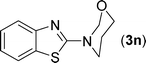
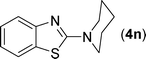
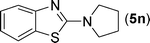
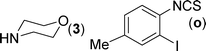
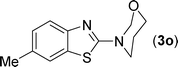
After the successful synthesis of the above scaffold with primary amines, to bring more variety, the coupling partner for the synthesis of thiourea was changed. In this strategy the thioureas were generated by reacting 2-iodophenyl isothiocyanate (n) with various secondary amines (Table 3). When the in situ generated thiourea obtained by the reaction of 2-iodophenyl isothiocyanate (n) and morpholine (3) was treated under the optimised reaction conditions, corresponding product (3n) was obtained in 92% yield. Similarly, reaction with other secondary amines such as piperidine (4) and pyrrolidine (5) also gave their corresponding 2-aminobenzothiazole derivatives (4n) and (5n), respectively, in excellent yields. Similarly the thiourea derived from morpholine (3) and 4-methyl-2-iodophenyl isothiocyanate (o) yielded the corresponding 2-aminobenzothiazole (3o) in high yield.
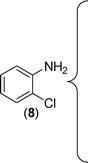
Finally, we wished to study the affect of relatively inert 2-bromo and 2-chloro substituents on the present coupling reaction. Most often C–Br and C–Cl reagents are overlooked due to their inertness toward coupling reactions although they are inexpensive and commercially available. So, in our ongoing project, when the in situ generated thiourea obtained by the reaction of 2-bromoaniline (7) and phenyl isothiocyanate (a) was treated with CuI (5 mol%), only 15–20% conversion to the product was observed after 5 h. Addition of K2CO3 (2 equiv.) improved the yield to 45%. Taking cues from our earlier ligand mediated C–S,24a double C–S,24c and C–N24b bond formation work, we considered using 1,10-phenanthroline for this coupling reaction. Interestingly, the use of 1,10-phenanthroline (5 mol%), K2CO3 (1 equiv.) along with CuI (5 mol%) at 90 °C led to complete disappearance of the starting materials after 2.5 h and the product was isolated in high yield (81%). Utilizing this optimised condition, the in situ generated thioureas derived from 2-bromoaniline (7) and aromatic isothiocyanates (a, c, f) furnished their corresponding benzothiazoles (1a, 1c, 1f) in high yields as shown in Table 4.
| Primary amine | Isothiocyanate | Productb | t/h | Yieldc (%) |
|---|---|---|---|---|
| a Reactions were monitored by TLC. b Confirmed by IR, 1H and 13C NMR. c Isolated yields.X = Br, Condition A: CuI (5 mol%), K2CO3 (1 equiv.), 1,10-phen (5 mol%), 90 °C. X = Cl, Condition B: CuI (10 mol%), K2CO3 (2 equiv.), 1,10-phen (20 mol%), 90 °C. | ||||
|
|
|
2.5 | 81 |
|
|
2 | 83 | |
|
|
10 | 75 | |
|
|
|
28 | 52 |
|
|
26 | 53 | |
|
|
40 | 48 | |
2-Chloroaniline, on the other hand, showed its sluggish tendency in coupling reaction as the C–Cl bond is much more inert than the C–Br bond. Reaction with CuI (5 mol%), K2CO3 (2 equiv.) and 1,10-phenanthroline (5 mol%) furnished only 30% conversion while an increase in the catalyst loading (10 mol%) as well as an increase in the amount of ligand (20 mol%) improved the conversion to 52% at 90 °C. Similarly, 2-aminobenzothiazoles (1a, 1b, 1e) have been synthesised from in situ generated thioureas of 2-chloroaniline and aromatic isothiocyanates under this condition in moderate yield as shown in Table 4. The thiourea derived from 2-fluorophenyl isothiocyanate and aniline failed to provide the desired product under the above condition possibly because of the inertness of sp2 C–F bond.
Taking cues from the earlier reports a plausible mechanism for the formation 2-aminobenzothiazole from 2-halothiourea is shown in Scheme 5.18d,e In path a, an oxidative insertion of the Cu(I)-ligand (1,10-phenanthroline) complex to the halo group of thiourea afforded an intermediate complex B. The complex B in the presence of base forms an intramolecular Cu–S bond, resulting in the formation of complex C. Subsequent reductive elimination provides 2-aminobenzothiazole and regenerates the catalytic copper species to maintain the catalytic cycle (path a, Scheme 5). However, in an alternative path (path b) pre-coordination of the sulfur atom of thiourea to the Cu(I)-ligand complex in the presence of a base leads to the formation of intermediate complex B′ and ultimately to complex Cvia an oxidative insertion. 2-Halo (Br and –Cl) thioureas follow the above reaction mechanism while a ligand-free condition is enough for 2-iodo-substituted thioureas. Base K2CO3 helps to form the intermediate complex C (or B′) while ligand 1,10-phenanthroline helps to stabilize the complex, prevent catalyst decomposition and improve the solubility of the in situ copper complex, thus increasing the reaction rate. Furthermore, an orthogonal differential selectivity and increasing reactivity in the Cu-catalysed Ullman reaction when assisted by a ligand has been demonstrated by Buchwald and our group.24,27
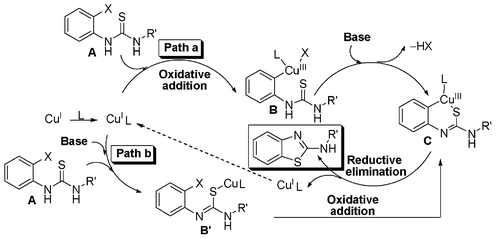 | ||
| Scheme 5 Plausible mechanism for the formation of 2-aminobenzothiazoles. | ||
On the other hand, when water is used as the reaction medium, a repulsive hydrophobic interaction arises between the small organic molecules and water. Small organic molecules due to their insolubility in water are forced to form aggregates in order to decrease the organic surface area exposed to water. In case of ‘on water’ reaction, these aggregates are shifted to the water–organic molecules boundary region. These small aggregates then interact with surface water molecules (having free –OH group) through hydrogen bonding. Under vigorous agitation, penetration of this boundary by the means of hydrogen-bonding interaction provides suitable catalytic site for reaction. The combined effect of hydrophobic interaction and hydrogen bonding at the boundary accelerates the reaction and thus gives high yields.
To check the activity of the catalyst (CuI) after one cycle, the product was extracted out with ethyl acetate layer leaving behind the catalyst in the aqueous layer. To the catalyst present in the aqueous layer was added 2-iodoaniline (1) and phenyl isothiocyanate (a) and the in situ generated thiourea was heated at 90 °C. The reaction gave 45% of the product after 24 h suggesting reduced catalytic efficiency.
Conclusions
In conclusion, we have achieved a simple and efficient Cu-catalysed method for the synthesis of 2-aminobenzothiazoles in water. This simple one-pot methodology delivers a range of target compounds in good to excellent yields from readily available starting materials. The in situ generated thioureas from 2-chloroanilne and 2-bromoaniline and various isothiocyanates underwent smooth reaction under optimised conditions along with their 2-iodoaniline analogues. An unprecedented demethoxylation of o-methoxy-o-iodoarylthiourea and demethoxylation followed by a Friedel–Crafts type methylation (para to nitrogen) was observed in the aryl ring bearing the original methoxy group. Such kind of result is rare in literature. Experimental simplicity, good substrate generality, use of cheap catalyst and water as the solvent are some special attributes of the present method.General procedure for the preparation of N-phenylbenzo[d]thiazol-2-amine (1a)
A mixture of 2-iodoaniline 1 (1 mmole, 219 mg) and phenyl isothiocyanate a (1 mmol, 135 mg) was stirred in water (6 mL) at 90 °C. White thiourea was formed within 20 min as judged from TLC. Under this stirring condition CuI (5 mol%, ∼10 mg) was added to it and the reaction was stirred for a further period of 13 h at 90 °C. After completion of the reaction as indicated by TLC, the reaction mixture was cooled to room temperature and extracted with ethyl acetate (2 × 10 mL). The combined ethyl acetate layer was then dried over anhydrous Na2SO4 and removed under reduced pressure. The crude product was purified over a column of silica gel eluting with EtOAc–petroleum ether (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) to give the pure product 1a in 85% yield (192 mg).
8) to give the pure product 1a in 85% yield (192 mg).
General procedure for the preparation of 2-aminobenzothiazole from 2-bromoaniline (7) and various isothiocyanates
In situ generated thiourea in water obtained by the reaction of 2-bromoaniline 7 (1 mmol, 172 mg) and phenylisothiocyanate a (1 mmol, 135 mg) was treated with CuI (5 mol%, ∼10 mg), K2CO3 (1 equiv., 138 mg) and 1,10-phen (5 mol%, 10 mg). The resulting mixture was reacted for a further period of 2.5 h at 90 °C. After completion of the reaction as indicated by TLC, the reaction mixture was cooled to room temperature and extracted with ethyl acetate (2 × 10 mL). The combined ethyl acetate layer was then dried over anhydrous Na2SO4 and removed under reduced pressure. The crude product was purified over a column of silica gel eluting with (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, EtOAc–petroleum ether) to give the pure product 1a in 81% yield (183 mg).
8, EtOAc–petroleum ether) to give the pure product 1a in 81% yield (183 mg).
Acknowledgements
B. K. P acknowledges the support of this research by the Department of Science and Technology (DST) (SR/S1/OC-79/2009), New Delhi, and the Council of Scientific and Industrial Research (CSIR) (01(2270)/08/EMR-II). N. K. and L. J. thank CSIR for fellowships and M. G. thanks UGC. Thanks are due to Central Instruments Facility (CIF) IIT Guwahati for NMR spectra and DST-FIST for XRD facility.References
- (a) K. H. Shaughnessy and R. B. Devasher, Curr. Org. Chem., 2005, 9, 585 CrossRef CAS; (b) N. E. Leadbeater, Chem. Commun., 2005, 2881 RSC; (c) A. Rahmati and K. Vakili, Amino Acids, 2010, 39, 911 CrossRef CAS; (d) M. C. Pirrung and K. D. Sarma, J. Am. Chem. Soc., 2004, 126, 444 CrossRef CAS; (e) A. Shaabani, A. Rahmati and E. Farhangi, Tetrahedron Lett., 2007, 48, 7291 CrossRef CAS; (f) S. Venkatraman and C.-J. Li, Tetrahedron Lett., 2001, 42, 781 CrossRef CAS; (g) S. Narayan, J. Muldoon, M. G. Finn, V. V. Fokin, H. C. Kolb and K. B. Sharpless, Angew. Chem., Int. Ed., 2005, 44, 3275 CrossRef CAS , and references therein; (h) C.-J. Li, Chem. Rev., 2005, 105, 3095 CrossRef CAS; (i) R. N. Butler and A. G. Coyne, Chem. Rev., 2010, 110, 6302 CrossRef CAS; (j) C.-J. Li, Chem. Rev., 1993, 93, 2023 CrossRef CAS; (k) C.-J. Li and T.-H. Chan, Comprehensive Organic Reaction in Aqueous Medium, Wiley, New York, 2007 Search PubMed; (l) A. Chanda and V. V. Fokin, Chem. Rev., 2007, 107, 2546 CrossRef; (m) M. C. Purrung, Chem.–Eur. J., 2006, 12, 1312 CrossRef; (n) S. Otto and J. Engberts, Org. Biomol. Chem., 2005, 9, 585 Search PubMed.
- (a) J. P. Genet and M. J. Savignac, J. Organomet. Chem., 1999, 576, 305 CrossRef; (b) Green Catalysis, vol. 1: Homogeneous Catalysis, ed. P. T. Anastas, Wiley-VCH, New York, 2009 Search PubMed; for other metal catalysed protocols, see: (c) M. C. Willis, D. Taylor and A. T. Gillmore, Org. Lett., 2004, 6, 4755 CrossRef CAS; (d) R. Sanz, M. P. Castroviejo, Y. Fernandez and F. J. Fananas, J. Org. Chem., 2005, 70, 6548 CrossRef CAS; (e) I. Nakamura, Y. Mizushima and Y. Yamamoto, J. Am. Chem. Soc., 2005, 127, 15022 CrossRef CAS; (f) N. B. Richard and G. C. Anthony, Chem. Rev., 2010, 110, 6302 CrossRef.
- A. W. Hofmann, Ber. Dtsch. Chem. Ges., 1887, 20, 1788 CrossRef.
- (a) H. Hugerschoff, Ber. Dtsch. Chem. Ges., 1901, 34, 3130 CrossRef; (b) H. Hugerschoff, Ber. Dtsch. Chem. Ges., 1903, 36, 3121 CrossRef; (c) J. M. Sprague and A. H. Land, in Heterocyclic Compounds, ed. R. C. Elderfield, J.Wiley, New York, 1957, vol. 5, ch. 8, pp. 484–721 Search PubMed.
- (a) A. C. Gyorkos, C. P. Corrette, S. Y. Cho, T. M. Turner, S. A. Pratt, K. Aso, M. Kori and M. Gyoten, World. Pat., 044793, 2005 Search PubMed(Chem. Abstr., 2005, 142, 482); (b) A. D. Jordan, C. Luo and A. B. Reitz, J. Org. Chem., 2003, 68, 8693 CrossRef CAS; (c) A. R. Katritzky, D. O. Tymoshenko, D. Monteux, V. Vvedensky, G. Nikonov, C. B. Cooper and M. Deshpande, J. Org. Chem., 2000, 65, 8059 CrossRef CAS; (d) R. A. Glennon, J. J. Gaines and M. E. Rogers, J. Med. Chem., 1981, 24, 766 CrossRef CAS.
- (a) C. Beaulieu, Z. Wang, D. Denis, G. Greig, S. Lamontagne, G. O'Neill, D. Slipetz and J. Wang, Bioorg. Med. Chem. Lett., 2004, 14, 3195 CrossRef CAS; (b) A. Kling, G. Backfisch, J. Delzer, H. Geneste, C. Graef, W. Hornberger, U. Lange, A. Lauterbach, W. Seitz and T. Subkowski, Bioorg. Med. Chem., 2003, 11, 1319 CrossRef CAS; (c) F. Janssens, J. Torremans, M. Janssen, R. A. Stokbroekx, M. Luyckx and P. A. Janssen, J. Med. Chem., 1985, 28, 1925 CrossRef CAS; (d) J. Van Heusden, R. Van Ginckel, H. Bruwiere, P. Moelans, B. Janssen, W. Floren, B. J. Van der Leede, J. Van Dun, G. Sanz, M. Venet, L. Dillen, C. Van Hove, G. Willemsens, M. Janicot and W. Wouters, Br. J. Cancer, 2002, 86, 605 CrossRef CAS; (e) M. S. Gomaa, J. L. Armstrong, B. Bobillon, G. J. Veal, A. Brancale, C. P. F. Redfern and C. Simons, Bioorg. Med. Chem., 2008, 16, 8301 CrossRef CAS; (f) R. S. Chopade, R. H. Bahekar, P. B. Khedekar, K. P. Bhusari and A. R. R. Rao, Arch. Pharm., 2002, 335, 381 CrossRef CAS; (g) P. Yogeeswari, D. Srisam, L. Suniljit, S. Kumar and J. Stables, Eur. J. Med. Chem., 2002, 37, 231 CrossRef CAS; (h) P. Yogeeswari, D. Sriram, S. Mehta, D. Nigam, M. Mohan Kumar, S. Murugesan and J. Stables, Farmaco, 2005, 60, 1 CrossRef CAS; (i) N. Siddiqui, S. Pandeya, S. Khan, J. Stables, A. Rana, M. Alam, M. Arshad and M. Bhat, Bioorg. Med. Chem. Lett., 2007, 17, 255 CrossRef CAS; (j) N. Siddiqui, A. Rana, S. Khan, M. Bhat and S. Haque, Bioorg. Med. Chem. Lett., 2007, 17, 4178 CrossRef CAS; (k) H. Suter and H. Zutter, Helv. Chim. Acta, 1967, 50, 1084 CrossRef CAS; (l) S. J. Hays, M. J. Rice, D. F. Ortwine, G. Johnson, R. D. Schwartz, D. K. Boyd, L. F. Copeland, M. G. Vartanian and P. A. Boxer, J. Pharm. Sci., 1994, 83, 1425 CrossRef CAS; (m) Y. Hey, A. Benz, T. Fu, M. Wang, D. F. Covey, C. F. Zorumski and S. Mennick, Neuropharmacology, 2002, 42, 199 CrossRef.
- P. Jimonet, F. Audiau, M. Barreau, J. C. Blanchard, J. M. Stutzmann and S. Mignani, J. Med. Chem., 1999, 42, 2828 CrossRef CAS.
- (a) C. Liu, J. Lin, S. Pitt, R. F. Zhang, J. S. Sack, S. E. Kiefer, K. Kish, A. M. Doweyko, H. Zhang, P. H. Marathe, J. Trzaskos, M. Mckinnon, J. H. Dodd, J. C. Barrish, G. L. Schieven and K. Leftheris, Bioorg. Med. Chem. Lett., 2008, 18, 1874 CrossRef CAS; (b) K. Takagi, Chem. Lett., 1986, 265 CrossRef CAS.
- T. Soneda, H. Takeshita, Y. Kagoshima, Y. Yamamoto, T. Hosokawa, T. Konosu, N. Masuda, T. Uchida, I. Achiwa, J. Kuroyanagi, T. Fujisawa, A. Yokomizo and T. Noguchi, World Pat., WO 2009084614, 2009.
- A. Itai, S. Muto, R. Tokuyama, H. Fukazawa, T. Ohara and T. Kato, World Pat., WO 2007023882, 2007.
- PPAR = peroxisome proliferator-activated receptor: L. A. Black, M. D. Cowart, G. A. Gfesser, B. D. Wakefield, R. J. Altenbach, H. Liu, C. Zhao and G. C. Hsieh, World Pat., WO 2009085945, 2009.
- A. Tehim, B. Herbert, T. M. Nguyen, W. Xie and C. M. Gauss, World Pat., WO 2004029050, 2004.
- Z.-G. Le, J.-P. Xu, H.-Y. Rao and M. Ying, J. Heterocycl. Chem., 2006, 43, 1123 CrossRef CAS.
- (a) T. Suzuki, S. Igari, A. Hirasawa, M. Hata, M. Ishiguro, H. Fujieda, Y. Itoh, T. Hirano, H. Nakagawa, M. Ogura, M. Makishima, G. Tsujimoto and N. Miyata, J. Med. Chem., 2008, 51, 7640 CrossRef CAS; (b) H. F. Motiwala, R. Kumar and A. K. Chakraborti, Aust. J. Chem., 2007, 60, 369 CrossRef CAS; (c) F. Delmas, A. Avellaneda, C. Di Giorgio, M. Robin, E. De Clercq, P. Timon-David and J. P. Galy, Eur. J. Med. Chem., 2004, 39, 685 CrossRef CAS; (d) J. Das, R. V. Moquin, J. Lin, C. J. Liu, A. M. Doweyko, H. F. DeFex, Q. Fang, S. Pang, S. Pitt, D. R. Shen, G. L. Schieven, J. C. Barrish and J. Wityak, Bioorg. Med. Chem. Lett., 2003, 13, 2587 CrossRef CAS; (e) H. Tanikawa, K. Ishii, S. Kubota, S. Yagai, A. Kitamura and T. Karatsu, Tetrahedron Lett., 2008, 49, 3444 CrossRef CAS.
- (a) Z. Li, S. X. Xiao, G. Q. Tian, A. G. Zhu, X. Feng and J. Liu, Phosphorus, Sulfur Silicon Relat. Elem., 2008, 183, 1124 CrossRef CAS; (b) Q. L. Shen, T. Ogata and J. F. Hartwig, J. Am. Chem. Soc., 2008, 130, 6586 CrossRef CAS; (c) J. J. Yin, M. M. Zhao, M. A. Huffman and J. M. McNamara, Org. Lett., 2002, 4, 3481 CrossRef CAS.
- (a) D. Fajkusova and P. Pazdera, Synthesis, 2008, 1297 CrossRef CAS; (b) R. Yella and B. K. Patel, J. Comb. Chem., 2010, 12, 754 CrossRef CAS; (c) X. Zhang, X. Jia, J. Wang and X. Fan, Green Chem., 2011, 13, 413 RSC.
- (a) J.-W. Qiu, X. G. Zhang, R. Y. Tang, P. Zhong and J. H. Li, Adv. Synth. Catal., 2009, 351, 2319 CrossRef CAS; (b) Q. Ding, B. Cao, X. Liu, Z. Zong and Y. Peng, Green Chem., 2010, 12, 1607 RSC.
- (a) C. Benedi, F. Bravo, P. Uriz, E. Fernandez, C. Claver and S. Castillon, Tetrahedron Lett., 2003, 44, 6073 CrossRef CAS; (b) L. L. Joyce, G. Evindar and R. A. Batey, Chem. Commun., 2004, 446 RSC; (c) G. Evindar and R. A. Batey, J. Org. Chem., 2006, 71, 1802 CrossRef CAS; (d) J.-K. Wang, F. Peng, J.-L. Jiang, Z.-J. Lu, L.-Y. Wang, J. F. Bai and Y. Pan, Tetrahedron Lett., 2008, 49, 467 CrossRef CAS; (e) Q.-P. Ding, X.-D. He and J. Wu, J. Comb. Chem., 2009, 11, 587 CrossRef CAS; (f) G. D. Shen, X. Lv and W. L. Bao, Eur. J. Org. Chem., 2009, 5897 CrossRef CAS; (g) Y.-J. Guo, R.-Y. Tang, P. Zhong and J.-H. Li, Tetrahedron Lett., 2010, 51, 649 CrossRef CAS; (h) J. Yang, P. Li and L. Wang, Tetrahedron, 2011, 67, 5543 CrossRef CAS; (i) P. Saha, T. Ramanna, N. Purkait, M. A. Ali, R. Paul and T. Punniyamurthy, J. Org. Chem., 2009, 74, 8719 CrossRef CAS; (j) E. A. Jaseer, D. J. C. Prasad, A. Dandapat and G. Sekar, Tetrahedron Lett., 2010, 51, 5009 CrossRef CAS; (k) S. K. Rout, S. Guin, J. Nath and B. K. Patel, Green Chem., 2012, 14, 2491 RSC.
- (a) E. Feng, H. Huang, Y. Zhou, D. Ye, H. Jiang and H. Liu, J. Comb. Chem., 2010, 12, 422 CrossRef CAS; (b) R. Cano, D. J. Ramon and M. Yus, J. Org. Chem., 2011, 76, 654 CrossRef CAS.
- W. Leitner, Top. Curr. Chem., 1999, 206, 107 CrossRef.
- (a) D. P. Curran, Pure Appl. Chem., 2000, 72, 1649 CrossRef CAS; (b) C. S. Consorti, M. Jurisch and J. A. Gladysz, Org. Lett., 2007, 9, 2309 CrossRef CAS; (c) I. Ryu, H. Matsubara, S. Yasuda, H. Nakamura and D. P. Curran, J. Am. Chem. Soc., 2002, 124, 12946 CrossRef CAS.
- (a) T. Welton, Chem. Rev., 1999, 99, 2071 CrossRef CAS; (b) Ionic Liquid: A Green Solvent for Organic Transformations Ionic Liquids in Organic Synthesis , S. L. Chen, G. L. Chua, S. J. Ji and T. P. Loh, ch. 13, pp 161–176, ch. 14, pp. 177–193 Search PubMed.
- (a) C.-J. Li and L. Chen, Chem. Soc. Rev., 2006, 35, 68 RSC; (b) Organic Reactions in Water, ed. U. M. Lindström, Blackwell, Oxford, UK, 2007 Search PubMed; (c) C. I. Herrerıas, X. Yao, Z. Li and C.-J. Li, Chem. Rev., 2007, 107, 2546 CrossRef; (d) D. G. Cruz, D. Tejedor, P. Arms, E. Q. Morales and E. G. -Tellado, Chem. Commun., 2006, 2978 Search PubMed; (e) Y. Hayashi, Angew. Chem., Int. Ed., 2006, 45, 8103 CrossRef CAS; (f) D. G. Blackmond, A. Armstrong, V. Coombe and A. Wells, Angew. Chem., Int. Ed., 2007, 46, 3798 CrossRef CAS; (g) N. Shapiro and A. Vigalok, Angew. Chem., 2008, 120, 2891 CrossRef; (h) Y. Jung and R. A. Marcus, J. Am. Chem. Soc., 2007, 129, 5492 CrossRef CAS; (i) M. Raj and V. K. Sing, Chem. Commun., 2009, 6687 RSC.
- (a) S. Murru, P. Mondal, R. Yella and B. K. Patel, Eur. J. Org. Chem., 2009, 5407 Search PubMed; (b) S. Murru, B. K. Patel, J. L. Bras and J. Muzart, J. Org. Chem., 2009, 74, 2217 CrossRef CAS; (c) S. Murru, H. Ghosh, S. K. Sahoo and B. K. Patel, Org. Lett., 2009, 11, 4254 CrossRef CAS.
- R. Yella, S. Murru, A. R. Ali and B. K. Patel, Org. Biomol. Chem., 2010, 8, 3389 CAS.
- J. Zhao, Y. Zhao and H. Fu, Org. Lett., 2012, 14, 2710 CrossRef CAS.
- (a) A. Sharif, P. A. Lichtor and S. L. Buchwald, J. Am. Chem. Soc., 2007, 129, 3490 CrossRef; (b) S. K. Sahoo, L. Jamir, S. Guin and B. K. Patel, Adv. Synth. Catal., 2010, 352(14–15), 2538 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2ra21826g |
| This journal is © The Royal Society of Chemistry 2012 |