 Open Access Article
Open Access ArticleDevelopment and validation of a presumptive colour spot test method for the detection of piperazine analogues in seized illicit materials
Morgan Philpa,
Ronald Shimmona,
Natasha Stojanovskaa,
Mark Tahtouhb and
Shanlin Fu*a
aCentre for Forensic Science, School of Chemistry and Forensic Science, University of Technology, Sydney, (UTS), Ultimo, NSW 2007, Australia. E-mail: Shanlin.Fu@uts.edu.au; Fax: +61 2 9514 1460; Tel: +61 2 9514 8207
bAustralian Federal Police, 110 Goulburn St, Sydney, NSW 2000, Australia
First published on 30th July 2013
Abstract
The increasingly large quantities of potentially illicit samples received for confirmatory analysis highlights the importance and demand for preliminary testing procedures that are simple, rapid, selective, inexpensive and able to be used in the field. Colour testing fulfils the aforementioned requirements and is a technique frequently employed to achieve presumptive identification. Piperazine analogues (often marketed as ‘legal ecstasy’) are a group of psychoactive substances that have recently become established on the illicit drug market and are not effectively discriminated or identified by current colour testing methods. Herein, we report on the development and validation of a chemical spot test for piperazine analogues present in unknown seized materials using the spectrophotometric reagent, sodium 1,2-naphthoquinone-4-sulphonate (NQS). Primary testing revealed that NQS reacts almost instantly to form an intense, bright orange-red coloured complex with the representative piperazine 1-benzylpiperazine (BZP) at room temperature. The results of the test, assessed by colour development, were evaluated visually and variables affecting the coloured reaction were optimised. The colour test method was validated to meet requirements for use in preliminary screening, providing qualitative and reliable presumptive test results. Validation studies show that the characteristic colour change is unique to the piperazine class at room temperature, and is unaffected by the presence of common cutting agents, i.e. glucose and caffeine, in test samples of 5% purity, and other drugs such as N-methyl-3,4-methylenedioxyamphetamine (MDMA). The NQS reagent stability was found to be limited to storage in a refrigerated environment for no more than one week before results were affected. The operational limit of detection was found to be 40 μg.
1 Introduction
The most recent drug seizure data from the Australian Federal Police (AFP) for the period 2010–2011 reported a 316% increase in seized illicit drugs and precursors from the previous year.1 This statistic alone demonstrates the current prominent drug situation in Australia, while figures provided by the United Nations Office on Drugs and Crime (UNODC) highlight a similar growth pattern globally.2Synthetic piperazine analogues are newly established drugs on the market that have seen a remarkable increase in abuse worldwide owing to the ease of access afforded by internet availability.3 Piperazine analogues are central nervous system stimulants and many possess hallucinogenic properties. They are commonly used in combination with other piperazine analogues or illicit substances including MDMA, cocaine or ketamine.4
A number of piperazine analogues are currently not under international control. Many countries (including Australia) have introduced national controls to prevent the sale and distribution of 1-benzylpiperazine (BZP) in particular.5 Following the changing legal status of piperazine analogues, the number of synthetic drugs being produced and made available on the illicit drug market predictably increased to include piperazines that were not scheduled.6
Piperazine is a heterocyclic compound containing four carbon atoms and two nitrogen atoms at the 1,4 position (also called 1,4-hexahydropyrazine).7 Piperazine analogues can be divided into two classes (Scheme 1): phenylpiperazines (1) and benzylpiperazines (2). BZP is the most prevalent benzylpiperazine analogue while phenylpiperazine analogues include 1-[3-(trifluoromethyl)phenyl]piperazine (TFMPP), 1-(3-chlorophenyl)piperazine (mCPP), and 1-(4-methoxyphenyl)piperazine (MeOPP).8
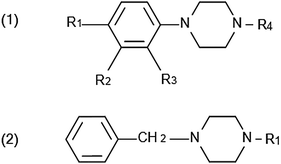 | ||
| Scheme 1 Chemical structures of phenylpiperazine (1) and benzylpiperazine (2) analogues. | ||
The confirmatory analysis of seized materials, and more specifically, piperazines, by techniques such as mass spectrometry (MS)9–12 and nuclear magnetic resonance (NMR) spectroscopy11,12 is well documented in the literature. However, due to the large amounts of unknown, seized materials received, confirmatory testing techniques can be time-consuming and costly.
Preliminary testing of seized materials is a vital first step in the identification of potentially illicit drugs. These preliminary tests combine techniques such as Fourier Transform Infrared spectroscopy (FTIR), Raman Spectroscopy and Thin Layer Chromatography (TLC) with presumptive colour testing methods. Presumptive colour tests are rapid, cheap, effective and, in contrast to FTIR and Raman, particularly useful in the detection of target analytes within mixtures. They can also be specific, require minimal sample preparation and able to be used by untrained personnel given a sequence of steps and colour charts. These colour tests are generally performed as chemical spot tests on a small sample of the unknown material and evaluated visually in white, porcelain spotting well plates, or commercially in polyethylene cartridges containing glass ampoules.
On-site analysis is becoming one of the most important fields of modern analytical chemistry, while preliminary screening in the laboratories is also becoming necessary due to the increasing number of samples being submitted.
The requirements for colour testing have been described in detail by Zolotov et al.13 These requirements include selectivity toward the analyte to be determined, high contrast and rate of colouring with the analyte, good reagent stability on storage, and a sufficiently stable analytical effect.
The need for research into novel chemical testing procedures governing piperazine analogues can be realised by looking at the unsuccessful results afforded by current preliminary testing methods. The typical colour screening tests used for on-site field testing by frontline personnel are not able to be used reliably or with any degree of specificity on piperazine analogues.9
The commonly encountered piperazine analogues BZP, TFMPP, mCPP, pCPP and MeOPP each possess one cyclo secondary amine functional group as part of their structure. For the purpose of developing a test that will react with each of these compounds, this secondary amine group is the likely target.
An estimation of amines using the spectrophotometric reagent NQS has previously been described in the literature in an ad-hoc manner.14–16 Dessouky and Ismaiel17 used the formation of a red coloured product with NQS in the detection of piperazine in pharmaceutical preparations. Supplementary to this, Cabeza et al.18 studied the reaction between NQS and primary or secondary amines in the presence of a non-ionic surfactant with favourable results.
The literature contains no reports of NQS use in the detection of illicit piperazine analogues such as BZP. This work aims to investigate, modify and develop the potential use of NQS as a colour test reagent in the detection of piperazine analogues. Method validation procedures were conducted for the proposed colour test by considering the reaction with other drugs of interest, reaction of common interferents, inherent sensitivity of the method, stability of reagents and coloured products, and the method's precision. The test exhibits excellent selectivity, sensitivity and repeatability towards piperazine analogues.
2 Experimental
2.1 Reagents and reference standards
Chemicals used in the development of a novel colour test method were sodium 1,2-naphthoquinone-4-sulphonate (NQS) and triton-X-100, both purchased from Sigma-Aldrich (Castle Hill, NSW, Australia), and sodium hydrogen carbonate (NaHCO3) and sodium hydroxide were supplied by Chem Supply (Gillman, SA, Australia).Working solutions of NQS at various concentrations were freshly prepared each day and stored in the absence of light in a refrigerator kept at 7 °C while not being used. A 0.1% (v/v) triton-X-100 working solution was also prepared. A NaHCO3 buffer solution was prepared by adjusting the pH of a 0.1 M NaHCO3 solution to 10.8 with 5 M NaOH.
In addition to the synthesis of BZP and 4-methylmethcathinone (4-MMC) in house, a large number of certified drug reference standards were obtained from the National Measurement Institute (NMI, North Ryde, NSW, Australia) through the AFP for a selectivity study. A list of these analytes can be found in the selectivity results section.
Caffeine, ephedrine hydrochloride, codeine phosphate, starch, glucose, sucrose and an extensive range of powdered substances were obtained from Ajax Chemicals (Sydney, NSW, Australia), BDH Chemicals (Sydney, NSW, Australia), Sigma Chemical Company (Perth, WA, Australia), Glaxo Australia (Sydney, NSW, Australia), Mallinckrodt (Lane Cove, NSW, Australia), Chem-Supply and Sigma-Aldrich. Plain flour, caster sugar, protein powder and artificial sweetener were from a local supermarket. A full list of these chemicals analysed can be found in the selectivity results section.
For colour tests on drugs in solution, working standard solutions of BZP HCl and 4-MMC HCl were prepared at a concentration of 500 μg mL−1 in distilled water. Working standard solutions of starch, glucose, caffeine, sucrose, codeine and ephedrine HCl were prepared at a concentration of 1000 μg mL−1 in distilled water.
2.2 Apparatus
Polypropylene flat bottom 96-well microplates were obtained from Greiner Bio-One and porcelain spotting well plates were supplied by the AFP laboratory. A Simmerstat Plain Top 240V AC from Industrial Equipment & Control Pty Ltd was used as the hot plate during colour test procedures. All pH measurements were carried out with a pH 211 Microprocessor from HANNA instruments. A digital CANON EFS 17-85 mm Single Lens Reflex (SLR) camera, DS126181, was employed to record all test results.2.3 Preparation of BZP HCl and 4-MMC HCl
BZP HCl was synthesised following the method outlined by Craig and Young.19 Equal molar piperazine hexahydrate and piperazine dihydrochloride monohydrate were dissolved (to form piperazine hydrochloride) in ethanol at 65 °C and then reacted with equal molar benzyl chloride for 25 minutes at 65 °C. The reaction solution was cooled on ice and the piperazine dihydrochloride monohydrate crystals were recovered by filtration. The filtrate was cooled on ice and treated with ethanolic HCl. BZP HCl was collected as a white precipitate. Its identity was confirmed by NMR, MS, and FTIR analysis. 1H NMR (500 MHz, CDCl3): δ 1.52 (1H, s), 2.41 (4H, t), 2.85 (4H, t), 3.49 (2H, s), 7.26 (5H, m) ppm. 13C NMR (125 MHz, CDCl3): δ 46.12, 54.54, 63.68, 126.94, 128.13, 129.17, 138.10 ppm. Electron impact MS: m/z 176 (M+, 16), 134 (60), 91 (100), 85 (10), 65 (14), 56 (23). IR νmax (cm−1): 3241, 2993, 2759, 1631, 1557, 1444, 1063.4-MMC HCl was synthesised in two stages following a modified method given by Camilleri et al.20 The first α-bromination step involved reacting 4-methylpropiophenone with excess bromine (to form 2-bromo-4-methylpropiophenone) in the presence of glacial acetic acid at 25 °C for 1 hour. The reaction solution was poured into ice-cold water and the 2-bromo-4-methylpropiophenone was extracted with dichloromethane and concentrated under vacuum to form yellow, fluffy crystals. The final methamination step involved combining equal molar NaOH and methylamine hydrochloride solutions. This solution was then added dropwise over 1 hour to a stirred solution of 2-bromo-4-methylpropiophenone in toluene. The mixture was allowed to stir for 32 hours at 25 °C and poured into ice-cold water. The toluene layer was separated and acidified with dilute HCl solution. The acidic extracts were washed with toluene before evaporating the aqueous layer to dryness to afford the crude 4-MMC HCl product as mottled light brown/brown coloured, flaky crystals. A fine, white powder was collected following recrystallisation from isopropanol. Its identity was confirmed by NMR, MS, and FTIR analysis. 1H NMR (500 MHz, D2O): δ 1.62 (3H, d), 2.46 (3H, s), 2.82 (3H, s), 5.08 (1H, q), 7.47 (2H, d), 7.94 (2H, d) ppm. 13C NMR (125 MHz, D2O): δ 18.3, 23.8, 33.8, 62.4, 131.9, 132.6, 132.8, 150.3, 200.2 ppm. Electron impact MS: m/z 177 (M+, 2), 119 (6), 91 (12), 65 (8), 58 (100), 56 (8). IR νmax (cm−1): 2912, 2740, 2453, 1686, 1605.
2.4 Colour spot test method development
| Test no. | Sample 500 μg mL−1 | NaHCO3 buffer | Triton-X-100 0.1% (v/v) | NQS 6.4 × 10−3 M |
|---|---|---|---|---|
| 1 | 0.5 mL | 0.5 mL | 0.5 mL | 1.0 mL |
| 2 | 1.0 mL | 0.3 mL | 0.2 mL | 1.0 mL |
| 3 | 1.0 mL | 0.4 mL | 0.1 mL | 1.0 mL |
| 4 | 1.0 mL | 0.1 mL | 0.4 mL | 1.0 mL |
Testing was performed on BZP HCl, 4-MMC HCl, ephedrine HCl, codeine, caffeine, glucose, sucrose and starch sample solutions alongside a control reagent blank.
2.5 Method validation
A series of tests were performed in order to determine the method's reliability. These tests were chosen based on the nature and purpose of the qualitative method.The developed test method was also performed in triplicate on different days, using different reagents and in different laboratories and by different operators. Certified reference samples were tested in triplicate at the AFP laboratory both intra-day and inter-day.
• Controlled drugs in the target group, i.e. piperazine analogues.
• Controlled drugs from other groups.
• Mixtures of piperazine analogues and other controlled drugs.
• Common precursors to illicit drugs.
• Common diluents/excipients in the matrix of seized drugs.
• Miscellaneous powdered substances and household tablets.
The drugs and other analytes were classified as drug standards, crystals, powders or tablets. Drug standards, crystals and powders were tested without further processing, and tablets were crushed into a fine powder using a mortar and pestle.
A small pin-head sized amount of each analyte to be tested was added to the well of a porcelain spotting plate, or plastic micro-well plate. The general recommended test procedure was applied. The final colours observed after the required two minutes standing time were recorded. Each analyte was tested in triplicate.
Methanolic solutions of BZP HCl at a concentration of 200 μg mL−1 and methanolic solutions of caffeine and glucose at 2000 μg mL−1 were prepared.
Aliquots of 100 μL of the drug standard solution were pipetted into eleven consecutive wells of a micro-well plate and the solvent was allowed to evaporate under the fume hood. To each of these wells was added, a specified aliquot of the methanolic caffeine solution: 0, 2.5, 5, 10, 15, 20, 25, 32.5, 50, 65, 100 and 200 μL (0–95% (w/w) impurity). The solvent was again left to evaporate. This was performed in triplicate. The developed NQS colour test procedure was applied to each well. The resulting colours were observed and recorded.
The process was repeated using the methanolic glucose solution in place of the caffeine solution.
A 200 μg mL−1 methanolic solution of BZP HCl was prepared. Five replicate aliquots of this solution of 0, 5, 10, 15, 20, 25, 30, 50, 100, 150, 200 and 250 μL were pipetted into a micro well plate and the solvent was allowed to evaporate under a fume hood. The general recommended test procedure was applied. The colours were observed and recorded. The lowest sample size producing a colour change noticeably different from that of the blank and characteristic for the target analyte for all five replicates was regarded as the limit of detection.
A small survey was undertaken asking seven volunteers to identify the sample size they considered to be the point at which a noticeable colour change was apparent. This survey was conducted over two days on two separate trials.
• Laboratory bench top
• Laboratory cupboard, covered in aluminium foil
• Refrigerator set at 7 °C, covered in aluminium foil
• Digital water bath set at 35 °C, sealed and covered in aluminium foil
Each reagent solution was employed in the developed colour testing procedure by applying to triplicate samples of the target analyte, BZP HCl, alongside a blank reagent weekly for up to three months. The colours produced in each test were observed and recorded.
3 Results and discussion
3.1 Colour spot test method development and optimisation study
 | ||
| Fig. 1 Preliminary NQS colour testing performed in test tubes for reagent blank (a) and BZP HCl sample solution (b). | ||
 | ||
| Fig. 2 Reduced scale NQS colour testing performed in plastic micro-well plates on control reagent blank (a) and BZP HCl sample solution (b) at room temperature. | ||
In contrast, results show that the reaction, and hence the colour change will not take place without the addition of the NaHCO3 buffer.
Seven common cutting agents, diluents and other white powdered drugs tested did not react with the NQS at room temperature, with the exception of ephedrine which reacted to produce a yellow-orange colour. This was the only sample that exhibited a possible interfering reaction; however, the colour produced was distinctly different from the orange-red product resulting with solid BZP HCl.
The NQS concentration at 2.0 × 10−3 M was chosen as the optimal concentration to afford an intense orange-red coloured product with BZP HCl, and a pale, light yellow coloured product with the reagent blank.
3.2 General recommended procedure
Optimum working conditions were selected by considering the influence of reaction parameters such as reaction time, pH, NQS concentration and temperature. Reaction times were selected based on general observations in order to provide for consistent results amongst tests.To a pin head sized amount of unknown powder sample:
1. Add 5 drops of 0.1 M NaHCO3–NaOH buffer solution, ensuring adequate mixing for a few seconds,
2. Followed by addition of 4 drops of 2.0 × 10−3 M NQS solution, and
3. Record the colour change after 2 minutes.
3.3 Evaluation of validation data
The reproducibility was determined by varying the conditions of testing. The test performance on different days, using different reagents and even in different laboratories with different heating apparatus and well plates showed no effect on the NQS colour test results, thus warranting the reproducibility of this method.
| Sample analyte | Spot test coloura |
|---|---|
| a NR describes a spot test whereby no reaction occurred and therefore no change in colour was observed. | |
| 1-Benzylpiperazine (BZP) HCl | Brilliant orange-red |
| 1-[3-(Trifluoromethyl)phenyl]piperazine (TFMPP) HCl | Orange-red |
| 1-(3-Chlorophenyl)piperazine (mCPP) HCl | Orange-red |
| 1-(4-Chlorophenyl)piperazine (pCPP) HCl | Orange-red |
| 1-(4-Methoxyphenyl)piperazine (MeOPP) HCl | Orange-red |
| Piperazine hexahydrate | Orange-red |
| 5-Methoxy-N,N-diallyltryptamine | NR |
| p-Fluorococaine HCl | NR |
| (−)-Methylephedrine HCl | NR |
| (2Sa,3Ra)-2-Methyl-3-[3,4-(methylenedioxy)phenyl]glycidic acid methyl ester | NR |
| (+)-Cathine HCl | Dark brown |
| Heroin HCl | NR |
| Cocaine HCl | NR |
| (+/−)-N-Methyl-3,4-methylenedioxyamphetamine (MDMA) HCl | Pink-orange |
| (+/−)-N-Ethyl-3,4-methylenedioxyamphetamine (MDEA) HCl | NR |
| (+/−)-3,4-MDA HCl | Light orange |
| (+/−)-N-Methyl-1-(3,4-methylenedioxyphenyl)-2-butylamine HCl | NR |
| Amphetamine sulphate | Light orange-yellow |
| (+/−)-Methamphetamine HCl | Light pink-orange |
| Gamma-hydroxy butyrate | NR |
| (−)-Ephedrine HCl | Light pink-orange |
| 4-Bromo-2,5-dimethoxyphenethylamine HCl | Orange |
| Phencyclidine HCl | NR |
| Phentermine | NR |
| Phenylpropanolamine (racemic mixture) | Brown |
| 3,4-Methylenedioxyphenyl-2-propanone (MDP2P) | Light brown swirls |
| Ketamine | NR |
| Dimethamphetamine (DMA) | NR |
| 2,5-Dimethoxy-4-propylthio-phenylethylamine | Light orange-pink |
| 2,5-Dimethoxy-4-iodophenylethylamine HCl | Orange-pink |
| Methylamine HCl | Light brown |
| 4-Methylpropiophenone | Light yellow |
| 2-Bromo-4-methylpropiophenone | Light yellow |
| Triethylamine | Light orange |
| 4-Fluoromethamphetamine | NR |
| 4-Fluoroamphetamine | Light yellow |
| 4-Methoxymethamphetamine (PMMA) | Light orange |
| 2,5-Dimethoxyamphetamine | Yellow-orange |
| 3,4-Dimethoxyamphetamine | Yellow-orange |
| 4-Methoxyamphetamine (PMA) | Orange-yellow |
| 4-Methylmethamphetamine | Light yellow-pink |
| 2-Fluoromethamphetamine | Light yellow-pink |
| 2-Fluoroamphetamine | Light yellow |
| 4-Hydroxyamphetamine | Brown-orange |
| 4-Bromo-2,5-dimethoxyamphetamine HCl | Orange |
| 2,5-Dimethoxy-4-methylamphetamine | Orange |
| 4-Methylmethcathinone (4-MMC) | NR |
| 3,4-Methylenedioxymethcathinone (3,4-MDMC) | NR |
| 3,4-Methylenedioxypyrovalerone (3,4-MPDV) | NR |
| 3,4-Dimethylmethcathinone (3,4-DMMC) | NR |
| 1-(3,4-Methylenedioxyphenyl)-2-(methylamino)butan-1-one | NR |
| 3-Fluoromethcathinone | NR |
| Ethylcathinone | NR |
| Cathinone HCl | Brown swirls |
| 4-Methoxymethcathinone | NR |
| N,N-Diethylcathinone HCl | NR |
| N,N-Dimethylcathinone HCl | NR |
| Methcathinone HCl | NR |
| 4-Methyl-α-pyrrolidinopropiophenone HCl | NR |
| Sample analyte | Spot test coloura |
|---|---|
| a NR describes a spot test whereby no reaction occurred and therefore no change in colour was observed. | |
| Caffeine | NR |
| Benzoic acid | NR |
| Paracetamol | NR |
| Ephedrine HCl | Yellow-orange |
| O-Acetylsalicylic acid | NR |
| Phenolphthalein | Brilliant pink |
| Codeine HCl | NR |
| Phenethylamine | Light orange |
| Ascorbic acid | Colourless |
| Boric acid | NR |
| Calcium chloride | NR |
| Levamisole | NR |
| Citric acid | NR |
| Dimethylsulfone | NR |
| Glucose | NR |
| Glycine | Dark brown |
| Lactose | NR |
| Mannitol | NR |
| Magnesium sulphate | NR |
| Potassium carbonate | NR |
| Sodium carbonate | NR |
| Caster sugar/icing sugar/brown sugar | NR |
| Sodium chloride | NR |
| Starch/cellulose | NR |
| Sucrose | NR |
| Tartaric acid | NR |
| Stearic acid | NR |
| Maltose | NR |
| Artificial sweetener | NR |
| Protein powder | Dark yellow |
| Self raising flour/plain flour | NR |
The selectivity of a colour test used in the preliminary identification of drugs is particularly important, as interferences from substances other than the analyte of interest may lead to false positives. Ideally, the NQS will only react with piperazine analogues, i.e. it is selective to piperazine analogues.
The application of the procedure to seven discrete aqueous samples of diluents, excipients and other drugs at room temperature showed no colour change from the reagent blank and therefore no reaction occurred within the two minute standing period. The potential of NQS as a colour test reagent for BZP specifically was realised.
The addition of heat, with a controlled water bath, to the colour testing of these particular substances had no effect on the reactions of starch, glucose, caffeine, sucrose and codeine which did not react and showed the same yellow colour as the reagent blank. Ephedrine and 4-MMC, however, reacted to produce a colour change of red-brown and brown, respectively (see Fig. 3). It should be noted that during heating, ephedrine initially produced a colour change to orange-yellow which subsequently darkened within a short time. The positive reactions with other substances allow for the potential application of the NQS reagent in their detection. It was decided to omit the heating stage and keep the reaction standing time as two minutes for testing in future in order to increase the selectivity.
 | ||
| Fig. 3 Small scale NQS colour testing with addition of heat performed in plastic micro-well plates on a blank control reagent (a), BZP HCl (b), ephedrine HCl (c), and 4-MMC HCl (d) sample solutions. | ||
The NQS reagent was found to produce an incredibly bright orange-red colour complex specific to BZP only. The five other piperazine analogues tested, TFMPP, mCPP, pCPP, MeOPP and piperazine, also produced orange-red colour. The results of BZP, TFMPP and mCPP alongside a reagent control blank can be seen in Fig. 4. Although not obvious in the image, the apparent brilliance of BZP made it distinguishable from the others upon visual examination.
 | ||
| Fig. 4 Selectivity results performed in white glazed porcelain spotting plates on blank control reagent (a) and piperazine analogues: BZP HCl (b), TFMPP HCl (c) and mCPP HCl (d). | ||
The bright, intense orange-red colour exhibited by the piperazine analogues was not produced by other analytes tested. This result is highly desirable for any colour test reagent as it shows the highly selective nature of the colour test. In addition, the specificity of the NQS colour test toward the single analyte, BZP, is also apparent by the uniquely bright colour as the result.
No common cutting agent, excipient or diluent was found to react with the NQS reagent at room temperature. This is a significant and important factor that can determine the practicality of a colour test when used for the purpose of identification of unknown solid sample mixtures. Two further tests involving multiple control substances were performed: a BZP and TFMPP mixed solid sample, and a BZP and MDMA solid mixture. Both of these samples turned to an orange-red colour, testing positive for the presence of piperazine and thus showing the potential for application of the test in case work.
A number of non-piperazine compounds reacted with the NQS reagent at room temperature to afford a range of dull colours. This difference in colour between reacting amines could be explained by the location of the amine group in piperazine analogues being in a cyclo group.
The NQS is known to react with the amino group of target analytes. As a result, the interference of endogenous amines remains a concern.
A given common sample matrix may contain endogenous or exogenous interfering substances, purity testing is carried out to determine the effects that these interfering substances have on the colour produced. Purity data for piperazine analogues in seized materials is not available in the Australian Crime Commission's latest Illicit Drug Data Report, nor can they be found in the UNODC's World Drug Report 2012.
 | ||
| Fig. 5 Results of limit of detection testing of BZP HCl using the NQS colour test method performed in plastic micro-well plates. Amounts used are 0, 1, 2, 4, 5, 7, 9, 10, 20, 30, 40 and 50 μg, respectively. | ||
The lowest concentration of BZP that afforded a colour change distinguishable from the background noise was determined to be 4 μg. This concentration was then multiplied by ten and recorded as the ‘operational drug detection limit’ at 40 μg.
It should be noted that LOD is not a particularly robust parameter and may be affected by minor changes in operational conditions. In addition, the subjective nature of the determination of the detection limit adds further room for inadequacies. In an attempt to add further validity to the proposed limits of detection, seven random participants were asked their opinion as to which concentration afforded the lowest detectable colour change. The results were in unanimous agreement with the proposed limits of detection.
For comparative purposes, the LOD of D-amphetamine HCl and D-methamphetamine HCl using the well established Marquis reagent is 20 μg and 100 μg, respectively.22 It follows that these determined limits are well within an acceptable range for use as colour test reagents.
Ruggedness is the ability to withstand small uncontrolled or unintentional changes in operating conditions and assesses the reproducibility of results obtained by analysis of the same samples under a variety of conditions.
The results of robustness testing on NQS showed that at each tested pH, with the exception of pH 14.1, the desired coloured product was observed. The time taken for development of this colour, however, varied. In the pH range of 9.0–11.0 the appropriate colour changes were developed within two minutes at room temperature, with the optimum pH level towards the end of the range. While between pH 11.1–13.3 the development time took from three minutes to six minutes. It was evident that the test method was significantly robust in regards to slight variations in pH.
The NQS reagent showed limited stability in regards to storage conditions and consequently, the storage time period. The NQS reagent has a light yellow colour when freshly prepared. Following storage in the 35 °C water bath for one week, the reagent itself turned to an intense brown colour and thus failed to produce coloured reactions with the target analytes. Similarly, the stored laboratory bench top and cupboard reagents developed dark brown precipitates after one month. After three weeks of storage in a refrigerator, the reagent had a dark orange colour and was no longer suitable for further monitoring. Colour tests were performed weekly on samples and control reagent blanks until the solution was considered unusable based on resulting colour changes.
The application of NQS as a colour test reagent would require the preparation of fresh test solutions weekly or just prior to use and be stored covered in the refrigerator in the meantime. This is not an ideal situation, however, preparation of this aqueous solution is considerably easy using distilled water only.
The stability of the BZP-NQS coloured product was demonstrated by examining the spotting well after a 24 hour standing period. The BZP-NQS complex remained slightly coloured and a red coloured precipitate crashed out of solution.
3.4 Proposed reaction mechanism
The influences of pH can be understood by investigation of the reaction mechanism involved. The buffer solution employed is alkaline to provide the means for turning the protonated amine salt (–NH2+) into an amino group (–NH). In general, a higher pH will more effectively remove this proton.The reaction of the primary amine salt, amantadine hydrochloride, in alkaline medium with NQS was described by Mahmoud. et al.15 In their proposed mechanism, the sulphonate group of the NQS is replaced by the amino group of the amine. This nucleophilic substitution reaction has also been proposed by Ali and Elbashir16 in their validated method for determining olanzapine using NQS at pH 13.
The effect of temperature on the reaction of NQS with BZP was studied by carrying out the reaction at different temperatures (25 °C–85 °C). It was found that the reaction of BZP with NQS was not affected by the increasing temperature; no changes in the coloured product were observed upon heating. The reaction at room temperature went to completion in two minutes. Application of the test to samples other than BZP led to the increase of reaction time to eight minutes at 85 °C to provide for a wider range of coloured reactions.
The chemical structure of the coloured product formed upon reaction between NQS and BZP has been proposed in Scheme 2. Similarly, the amino groups of those substances affording a colour change with the NQS test will be the functional group involved in the reaction. The highly coloured complexes produced through reaction with NQS are the result of a high degree of conjugation afforded in the newly prepared molecule. Full structural elucidation of the product is of interest for future studies.
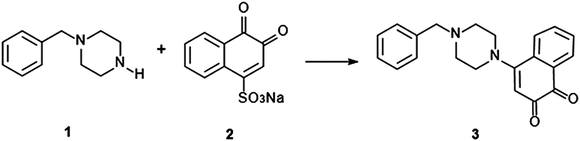 | ||
| Scheme 2 Scheme for the proposed reaction pathway of BZP (1) and NQS reagent (2) to form an orange-red product (3). | ||
4 Conclusions
Preliminary investigation on the use of NQS as a colour test reagent for piperazine analogues revealed that there exists great potential for further application. A bright, orange-red product formed during the reaction of NQS with BZP is not only distinctly different from the light yellow coloured reagent blank, but selectivity tests show that this characteristic colour change and colour intensity did not match with any other substance tested.The proposed colour test procedure fulfills laboratory screening colour test requirements, using small quantities of non-toxic reagents on limited amounts of solid samples, resulting in a characteristic colour change to orange-red within two minutes at room temperature in the presence of the piperazine analogues.
The suitability of NQS as a novel colour testing reagent for the purpose of preliminary detection of piperazine analogues was effectively evaluated through the examination of method validation parameters. The excellent selectivity of the NQS colour test toward piperazine analogues, combined with the lack of interference from the majority of analytes tested and its superior reproducibility, affords the reagent great potential for exploitation as a colour test reagent. The addition of heat provides the test with an even greater scope for identification of other reacting analytes. The results of the investigation allow for suggested additions to current preliminary testing workflows.
Future work would involve NQS reagent stability studies to investigate the means of suppressing the instability of the reagent without affecting its use as a colour test reagent. This would allow for its use in field testing kits.
Acknowledgements
The authors would like to thank Dr Sonia Taflaga, Australian Federal Police for helpful discussion during the study.Notes and references
- Australian Federal Police, Annual Report 2010-2011. Commonwealth of Australia, 2011, p. 71.
- UNODC, World Drug Report, United Nations Publication, New York, 2011 Search PubMed.
- M. D. Arbo, M. L. Bastos and H. F. Carmo, Drug Alcohol Depend., 2012, 122, 174–185 CrossRef CAS PubMed.
- WHO Secretariat, Essential Medicines and Health Products and Medicines Access and Rational Use Unit, Thirty-Fifth Meeting of the Expert Committee on Drug Dependence, Hammamet, 2012.
- UNODC, World Drug Report – The ATS Market, New York, 2011 Search PubMed.
- R. F. Staack, Lancet, 2007, 369, 1411–1413 CrossRef.
- WHO Secretariat, Essential Medicines and Health Products and Medicines Access and Rational Use Unit, Thirty-Fifth Meeting of the Expert Committee on Drug Dependence, Hammamet, 2012.
- Drug Enforcement Administration (DEA), Diversion Control Program, US Department of Justice DEA website, 2011.
- J. E. Aunan and R. A. Ely, Paper Presented at 9th Annual Clandestine Laboratory Investigating Chemists Association Technical Training Seminar, Toronto, Ontario, Canada, 1999.
- S. Davies, Drug monograph: analytical profiles of the piperazines; revised, London Toxicology Group, 2009 Search PubMed.
- F. Westphal, T. Junge, U. Girreser, S. Stobbe and S. B. Pérez, Forensic Sci. Int., 2009, 187, 87–96 CrossRef CAS PubMed.
- S. Davies, R. Archer and J. Ramsey, Drug monograph: 1-methyl-4-benzylpiperazine (MBZP), London Toxicology Group, 2009 Search PubMed.
- Y. A. Zolotov, V. M. Ivanov and V. G. Amelin, TrAC, Trends Anal. Chem., 2002, 21, 302–319 CrossRef CAS.
- D. H. Rosenblatt, P. Hlinka and J. Epstein, Anal. Chem., 1955, 27, 1290–1293 CrossRef CAS.
- A. M. Mahmoud, N. Y. Khalil, I. A. Darwish and T. Aboul-Fadl, Int. J. Anal. Chem., 2009, 2009, 1–8 Search PubMed.
- A. A. A. Ali and A. A. Elbashir, American Academic & Scholarly Research Journal, 2012, 4, 51–66 Search PubMed.
- Y. M. Dessouky and S. A. Ismaiel, Analyst, 1974, 99, 482–486 RSC.
- A. S. Cabeza, P. C. Falcó and C. M. Legua, Anal. Lett., 1994, 27, 1095–1108 CrossRef.
- J. C. Craig and R. J. Young, Org. Synth., 1973, 5, 88 Search PubMed.
- A. Camilleri, M. R. Johnston, M. Brennan, S. Davis and D. G. E. Caldicott, Forensic Sci. Int., 2010, 197, 59–66 CrossRef CAS PubMed.
- Clarke's Analysis of Drugs and Poisons, A. C. Moffat, M. D. Osselton, B. Widdop and J. Watts, Pharmaceutical Press, London, UK, 2011 Search PubMed.
- The Law Enforcement and Corrections Standards and Testing Program, National Institute of Justice, Washington, July 2000, vol. 0604, p. 01 Search PubMed.
- United States, Drug Enforcement Administration, SWGDRUG: Scientific Working Group for the Analysis of Seized Drugs Recommendations for: Education and Training, Quality Assurance, Methods of Analysis, U.S. Drug Enforcement Administration, 2011 Search PubMed.
| This journal is © The Royal Society of Chemistry 2013 |
