Silver colloidal pastes for dye analysis of reference and historical textile fibers using direct, extractionless, non-hydrolysis surface-enhanced Raman spectroscopy†
Ambra Idoneab,
Monica Gulminic,
Anne-Isabelle Henryd,
Francesca Casadioe,
Lauren Change,
Lorenzo Appoloniab,
Richard P. Van Duyned and
Nilam C. Shah*d
aUniversità degli Studi del Piemonte Orientale “A. Avogadro”, Dipartimento di Scienze e Innovazione Tecnologica, Via T. Michel 11, 15121 Alessandria, Italy. E-mail: ambra.idone@gmail.com
bRegione Autonoma Valle d'Aosta, Direzione Ricerca e Progetti Cofinanziati, Laboratorio Analisi Scientifiche, Fraz. Lillaz 7, 11020 Villair di Quart (AO), Italy. E-mail: l.appolonia@regione.vda.it
cUniversità degli Studi di Torino, Dipartimento di Chimica, Via P. Giuria 5, 10125 Torino, Italy. E-mail: monica.gulmini@unito.it
dDepartment of Chemistry, Northwestern University, 2145 Sheridan Road, Evanston, Illinois, USA. E-mail: nilamshah2010@u.northwestern.edu
eThe Art Institute of Chicago, 111 S. Michigan Ave., Chicago, Illinois, USA. E-mail: fcasadio@artic.edu
First published on 26th July 2013
Abstract
Surface-enhanced Raman spectroscopy (SERS) is an ideal tool for analyzing dyes on historical textiles because it requires very little sample compared to other available analytical methods and analysis can be done directly on the fiber. This paper reports on the first systematic study of the use of citrate-reduced silver colloidal pastes for the direct, extractionless, non-hydrolysis detection of dyes directly on wool, silk, cotton, and flax fibers. This type of study provides greater insight into the optimal conditions required for accurate analysis of dyes in historical samples. In this work, Ag colloidal pastes were characterized using localized surface plasmon resonance and scanning electron microscopy. The pastes were then employed for SERS analysis of twelve reference samples of different vegetal and animal fibers dyed with cochineal and eleven dyed with brazilwood. Furthermore, six historical textiles from an important collection of Mariano Fortuny (1871–1949) textiles at the Art Institute of Chicago were also examined, to test the efficacy of the paste on aged samples, and to shed light on Fortuny's fascinating production techniques. A mixture of cochineal and brazilwood was detected in some of the historical samples demonstrating, for the first time, simultaneous identification of these colorants used in combination. In addition, the findings give substance to the claim that Fortuny kept using natural dyes at a time when many new and attractive synthetic products became available.
Introduction
In recent years, surface-enhanced Raman spectroscopy (SERS) has emerged as a powerful alternative method for the study of organic colorants of historical and artistic significance. SERS is an ideal tool for probing dyes because it requires very little sample, dramatically quenches fluorescence and significantly enhances Raman signals of target analytes. It has been demonstrated that the SERS signals of ensemble-averaged molecules exhibit enhancements up to eight orders of magnitude over the normal Raman signal.1One important focus of SERS research is fabrication of SERS substrates that have high enhancement factors and are robust, stable, and reproducible. A variety of SERS substrates have been proposed for Cultural Heritage applications including metal films over nanospheres (FONs),2,3 nanoparticles obtained by in situ photo-reduction4 or laser ablation,5–7 and chemically reduced silver colloids.8–11 Among them, silver colloids remain the most popular substrates for art analysis.
Several different methods have been examined for fabricating reproducible colloids. These include reducing silver colloids with citrate,12 borohydride,13 or hydroxylamine14 as well as laser photoreduction of silver nitrate.4 These methods are popular for use in art analysis because they produce substrates that are simple to make, relatively inexpensive, and stable for some time. However, in many cases, SERS analysis requires extraction of the dyestuff.15 For example, Chen et al. extracted dyes from a silk textile and used an Ag–Al2O3 substrate for detection of anthraquinones.16 Leona et al. used hydrofluoric acid to hydrolyze the dye from the mordant and were able to detect alizarin from a sixteenth-century tapestry fiber sample.17 A different approach exploits active hydrogels that are applied directly on the work of art in order to extract minimal amounts of the dyes for SERS analysis.18–20
There are only a few published works dedicated to direct on-the-fiber analysis without the need of extraction or hydrolysis pre-treatments. On-the-fiber SERS can be very appropriate for valuable samples such as archaeological or historical textiles, because it strongly reduces the amount of sample required. The main issue for on-the-fiber application lies on the difficulty in coating the three-dimensional fibers with silver nanoparticles, in order to easily obtain spectra with identifiable peaks. Jurasekova et al. developed a method for on-the-fiber analysis using silver nanoparticles fabricated by laser photoreduction.4,21 This method was used to detect flavonoids in weld-dyed wool, silk textiles, and linen fibers. Recently, Brosseau et al. developed a method to perform on the fiber detection of dyes using silver nanoparticle colloidal pastes (Ag colloidal pastes). This method has proven to be very versatile and enabled the detection of highly fluorescing colorants from historical samples such as fibers, pastels, and watercolors.22,23
Although Ag colloidal pastes are promising for a variety of applications, a systematic study has not been performed to determine if Ag colloidal pastes can be used for the detection of dyes on different classes of fibers (such as wool, silk, cotton and flax). This type of study gives greater insight into the optimal conditions required for accurate analysis of dyes in historical fibers. Ag colloidal pastes are fabricated by centrifuging citrate-reduced silver colloids that are chemically synthesized using the Lee and Meisel procedure.12 In this study, Ag colloidal pastes were characterized using localized surface plasmon resonance (LSPR) and scanning electron microscopy (SEM). Then the pastes were employed for SERS analysis of four different types of fibers (wool, cotton, flax, and silk) and from historical aged fiber samples. More specifically, the Ag colloidal pastes were tested on a reference set of animal fibers (wool and silk) dyed with cochineal or brazilwood, as well as vegetable fibers (cotton and flax) tinted with brazilwood at various concentrations. Additionally, examples of historical textiles from an important collection of Mariano Fortuny textiles at the Art Institute of Chicago were examined, in order to compare the efficacy of the Ag colloidal pastes on newly dyed samples versus aged samples from historical textiles.
Innovation and creativity in historical textiles: the creations of Mariano Fortuny
Mariano Fortuny y de Madrazo (Granada, Spain, 1871–Venice, Italy 1949) was a veritable “Renaissance man” in early 20th Century Europe. Trained first as a painter, he worked in the Palazzo Pesara-Orfei in Venice (home today of the Fortuny Museum) creating one of a kind textiles and dresses with his lifelong companion Henriette Negrin and winning ardent admirers amongst the European elite. In 1919 Fortuny created an additional company, called Società Anonima Fortuny, which produced interior textiles, mostly made of cotton, with some mechanical printing.For fifty years Mariano Fortuny and Henriette Negrin continued to develop new and innovative techniques and after Henriette and Mariano passed away, his company along with his “secret” technology passed on to Countess Elsie Lee Gozzi, the American distributor of Fortuny's creations. Despite numerous patents, Fortuny's specific recipes and processes remain a mystery. From the literature, images of the studio, and extant textiles, equipment and pattern books, it is clear that Mariano and Henriette mixed their own dyes and dyed their own textiles, often in multiple and successive baths. Fortuny is also thought to have employed a wide variety of surface decoration techniques including color removal, direct printing, photo-lithography, stenciling, and a mixed method of painting and hand and screen printing. Blending of historical patterns and Fortuny's technical innovations result in textiles both reminiscent of the past and the contemporary. Today their production is still a guarded secret within the company. As a result, this artist, who has been called “the magician of Venice”,24 continues to interest and mesmerize textile historians, conservators, and enthusiasts through the beauty and complexity of his creations.
The Art Institute of Chicago houses an important collection of over 58 costumes and textiles produced by the various incarnations of Mariano Fortuny's creative enterprises. The collection of the Art Institute includes Delphos gowns (a hallmark production and symbol of refined extravagance, whose innovative mechanically produced pleated silk was patented in 1909), silk velvet coats, printed silk velvet panels, and several printed cotton lengths. A red cotton panel from the Società Anonima Fortuny production (AIC accession number 1987.375.2) is shown in Fig. 1. The technical process of creating the intricate, yet visually stunning decoration of these textiles is not well documented and has been little studied so far.
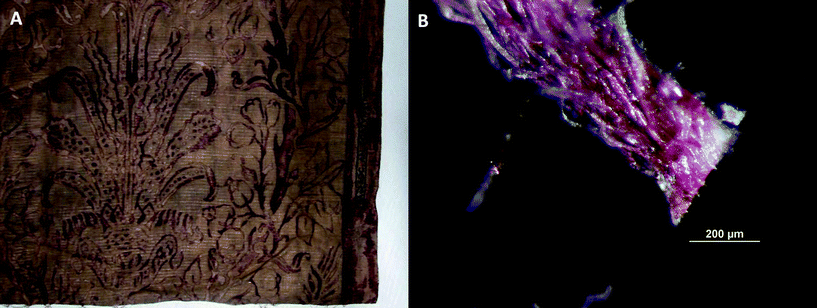 | ||
| Fig. 1 Red cotton panel from the Società Anonima Fortuny production, AIC accession number 1987.375.2; (A) detail of the panel and (B) microphotography of the fiber bundle sampled for SERS analysis. | ||
Relevant to this study is the fact that uncertainty still exists on whether the claim that Fortuny did not use synthetic dyes is true, at a time when the chemical industries were flooding the market with bright and attractive new industrial products. Only one previous example of analysis of Fortuny dyes is known.25 This study used absorption spectrophotometry and thin layer chromatography and identified indigo, brazilwood, cochineal, and a blend of yellow dyes, most likely old fustic with quercitron bark, on a limited number of samples. However, the techniques used do not provide unambiguous molecular information for identification of the colorants. In the present work, SERS is used for the direct molecular identification of colorants in selected examples from the Art Institute collection in order to shed further light on Fortuny's textiles.
Results and discussion
Localized surface plasmon resonance (LSPR) of Ag colloids and Ag colloidal pastes
Spectra showing LSPR of Ag colloids and Ag colloidal paste are reported as ESI (Fig. S1†). The LSPR of the Ag colloid is obtained in transmission mode and has a broad maximum absorption centered at 440 nm. The LSPR spectrum of the Ag colloidal paste is obtained in reflectance mode because it is an opaque sample and has a minimum centered at 514 nm. This shift in the LSPR is consistent with the fact that the Ag colloidal paste sample is concentrated with nanoparticles closer together as visualized at high magnification in the scanning electron microscope and described in detail below.Scanning electron microscopy (SEM) study of Ag colloids and Ag colloidal pastes
The Ag colloidal paste consists mostly of spherical nanoparticles, along with rods and even wires (aspect ratio comprised between 3 and 16). Spheres diameter ranges from 17 to 100 nm with an average diameter of 39 ± 17 nm (based on measuring the diameter of 40 particles). The length of the low axis of the rods was measured to range between 18 and 58 nm, while the long axis was measured to range between 143 and 457 nm.As expected, the composition in size and shape of the Ag colloid sample (not centrifuged) did not differ from the Ag colloidal paste sample. Only the nanoparticle concentration differed drastically. Representative SEM images of both samples taken at the same magnification are reported as ESI (Fig. S2†). The images clearly shows that the paste is much more concentrated than the initial colloidal solution and that randomly oriented nanoparticle superlattices are observed. It is also worth mentioning the presence of organic matter (the darker 100 nm spots) in the Ag colloid sample that is not observed in the paste. This is consistent with the fact that remaining surfactant in the colloidal solution is removed by collecting the supernatants in the several centrifugations during the paste preparation.
The micromorphology of the Ag nanoparticles coating was investigated by SEM imaging of fibers drop-coated with the Ag colloidal paste. Scanning electron micrographs of all four fibers coated with the Ag colloidal paste are shown in Fig. 2. Images reveal that the nanoparticle paste coverage shows some disparity from one fiber to another and on the same fiber. It should be emphasized that by using the word ‘paste’, it is only meant that the coverage of nanoparticles along the fibers is comparable to the one of a paste, not that the nanoparticles are coalesced and form themselves a paste. This can be seen more clearly in the higher magnification SEM images reported as ESI (Fig. S3†).
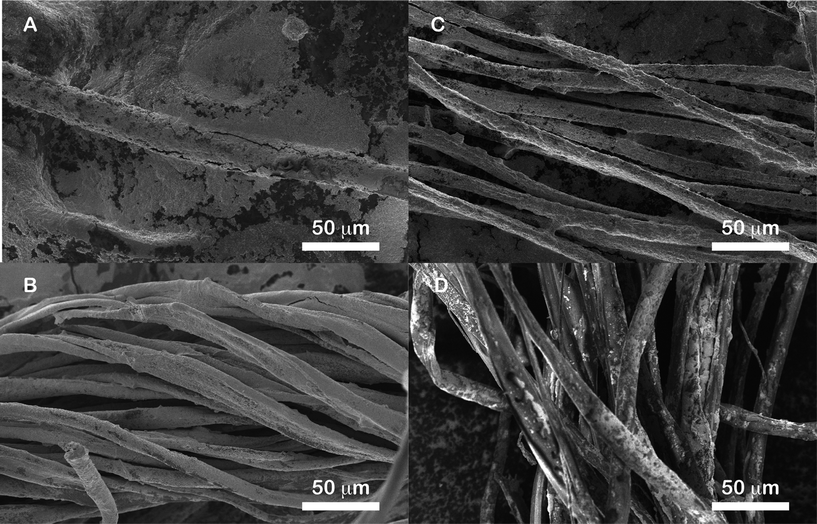 | ||
| Fig. 2 SEM micrographs of natural fibers coated with Ag colloidal paste: (A) wool, (B) silk, (C) cotton and (D) flax. | ||
The Ag colloidal paste forms a relatively dense nanoparticle coating around the wool fibers (Fig. 2A). High magnification observation of the samples (Fig. S3A†) allows confirmation of the load of nanoparticles on the fibers. In particular, what may appear as a smooth film at low magnification actually consists of thick multilayers of colloids whose shape and size are the same as the particles from the Ag colloidal paste. Along this smooth film though, sparse islands are observed. The thicker the colloid layer the better the coverage. At higher magnification (Fig. S3A†), it is clear that the structural integrity of the nanoparticles forming the paste is maintained. Thin covered areas on the wool sample consist of aggregated nanoparticles islands. The observation of islands and not film suggest some dewetting on the wool fiber surface. The coating of the silk fibers with the Ag colloidal paste (Fig. 2B) is observed to be excellent, both at low and high magnification (the latter shown in Fig. S3B†). Only very few bare areas were observed by SEM. The cotton samples (Fig. 2C) have excellent coverage both at low and high magnification (the latter shown in Fig. S3C†). In Fig. 2D charging in the SEM image of the flax fiber sample coated with the Ag colloidal paste is apparent, due to lower nanoparticle coverage. Because of charging effects, the flax fiber sample was re-imaged with an environmental SEM (ESEM) and is shown in Fig. 3A. The coverage of flax fibers differs drastically from the silk and cotton samples. Similar to the wool sample, at low and high magnification (the latter shown in Fig. S3D†) it can be seen that the Ag colloidal paste forms islands on the flax fiber whose size varies with thickness and may form film-like structure in areas with thickest coverage. While the majority of wool, cotton, and silk fibers were largely covered with Ag colloidal paste, nanoparticles coverage is low on the flax fiber with respect to the other types of fibers studied here.
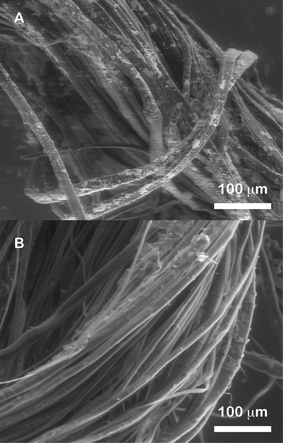 | ||
| Fig. 3 ESEM micrographs of flax fibers coated with (A) Ag colloidal paste and (B) Ag colloids. | ||
Furthermore, in order to compare the relative coating of fibers when using Ag colloids versus Ag colloidal paste, flax fiber samples were imaged with these two substrates. Due to relatively low coverage of nanoparticles, there was poor conductivity of the fibers resulting in images with high charging effects in the non-environmental SEM image. Therefore, the ESEM was used to image these samples as reported in Fig. 3A and B. Differences in the nanoparticle coverage are clearly observed between the Ag colloidal paste and the Ag colloids. While islands of aggregated nanoparticles are observed on the Ag colloidal paste coated sample, none was observed on the Ag colloid coated sample. Comparison of the two samples illustrates the superiority of the Ag colloidal paste at coating the fibers.
SERS measurements on reference samples
The Ag colloidal paste was effective in enhancing the signals of the dye molecules of cochineal (main component carminic acid) and brazilwood (main components brazilin and brazilein). As expected, when the analysis was performed without Ag colloidal paste, intense fluorescence dominated the spectrum. On the other hand, spectra obtained employing the Ag colloidal paste showed intense peaks related to the dye molecules. The absorption spectra of the main dyeing molecules of cochineal and brazilwood26,27 and reflectance spectra of the dyed reference fiber samples28 (Fig. S4†) display main peaks in the green region (500–550 nm). Since a 633 nm laser was employed in the present study, the enhanced signal is not attributed to resonance effects. Moreover, when employing Ag colloids prior to centrifugation the fiber is very poorly coated (Fig. 3B) and it is very difficult to obtain Raman signals.Fig. S5† shows the spectrum of the Ag colloidal paste with peaks at about 230, 690, 730, 810, 930, 1045, 1300, 1350, 1400 and 1470 cm−1; the intensities of these spurious signals are generally much lower than those of Raman signals arising from the dyes.
Fig. 4 to 6 show selected spectra that are representative of the many SER spectra obtained from the reference samples of dyed fibers coated with the Ag colloidal paste substrate. SER spectra obtained from wool and silk samples dyed with cochineal (Fig. 4) show a very strong peak at 1300 cm−1 and a less intense one, occasionally observed as a shoulder, at about 1225 cm−1. Other signals can be found at 460 (m) 1083 (w), 1425 (m), 1515 (w), 1630 (w) cm−1. All of them can be attributed to cochineal and to its major dyeing component, carminic acid.9,17,29 In particular, the intense peak at 1300 cm−1 is assigned to C–OH bending modes and it includes to a certain extent the contribution of the citrate used to prepare the nanoplasmonic substrate.
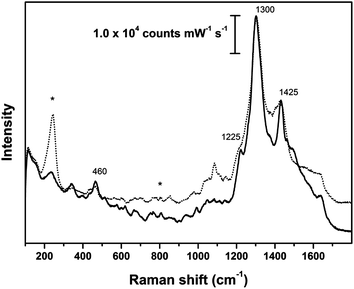 | ||
| Fig. 4 SER spectra of reference animal fibers dyed intense red with cochineal; dotted line: wool fleece; solid line: silk cloth; asterisks indicate peaks arising from Ag colloidal paste. SER spectra obtained using λex = 633 nm. | ||
The main peaks recorded for samples of both animal and vegetal fibers dyed with brazilwood (Fig. 5 and 6) are at 460, 1350 and 1560 cm−1; weaker signals were detected at about 1020, 1090, 1160, 1190, 1310, 1495 cm−1, together with weak peaks in the fingerprint region between 500 and 900 cm−1. These peaks can be attributed to the main dye components of brazilwood (brazilin and brazilein), whose Raman bands have been fully described by Edwards et al.30 and de Oliveira et al.31 As remarked by Bruni et al.,9 the intense peak at about 460 cm−1 is due to ring deformation. The peak centered at 1350 cm−1 superimposes to the signal from the citrate. The wool sample dyed with brazilwood has peaks that are slightly shifted.
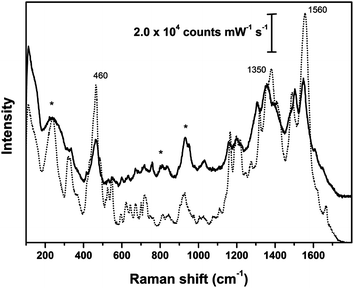 | ||
| Fig. 5 SER spectra of reference animal fibers dyed with brazilwood; solid line: silk cloth; dotted line: wool fleece; asterisks indicate peaks arising from Ag colloidal paste. SER spectra obtained using λex = 633 nm. | ||
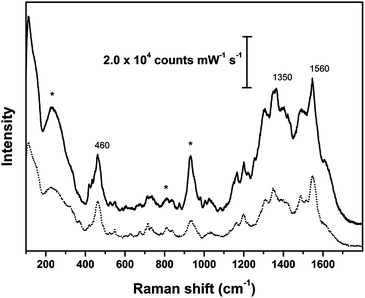 | ||
| Fig. 6 SER spectra of reference vegetal fibers dyed with brazilwood; solid line: cotton cloth; dotted line: flax cloth; asterisks indicate peaks arising from Ag colloidal paste. SER spectra obtained using λex = 633 nm. | ||
In general, SER spectra collected on the same sample are reproducible in terms of position of the peaks, while the relative intensity of the signals varies. This intensity variation is possibly due to the proximity of the dye near the SERS substrate or a varying concentration of dye and Ag colloidal paste at different locations on the fiber. In particular, the most intense signals were obtained from areas that appear coated by a thin film of silver nanoparticles when observed under the optical microscope coupled to the Raman spectrometer, whereas poor signals were obtained from islands showing a thicker coating. Besides the presence of spurious signals from the Ag colloidal paste substrate, the spectra obtained from the Ag-coated fibers (Fig. 4–6) do not show additional peaks that can be attributed to the proteinaceous or glycosidic fiber substrate. In addition, this study confirms that the substrate does not affect the position of the diagnostic peaks for the considered dyes, as previously highlighted by Jurasekova et al.21 for wool and flax dyed with madder or cochineal investigated through SERS employing Ag nanoparticles obtained by laser photoreduction. Furthermore, the Ag colloidal paste substrate is able to detect dye even in weakly dyed fibers. In fact, good SER spectra of light red samples dyed with cochineal in a bath previously used to dye other samples were successfully collected. As expected, the signal of the dye was less intense than those recorded for reference samples with higher color saturation. Fig. S6 in the ESI† shows some of the SER spectra obtained from light red reference samples.
SERS investigation of Fortuny samples
The SER spectra of Fortuny samples were recorded on fragments of fibers coated with Ag colloidal pastes and are shown in Fig. 7 and 8 and Fig. S7–S9 in the ESI.† In order to illustrate the variations that characterize SER spectra obtained with Ag colloidal pastes from historical textiles, each figure reports three spectra acquired from one sample.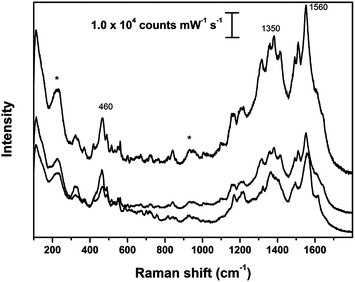 | ||
| Fig. 7 SER spectra obtained on sample 1978.174; asterisks indicate peaks arising from Ag colloidal paste. SER spectra obtained using λex = 633 nm. | ||
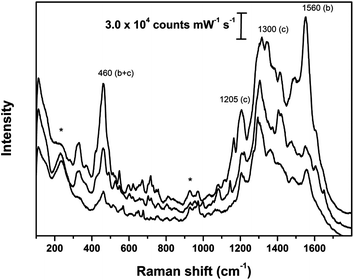 | ||
| Fig. 8 SER spectra obtained on sample 1987.375.2; asterisks indicate peaks arising from Ag colloidal paste; (b) and (c) indicate the most diagnostic peaks for brazilwood and cochineal, respectively. SER spectra obtained using λex = 633 nm. | ||
SER spectra of the sample of purplish-red silk (1978.173e) generally have an intense peak at about 1300 cm−1 and a shoulder (appearing as a distinctive peak in some of the repeated measurements) at about 1225 cm−1 as shown in Fig. S7.† The spectra also show the presence of medium intensity peaks at about 460 and 1425 cm−1. These peaks are attributed to carminic acid therefore the dyestuff employed to color this purplish-red sample can be identified as cochineal. The spectra obtained from this sample are generally reproducible, while the peak at 460 cm−1 remain sometimes undetected.
SER signals of the dye molecules were not detected from a very light pink pleated silk sample 1978.173f. Here, the lack of signal could possibly be a consequence of a very low concentration of dye. This sample is in fact much lighter than the lightest sample tested in the reference set. In this case, extraction of the coloring matter and analysis in solution as described by Bruni9 might be beneficial. However, the limited amount of sample prevented the possibility of performing further analytical attempts. On the other hand, pre-treatment with HF to release dye molecules from the complex used for mordanting would not be effective as it has been demonstrated to hydrolyze silk fibers.32
The spectra obtained from the sample of coral printed silk velvet (1978.174) display bands that can be attributed to the presence of brazilwood (Fig. 7). While variable features were observed in the region around the peak at 1350 cm−1, the relative intensity and shape of the major peaks at 460 and 1560 cm−1 were generally rather reproducible.
SER spectra recorded from the sample of a weft thread of the red cotton panel (1987.375.2) and the sample of silk pile of the red velvet panel (1986.47) are very similar although the intensity varies among the different measurements performed on the fibers belonging to the same sample (Fig. 8 and S8†). In general, the spectra show intense peaks at 460, 1300 or 1560 cm−1, while less intense signals can be detected at about 1020, 1080, 1090, 1160, 1190, 1205, 1350 and 1425 cm−1. Some of these peaks can be attributed to cochineal whose main component is carminic acid (e.g. 1080, 1205, 1300, 1425 cm−1) while the others arise from brazilwood (e.g. 1020, 1090, 1160, 1190, 1350, 1560 cm−1). The peak at about 460 cm−1 could be interpreted as a sum of contributions to the spectrum arising from ring deformations of the dye molecules deriving from both dyestuffs. To the best of the authors' knowledge, this is the first time that dyeing with multiple dyes have been detected on historical dyed fibers using on-the-fiber, extractionless SERS. Fig. 8 and S8† report three out of the many spectra which were collected from these two samples and show the variability of the spectral response for double-dyed samples.
SER spectra of the sample of cotton weft thread from a red panel (1985.681) show a very intense peak at about 1300 cm−1, medium intensity peaks at 460, 1205 and 1560 cm−1 and a weak one at 1080 cm−1 (Fig. S9†). Repeated SER measurements show in this case fairly reproducible spectra. Most of the signals can be attributed to cochineal. However, the presence of a peak at 1560 cm−1 and the intensity of the peak at 460 cm−1 suggest that brazilwood also contributes to the final hue of the sample.
Experimental
Materials
Hydrochloric acid, nitric acid, methanol, silver nitrate and sodium citrate were purchased from Carlo Erba reagents (Arese, Italy). Analytical grade carminic acid and chopped brazilwood (Caesalpinia sappan L.) were purchased from Sigma Aldrich (St. Louis, USA) and Kremer Pigments Inc. (New York, USA), respectively. Ultra High Quality (UHQ) water was obtained by a Millipore (Darmstadt, Germany) Direct-q 3 system.All the glassware employed was cleaned with a solution of aqua regia (HCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HNO3 3
HNO3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and then rinsed tenfold with deionized water, as previously specified in Chen et al.33 Methanol, followed by rinsing with deionized water was used to clean all the metallic tools.
1) and then rinsed tenfold with deionized water, as previously specified in Chen et al.33 Methanol, followed by rinsing with deionized water was used to clean all the metallic tools.
Instrumentation
Synthesis of Ag colloids and preparation of Ag colloidal paste
Citrate-reduced Ag colloids were synthesized by the procedure of Lee and Meisel.12 Briefly, 0.09 g of silver nitrate was added to deionized water and brought to a vigorous boil while magnetically stirring. Trisodium citrate (10 mL, 1%) was added and the solution was boiled for thirty minutes. The preparation of the Ag colloidal paste substrate was performed after cooling the Ag colloids at room temperature. More precisely, 1 mL aliquots of Ag colloids were centrifuged for 15 min (relative centrifugal force = 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), in order to concentrate the silver nanoparticles. Subsequent centrifugation cycles (10 times total) were performed after having discarded the supernatant and adding an additional 1 mL aliquot of Ag colloids into the centrifuge tube. This procedure resulted in approximately 10 mg of a dense Ag colloidal paste which was employed as a substrate for subsequent SERS analysis.
000), in order to concentrate the silver nanoparticles. Subsequent centrifugation cycles (10 times total) were performed after having discarded the supernatant and adding an additional 1 mL aliquot of Ag colloids into the centrifuge tube. This procedure resulted in approximately 10 mg of a dense Ag colloidal paste which was employed as a substrate for subsequent SERS analysis.
The Ag colloidal paste substrate was used to analyze reference dye solutions before being employed on textile fibers. In particular, a 10−3 mol L−1 solution of analytical grade carminic acid in methanol was used, while the dye components of brazilwood were obtained from a clear aqueous solution prepared by soaking approximately 0.5 g of chopped brazilwood in 50 mL of deionized water overnight, then boiling for one hour and filtering.
Reference samples
Reference samples were taken from three different sets of dyed wool fibers (flock or yarn) and one set of dyed silk, cotton and flax fibers. Each set of samples was dyed independently by different laboratories. In general, animal fibers (wool and silk) were dyed with cochineal (Dactylopius coccus Costa) or brazilwood (Caesalpinia sappan L.) using alum (KAl(SO4)2) and cream of tartar (KC4H5O6) as mordants; alum and sodium carbonate were instead employed for mordanting vegetal fibers (cotton and flax) before dyeing them with brazilwood. In some cases, the same dye-bath was subsequently used to dye new cloth, obtaining samples with decreasing color intensities, as shown in Fig. S10 reported in the ESI.† In this work, twelve reference samples dyed with cochineal (nine on wool and three on silk) and eleven samples dyed with brazilwood (seven on wool, two on silk, one on cotton and one on flax) were examined. Cochineal poorly works on vegetable fibers, therefore the reference set does not include samples of cotton or of flax dyed with this dyestuff.Fortuny textiles
Six samples were taken from the two productions of Fortuny's lifetime. Threads that were 1–2 mm long were cut from pink (AIC accession number 1978.173f) and purplish-red (AIC accession number 1978.173e) pleated silk swatches of fabric for Delphos gowns. Another sample was taken from the ground fabric of a coral printed silk velvet swatch (AIC accession number 1978.174). These three swatches were given to the museum by Countess Elsie Lee Gozzi, who also confirmed they were produced by Fortuny at the Palazzo Pesara-Orfei.34 A fourth sample was taken from the silk pile of a red velvet panel based on a 15th century Venetian design (AIC accession number 1986.47). This sample bears the Mariano Fortuny Deposé stamp of textiles produced at the Palazzo Pesara-Orfei in Venice. Two samples were also taken from cotton panels from the Società Anonima Fortuny production. The weft thread from a red panel almost entirely covered with printed metallic flake was analyzed (AIC accession number 1987.375.2, Fig. 1). The second sample was from the weft of another red panel printed with a 15th century Italian silk design (AIC accession number 1985.681). Both of these latter textiles have the Società Anonima Fortuny stamp in the selvage.Fiber preparation for SERS analyses
The reference dyed fibers were rinsed with methanol in order to completely remove any unmordanted dyes that possibly remained after the dyeing process; the samples were then dried in the laboratory hood prior to treatment with the Ag paste. The historical Fortuny samples were treated with Ag paste as they arrived in the laboratory. Small portions (about 0.5 mm in length) of a single fiber were cut with a scalpel and deposited on a pre-cleaned microscope slide. A 0.5 μL aliquot of the Ag colloidal paste was pipetted onto the fiber and gently brushed on the fiber with a golden wire. All the substrates employed for SER analysis were prepared the day before the measurement session. Raman investigation was performed the same day as sample slide preparation. A minimum of five measurements were performed on each sample, in order to check the reproducibility of the obtained spectra and account for possible inhomogeneity of the dyeing. Different portions of the same sample were analyzed on different days, thus employing Ag colloidal pastes obtained from different batches of colloid preparation to check reproducibility of results.Conclusions
Chemically reduced Ag colloids remain one of the most popular substrate for applications to art analysis. Centrifuging of colloids to make a thick paste has been found to simplify applications to works of art such as pigment particles or textile fibers. In this study, a systematic study of Ag colloidal pastes using LSPR, SEM, and SERS is conducted on a variety of animal and vegetable fibers dyed with two different natural dyes. The study sheds light on the extent and morphology of coverage of Ag colloidal pastes on different types of fibers. In addition, this work also demonstrates that it is possible to detect cochineal and brazilwood even with weakly colored fibers of both vegetable and animal origin using Ag colloidal pastes. The present SERS analysis of an important corpus of historical Fortuny textiles provided precise molecular identification of the red dyes used to tint their threads. In particular the exclusive use of natural colorants was confirmed for the samples examined, spanning the various creative enterprises of the Fortuny Empire throughout the history of the production. Furthermore, this was the first time that the direct, extractionless, non-hydrolysis SERS method was used to identify two dyes simultaneously on a historical sample. Cochineal and brazilwood were consistently found in both the silk velvets and cotton fibers that were analyzed. A skillful use of combinations of such dyes (possibly obtained through multiple baths) was clearly put in place to obtain a variety of different hues contributing to the enduring allure of such beautiful textiles.Acknowledgements
This work was carried out with the contribution of European Union, of the Regione Autonoma Valle d'Aosta and of the Italian Ministry of Labour and Social Policy. Support from the Andrew W. Mellon Foundation and the National Science Foundation through grants CHE-104182, CHE-0911145, and DMR-1121262 is also gratefully acknowledged. The RET program at Northwestern University, sponsored by the Materials Research Science and Engineering Center under NSF grant DMR 0520513, and its director, Prof. Monica Olvera de la Cruz are also gratefully acknowledged. The SEM work was performed in the EPIC facility of NUANCE Center at Northwestern University. NUANCE Center is supported by NSF-NSEC, NSF-MRSEC, Keck Foundation, the State of Illinois, and Northwestern University. Lisa Backus is thanked for preliminary SEM imaging.References
- B. Sharma, R. R. Frontiera, A.-I. Henry, E. Ringe and R. P. Van Duyne, Mater. Today, 2012, 15, 16 CrossRef CAS.
- N. G. Greeneltch, A. S. Davis, N. A. Valley, F. Casadio, G. C. Schatz, R. P. Van Duyne and N. C. Shah, J. Phys. Chem. A, 2012, 116, 11863 CrossRef CAS.
- A. V. Whitney, F. Casadio and R. P. Van Duyne, Appl. Spectrosc., 2007, 61, 994 CrossRef CAS.
- Z. Jurasekova, C. Domingo, J. V. Garcia-Ramos and S. Sanchez-Cortes, J. Raman Spectrosc., 2008, 39, 1309 CrossRef CAS.
- M. V. Cañamares, J. V. Garcia-Ramos, S. Sanchez-Cortes, M. Castillejo and M. Oujja, J. Colloid Interface Sci., 2008, 326, 103 CrossRef.
- M. Muniz-Miranda, C. Gellini and E. Giorgetti, J. Phys. Chem. C, 2011, 115, 5021 CAS.
- J. Neddersen, G. Chumanov and T. M. Cotton, Appl. Spectrosc., 1993, 47, 1959 CrossRef CAS.
- M. V. Cañamares, J. V. Garcia-Ramos, J. D. Gómez-Varga, C. Domingo and S. Sanchez-Cortes, Langmuir, 2005, 21, 8546 CrossRef.
- S. Bruni, V. Guglielmi and F. Pozzi, J. Raman Spectrosc., 2011, 42, 1267 CrossRef CAS.
- C. L. Brosseau, K. S. Rayner, F. Casadio, C. M. Grzywacz and R. P. Van Duyne, Anal. Chem., 2009, 81, 7443 CrossRef CAS.
- S. L. Kleinman, E. Ringe, N. Valley, K. L. Wustholz, E. Phillips, K. A. Scheidt, G. C. Schatz and R. P. Van Duyne, J. Am. Chem. Soc., 2011, 133, 4115 CrossRef CAS.
- P. C. Lee and D. Meisel, J. Phys. Chem., 1982, 86, 3391 CrossRef CAS.
- J. A. Creighton, C. G. Blatchford and M. G. Albrecht, J. Chem. Soc., Faraday Trans. 2, 1979, 75, 790 RSC.
- N. Leopold and B. Lendl, J. Phys. Chem. B, 2003, 107, 5723 CrossRef CAS.
- M. Leona, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 14757 CrossRef CAS.
- K. Chen, C. C. Vo-Dinh, F. Yan, M. B. Wabuyele and T. Vo-Dinh, Anal. Chim. Acta, 2006, 569, 234 CrossRef CAS.
- M. Leona, J. Stenger and E. Ferloni, J. Raman Spectrosc., 2006, 37, 981 CrossRef CAS.
- M. Leona, P. Decuzzi, T. A. Kubic, G. Gates and J. R. Lombardi, Anal. Chem., 2011, 83, 3990 CrossRef CAS.
- B. Doherty, B. G. Brunetti, A. Sgamellotti and C. Miliani, J. Raman Spectrosc., 2011, 42, 1932 CrossRef CAS.
- C. Lofrumento, M. Ricci, E. Platania, M. Becucci and E. Castellucci, J. Raman Spectrosc., 2013, 44, 47 CrossRef CAS.
- Z. Jurasekova, E. del Puerto, G. Bruno, J. V. Garcia-Ramos, S. Sanchez-Cortes and C. Domingo, J. Raman Spectrosc., 2010, 41, 1455 CrossRef.
- C. L. Brosseau, F. Casadio and R. P. Van Duyne, J. Raman Spectrosc., 2011, 42, 1305 CrossRef CAS.
- C. L. Brosseau, A. Gambardella, F. Casadio, C. M. Grzywacz, J. Wouters and R. P. Van Duyne, Anal. Chem., 2009, 81, 3056 CrossRef CAS.
- O. M. F. A. Kunst, Mariano Fortuny 1871–1949, Der Magier des Textilen Design, First, Museum fur Angewandte Kunst, 1985 Search PubMed.
- F. Pritchard, Dyes Hist. Archaeol., 2001, 16–17, 39 Search PubMed.
- G. Favaro, C. Miliani, A. Romani and M. Vagnini, J. Chem. Soc., Perkin Trans. 2, 2002, 1, 192 Search PubMed.
- K. Wongsooksin, S. Rattanaphani, M. Tangsathitkulchai, V. Rattanaphani and J. B. Bremner, Suranaree J. Sci. Technol., 2008, 15, 159 Search PubMed.
- M. Gulmini, A. Idone, E. Diana, D. Gastaldi, D. Vaudan and M. Aceto, Dyes Pigm., 2013, 98, 136 CrossRef CAS.
- M. V. Canamares, J. V. Garcia-Ramos, C. Domingo and S. Sanchez-Cortes, Vib. Spectrosc., 2006, 40, 161 CrossRef CAS.
- H. G. M. Edwards, L. F. C. de Oliveira and M. Nesbitt, Analyst, 2003, 128, 82 RSC.
- L. F. C. de Oliveira, H. G. M. Edwards, E. S. Velozo and M. Nesbitt, Vib. Spectrosc., 2002, 28, 243 CrossRef CAS.
- F. Pozzi, J. R. Lombardi, S. Bruni and M. Leona, Anal. Chem., 2012, 84, 3751 CrossRef CAS.
- K. Chen, M. Leona, K. C. Vo-Dinh, F. Yan, M. B. Wabuyele and T. Vo-Dinh, J. Raman Spectrosc., 2006, 37, 520 CrossRef CAS.
- Personal communication between Christa C. Mayer Thurman and Countess Elsie Lee Gozzi, letter from Countess Elsie Lee Gozzi, dated March 17, 1978, Archives of the Textile Department, the Art Institute of Chicago.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1: LSPR of Ag colloids recorded in transmission mode and of Ag colloidal paste recorded in reflectance mode. Fig. S2: SEM micrographs of Ag colloidal paste and Ag colloids. Fig. S3: SEM micrographs at high magnification of natural fibers coated with Ag colloidal paste: wool, silk, cotton and flax. Fig. S4: reflectance spectrum of a reference fiber (wool) dyed with cochineal and chemical structure of carminic acid; reflectance spectrum of a reference fiber (wool) dyed with brazilwood and chemical structures of brazilein and brazilin. Fig. S5: SER spectrum of Ag colloidal paste spread on a microscope slide. Fig. S6: SER spectra of reference animal fibers dyed light red with cochineal: wool fleece and silk cloth. Fig. S7: SER spectra obtained on sample 1978.173e. Fig. S8: SER spectra obtained on Fortuny sample 1986.47. Fig. S9: SER spectra obtained on sample 1985.681. Fig. S10: reference samples of wool and silk dyed with cochineal to obtain intense and light red. See DOI: 10.1039/c3an00788j |
| This journal is © The Royal Society of Chemistry 2013 |
