 Open Access Article
Open Access ArticleDetection of methamphetamine in indoor air using dynamic solid phase microextraction: a supplementary method to surface wipe sampling†
Elizabeth J. McKenzieab, Gordon M. Miskelly*a and Paul A. G. Butlerc
aForensic Science Programme, School of Chemical Sciences, The University of Auckland, Private Bag 92019, Auckland, New Zealand. E-mail: g.miskelly@auckland.ac.nz; Fax: +64-9-373-7422; Tel: +64-9-923-8338
bForensic & Industrial Science Ltd, PO Box 20103 Glen Eden, Auckland 0641, New Zealand
cSchool of Chemical Sciences, The University of Auckland, Private Bag 92019, Auckland, New Zealand
First published on 9th September 2013
Abstract
Surface wipe sampling for methamphetamine is a standard protocol in many jurisdictions for sampling at suspected or known former clandestine methamphetamine laboratories, but this method relies on samples being taken from representatively contaminated surfaces. We have investigated whether a rapid sampling method for airborne methamphetamine can be used to supplement surface sampling. A dynamic solid phase microextraction (SPME) field sampler was constructed and tested in the field and in the laboratory. This device enabled large volumes of air to be passed over SPME fibres exposed during the comparatively short time (<2 h) that a testing company might be present at a former clandestine laboratory. The collected samples were then analyzed by gas chromatography-mass spectrometry. Airborne methamphetamine was detected with this method at former clandestine methamphetamine laboratory sites where surface wipe sampling showed surface methamphetamine concentrations greater than 40 μg/100 cm2.
Introduction
The New Zealand Police have identified over 1700 clandestine amphetamine-type stimulant (ATS) laboratories between 1999–2011.1 Worldwide, 137![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 285 clandestine ATS laboratories were identified between 1999–2009, 96% of which were clandestine methamphetamine laboratories.2 The 2012 World Drug Report from the United Nations Office on Drugs and Crime states that 14
285 clandestine ATS laboratories were identified between 1999–2009, 96% of which were clandestine methamphetamine laboratories.2 The 2012 World Drug Report from the United Nations Office on Drugs and Crime states that 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 742 clandestine ATS laboratories were identified in 2009–2010, with 92% of these being clandestine methamphetamine laboratories and a further 6% either clandestine amphetamine or methamphetamine laboratories.3
742 clandestine ATS laboratories were identified in 2009–2010, with 92% of these being clandestine methamphetamine laboratories and a further 6% either clandestine amphetamine or methamphetamine laboratories.3In addition to the known problems associated with illicit methamphetamine production and use, the clandestine synthesis of methamphetamine presents risks to the public from exposure to the sites where it has been manufactured or waste material has been located. In many countries including the USA, New Zealand, and Australia, methamphetamine is often made on a small scale in makeshift facilities that can be located in residences, garages, or temporary accommodation such as hotels and motels. The previous presence of a clandestine laboratory may be known due to police searches, or the owner or occupant of a dwelling may be suspicious due to factors such as odours or stains within the dwelling or the behavior of the occupants at the suspected time of manufacture. Under these circumstances, it is important to establish whether manufacture of methamphetamine was likely to have occurred and the extent of any contamination due to this manufacture. However, it is also possible that heavy usage of methamphetamine can result in contamination of a structure. In many jurisdictions, measurement of the contamination at former clandestine laboratories is performed by taking surface wipes at selected locations throughout the structure, and then analyzing these for methamphetamine. The methamphetamine is determined both as a contaminant of concern and as a surrogate for other possible contaminants associated with clandestine manufacture.4 The acceptable level of methamphetamine contamination varies between jurisdictions, with some being based on an estimated health-based risk criterion5 while others are based on practical detection levels and the ability to decontaminate to those levels.6 This surface-wipe method, while pragmatic, suffers from some disadvantages such as not detecting the true extent of contamination if the substrates sampled are porous or if undetected pathways exist for mobilisation of the methamphetamine to or from other parts of the structure or the underlying substrate. For such reasons, jurisdictions such as the State of Minnesota have recommended the removal of all soft furnishings and wallpaper from premises that have been used for clandestine methamphetamine manufacture.7
Methods of methamphetamine manufacture favoured in New Zealand currently involve a pseudoephedrine/ephedrine precursor, iodine or hydriodic acid, and red phosphorus/phosphorous acid/hypophosphorous acid as a reducing agent. There can be up to four separate processes involved in this type of methamphetamine manufacture: precursor extraction, synthesis (‘cook’), methamphetamine extraction, and crystallisation (‘salt-out’). There is no information in the open literature on the emission of volatiles during the precursor extraction. During synthesis, little or no methamphetamine may be released,8,9 however iodine, unspecified acids, phosphine, 1-phenyl-2-propanone, 1,2 dimethyl-3-phenylaziridine, 1,3-dimethyl-2-phenylnaphthalene and 1-benzyl-3-methylnaphthalene have been detected.9 During extraction and crystallisation, iodine, hydrochloric acid and methamphetamine vapours can be released.8,9 The concentration of methamphetamine detected in air during extraction and salt-out is in the range 100–5000 μg m−3 (ref. 8 and 10) and these levels were reported to decrease to 70–210 μg m−3 the day after manufacture.10 Studies of remediated former clandestine laboratories 20–365 days after discovery report airborne methamphetamine in the range 0.1–1 μg m−3.11–13 This airborne material, together with spills and spatter, result in surface contamination within the structure being not just at the point of methamphetamine manufacture.
Methamphetamine is known to be stable under ambient conditions,14–20 however its long-term persistence within a structure has not been well-studied. The deposited methamphetamine acts as a reservoir for airborne methamphetamine, and this can result in elevated airborne concentrations if an activity disturbs the surfaces in the structure.10 Most airborne methamphetamine is present in the <1 μm fraction,10 well within the <10 μm limit for respirable particles. Characterisation of the distribution of airborne methamphetamine within the <1 μm size range has not been reported. Methamphetamine base is significantly more volatile than methamphetamine hydrochloride,21 and it is likely that it is the main airborne species present several months after manufacture. The determination of the concentration of airborne methamphetamine and the consequent estimation of methamphetamine exposure via inhalation can be used to test theoretical models5 that estimate inhalation exposure from surface-recoverable methamphetamine.
Previous determinations of methamphetamine vapour from powders, spills and spatter have employed direct ion mobility spectroscopy (IMS), however this method suffers from interference from nicotine, a common household contaminant, so that sample heating or derivatisation are required to discriminate methamphetamine by this method.22,23 IMS has also been used in combination with solid phase microextraction (SPME) for the analysis of methamphetamine from blood headspace.24 Dynamic planar SPME has been used with IMS for the analysis of headspace from ecstasy tablets at ppt levels and could potentially be used for the analysis of methamphetamine in indoor air.25 Amphetamines in exhaled breath have been determined by solid-phase extraction (SPE) followed by selected reaction monitoring (SRM) ultra-high pressure liquid chromatography (UPLC) tandem mass spectrometry (MS-MS).26 For methamphetamine in indoor air, trapping on acid-treated glass fibre filters coupled to an air sampling pump,10,27 with derivatisation and gas chromatography mass spectrometry (GC-MS) analysis,10 or liquid chromatography mass spectrometry (LC-MS) analysis of underivatised methamphetamine27 have been used.
Since, as noted above, it is possible that major sites of contamination within a dwelling might be missed if sampling was not undertaken on all surfaces, analysis of airborne methamphetamine has the potential to be used to supplement surface wipe sampling. While there are many standard methods for analyzing semivolatile compounds in air, many of these require extended sampling periods, such as 8 h or 24 h. In New Zealand, the evaluation of the degree of contamination in a known or suspected former clandestine laboratory is performed by commercial testing companies, that may only have access to a house for a limited time, and this testing may be done in the presence of owners or residents of the premises. For these reasons, a suitable sampling method for airborne methamphetamine needs to be rapid, representative, and sensitive.
SPME was introduced as a sample collection and introduction method by Pawliszyn in the early 1990s,28 and since then has been shown to be advantageous for analytes in both gaseous and condensed media. It allows rapid sample collection, does not use organic solvents, is easy to use, and is selective.29 SPME has been used with GC-MS for qualitative analysis of headspace vapors of surface wipes30,31 hair,32 and street methamphetamine33,34 but has not been evaluated for determination of methamphetamine in indoor air. Our preliminary studies showed that SPME met the criteria of sensitivity and rapidity, but in its passive mode SPME fibres could be exposed to varying volumes of air depending on air currents within the premises. Therefore, a portable dynamic SPME air sampler was developed that could be taken throughout a structure while air was drawn through it at a constant rate. The SPME fibres could then be returned to the laboratory for analysis by GC-MS. At all sites where this SPME sampling was conducted, wipe samples were also collected and then analyzed for methamphetamine, to allow comparison of these two forms of analysis.
Experimental
Dynamic SPME field sampler
A diagram and photograph of the dynamic SPME field sampler prototype are shown in Fig. 1 and 2.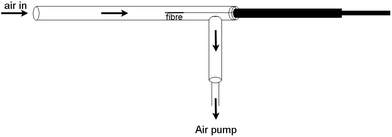 | ||
| Fig. 1 Diagram of the dynamic SPME field sampler. | ||
 | ||
| Fig. 2 Photograph of the dynamic SPME field sampler being used inside a suspected former clandestine methamphetamine laboratory. The air pump is generally not held during sampling but is housed in a washable carry bag. | ||
The main body of the dynamic SPME field sampler was constructed from Restek Silcosteel®-CR treated ⅜′′ outer diameter 0.277′′ inner diameter, 316L grade stainless steel tube. The side-arm of Silcosteel® was attached by drilling a hole with a diameter smaller than the inner diameter of the tube, shaping the sidearm connection to follow the curvature of the tube, then welding on the outside of the tube. A Supelco SPME fibre holder (Sigma Aldrich) was attached to the Silcosteel® tube by creating a thread on the outside of the SPME holder hub which could then be screwed into an adaptor plug inserted in the tubing.
The dynamic field sampler was coupled to an SKC Model 224-PCXR4 air sampling pump using a push-fit connector and tube adapter with Tygon 2275 ¼′′ inner diameter, ⅜′′ outer diameter tubing. The air pump had a built-in rotameter and flow rate was calibrated independently using a TSI 4100 series flow meter. A flow dampener and Anasorb/Tenax 226-171 sorbent tube (SKC) were used to even out the flow rate and to protect the air sampling pump from contamination. The air pump is specified to maintain flow to within 5% of its set point, and in calibration experiments using the TSI 4100 and with the dampener and sorbent tube connected we observed flow rates of 1.00 ± 0.01 L min−1 (standard deviation; n = 20) over 20 min. SPME fibres (Supelco 100 μm polydimethylsiloxane (PDMS) fibres and 75 μm Carboxen-PDMS fibres) were obtained from Sigma Aldrich. Storage tubes for the SPME fibres were fabricated from Silcosteel®-CR treated ⅜′′ outer diameter 0.277′′ inner diameter, 316L grade stainless steel tube fitted with ⅜′′ Swagelok 316 stainless steel caps.35 SPME fibres were placed in these storage tubes and stored in a cooled insulated container during transportation back to the laboratory and until analysis. All were analysed within 24 h of collection except for one set of site samples (first visit, site 25) which were analysed 72 h later after storage at 4 °C.
SPME GC-MS analysis
The GC-MS instrument parameters were based on published guidelines for SPME GC-MS.36 Samples were analysed on an HP 6890 gas chromatograph with a HP 5973 mass spectrometer in positive electron ionisation mode with 70 eV electron energy. The column was a 30 m HP-5MS 0.25 mm internal diameter, 0.25 μm 5%-phenyl-methylpolysiloxane stationery phase with He as the carrier gas at a linear velocity of 36 cm s−1. The GC oven temperature program started at 40 °C held 2.5 min, increased at 40 °C min−1 to 300 °C and held 3 min. The transfer line to the MSD was 300 °C. Mass spectra were acquired in scan mode from 38–300 amu. SPME fibres were introduced manually through an Agilent BTO septum at 250 °C in splitless mode using a 0.75 mm internal diameter deactivated glass SPME liner (Supelco), with a purge flow of 30 mL min−1 after 1.5 min. After SPME fibre desorption in the GC inlet, the fibre was left in the inlet during the chromatographic separation to desorb all analytes on the fibre and prepare it for the next sampling. Peak integration and identification was carried out using MSD Chemstation D01.02 and the NIST Mass Spectral Library (2008). Peak areas were measured from extracted ion chromatograms for the main ion fragment of underivatized methamphetamine (58 amu).Sampling at former clandestine laboratories
Sampling was performed by E. J. McKenzie during visits to former clandestine laboratories by Forensic and Industrial Science Ltd. The study sampled from sites close to the Auckland region. Sampling occurred between 12 August 2009–6 October 2010. Sites were limited to those where the property owner and occupier consented to the study. Samples were collected from 21 suspected former clandestine methamphetamine laboratories. Air sampling was carried out at 11 sites, however only 9 sites had sufficient wipe samples to enable data evaluation. Of the 9 sites, one was a caravan and 8 were houses, 3 were in a rural setting and 6 were urban. Owners and/or occupants of the premises were informed of the purpose of the sampling, and the project had approval from the Human Ethics Committee of the University of Auckland. The dynamic sampler was operated for 5–30 min at a flow rate of 1.00 L min−1, with the experimenter walking through the house with the sampler held at chest level, in order to sample from what might be the typical air intake zone for human breathing. A limited number of static SPME measurements were also performed, with the SPME fibre clamped about 30 cm above a given surface.During the same sampling visit, 6–12 wipe samples were collected using a protocol based on that reported by Abdullah.37 This involved surface wiping of areas 10 × 10 cm using a disposable card template. Wiping media was a Sartorius 1388![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 110 mm diameter filter paper cut into four pieces dampened with HPLC-grade methanol. The surface was wiped four times in concentric squares from the outside to the centre with the pieces of filter paper, both clockwise and anticlockwise, and folding and placing them in a clean 20 mL glass scintillation vial. On return to the laboratories the wipe samples were spiked with 0.1 μg d9-methamphetamine (ISOTEC 99%, Sigma Aldrich) and stored at 4 °C. Samples were processed using the method developed by Abdullah,37 which involved adding 4 mL of 4% sodium hydroxide to the wipe sample, tamping down firmly with a glass rod to submerge the sample, 5 min sonication, then a 20 or 40 mL glass syringe was used to squeeze out the filter papers, collecting the solution in a culture tube, and the process was repeated on the same filter paper and the extracts collected together. Dichloromethane (3 mL) was added to the sodium hydroxide extract, which was then vortexed for 3 min, centrifuged at 990 rpm for 5 min, then the bottom dichloromethane layer was transferred to another culture tube. This step was also repeated on the aqueous extract. The dichloromethane extract was passed through a short column of anhydrous sodium sulfate, evaporated down to ∼1 mL at 26 °C under nitrogen, then transferred to a GC vial and further evaporated to <50 μL. Ethyl acetate (100 μL) and trifluoroacetic anhydride (50 μL) were added, then the vial was shaken and incubated at 38 °C for 30 min. Following incubation, the sample was evaporated to near-dryness under nitrogen, 1 mL ethyl acetate was added, and the vial was shaken and flushed with nitrogen, then capped with a PTFE lined cap prior to GC-MS analysis. Samples were analysed on a Hewlett Packard 6890 gas chromatograph coupled to a Hewlett Packard 5973 mass spectrometer. The analytical parameters for GC-MS analysis were based on the method developed by Abdullah,37 with minor changes. Methamphetamine surface concentrations were calculated using the peak area of the most abundant ion fragment for TFA-derivatised methamphetamine (154) and methamphetamine-d9 (161). The response factor for methamphetamine to methamphetamine-d9 was determined from reference standards to be 1
110 mm diameter filter paper cut into four pieces dampened with HPLC-grade methanol. The surface was wiped four times in concentric squares from the outside to the centre with the pieces of filter paper, both clockwise and anticlockwise, and folding and placing them in a clean 20 mL glass scintillation vial. On return to the laboratories the wipe samples were spiked with 0.1 μg d9-methamphetamine (ISOTEC 99%, Sigma Aldrich) and stored at 4 °C. Samples were processed using the method developed by Abdullah,37 which involved adding 4 mL of 4% sodium hydroxide to the wipe sample, tamping down firmly with a glass rod to submerge the sample, 5 min sonication, then a 20 or 40 mL glass syringe was used to squeeze out the filter papers, collecting the solution in a culture tube, and the process was repeated on the same filter paper and the extracts collected together. Dichloromethane (3 mL) was added to the sodium hydroxide extract, which was then vortexed for 3 min, centrifuged at 990 rpm for 5 min, then the bottom dichloromethane layer was transferred to another culture tube. This step was also repeated on the aqueous extract. The dichloromethane extract was passed through a short column of anhydrous sodium sulfate, evaporated down to ∼1 mL at 26 °C under nitrogen, then transferred to a GC vial and further evaporated to <50 μL. Ethyl acetate (100 μL) and trifluoroacetic anhydride (50 μL) were added, then the vial was shaken and incubated at 38 °C for 30 min. Following incubation, the sample was evaporated to near-dryness under nitrogen, 1 mL ethyl acetate was added, and the vial was shaken and flushed with nitrogen, then capped with a PTFE lined cap prior to GC-MS analysis. Samples were analysed on a Hewlett Packard 6890 gas chromatograph coupled to a Hewlett Packard 5973 mass spectrometer. The analytical parameters for GC-MS analysis were based on the method developed by Abdullah,37 with minor changes. Methamphetamine surface concentrations were calculated using the peak area of the most abundant ion fragment for TFA-derivatised methamphetamine (154) and methamphetamine-d9 (161). The response factor for methamphetamine to methamphetamine-d9 was determined from reference standards to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Wipe sample results are reported as μg free base methamphetamine/100 cm2.
1. Wipe sample results are reported as μg free base methamphetamine/100 cm2.
Results and discussion
This project aimed to develop a sampler for airborne methamphetamine that could be used to supplement surface wipe sampling and that could be used within the limited time available for commercial testing of suspected or actual former clandestine laboratories. Preliminary testing showed that SPME sampling might suit these needs (Fig. 3 and S1, see ESI†). These initial tests also showed that PDMS fibres retained 3–4 times more methamphetamine when exposed in a controlled laboratory setting than did carboxen-PDMS fibres, and no methamphetamine was detected on carboxen-PDMS fibres in a former clandestine laboratory whereas it could be detected using PDMS fibres (see entry 1, Table 1).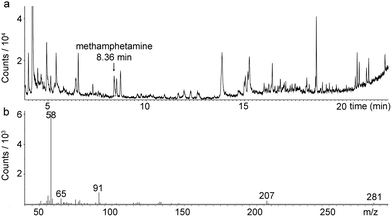 | ||
| Fig. 3 (a) GC-MS total ion chromatogram from a PDMS SPME fibre exposed to air at 1.00 L min−1 for 10 min the day after cleaning at a former clandestine methamphetamine laboratory showing methamphetamine (8.36 min). (b) The mass spectrum for the methamphetamine peak at 8.36 min. | ||
| Site | Date of visit | Surface methamphetamine concentration (μg/100 cm2) | Test type | Sampling time | SPME methamphetamine ion fragment 58 peak area/105 | TVOCPID (ppm, normal house <50 ppb) |
|---|---|---|---|---|---|---|
| 10 | 25 September 2009 | 40–653 | 1 Tedlar bag | 10 min | 4.3 (PDMS only) | 3–105 |
| 1 Carboxen/PDMS | ||||||
| 1 PDMS | ||||||
| 27 October 2009 | 0.6–137 | 3 PDMS | 10 min | 0.47–0.96 | Not tested | |
| 4 November 2009 | Not tested | 2 + field blank PDMS | 10 min | 5.2–22 | Not tested | |
| 5 November 2009 | bd–150 | 3 + field blank PDMS | 10 min | 1.6–2.2 | Not tested | |
| 13 | 30 September 2009 | 17–6093 | 3 + field blank PDMS | 10 min | 3.2–6.6 | 100–678 |
| 5 November 2009 | 1–545 | 3 PDMS | 10 min | 4.5–5.7 | 8–28 (after remediation) | |
| 16 | 20 January 2010 | 0.1–29 | 6 + field blank PDMS | 5, 10, 15 min | Not detected | 177–281 |
| 17 | 27 January 2010 | 0.02–2 | 3 PDMS | 10 min | Not detected | 174–517 |
| 19 | 16 March 2010 | 0.9–1 | 6 PDMS | 5, 10, 15 min | Not detected | 4–22 |
| 20 | 22 April 2010 | 0.1–14 | 5 + field blank PDMS | 10 min | Not detected | 200–270 (after remediation and freshly painted) |
| 23 | 1 June 2010 | 1–58 | 1 + field blank PDMS | 10 min | Not detected | 30–80 upstairs, 710–1200 downstairs |
| Polyethylene glycol | ||||||
| Carboxen-PDMS | ||||||
| 2× DVB/Carboxen/PDMS | ||||||
| 7 July 2010 | bd–2 | 5 + field blank PDMS | 10 min | Not detected | 2–7 upstairs, 34–57 downstairs (after remediation) | |
| 25 | 23 July 2010 | 0.5–41 | 3 PDMS | 15 min | 3.1–4.2 | 0 (evidence of fire, broken windows, attached garage without door) |
| 11 August 2010 | bd–57 | 5 PDMS | 15, 30 min | 0.13–0.16 | 0 (broken windows, attached garage without door) | |
| 26 | 16 August 2010 | 0.06–27 | 4 + field blank PDMS | 15 min | Not detected | 0 |
A drawback of passive SPME for short-term air sampling in a dwelling is that the fibre is exposed to a limited volume of air, and sampling will be impacted by uncontrolled air currents. Therefore, a dynamic SPME sampling device was constructed that would cause a higher volume of air (1.00 L min−1) to pass the fibre and that would introduce the air at a sufficient velocity that the response should not be affected by the air currents within a closed dwelling.
The dynamic SPME sampler was constructed so that all surfaces upstream and slightly downstream of the SPME fibre were inert materials, with the main structural material being Silcosteel, to reduce the potential for methamphetamine adsorption which could lead to both low results and cross-contamination. This construction also meant that the device was robust, which is an important consideration for application in the field. The air pump was located downstream of the SPME fibre with an in-line general adsorption trap to protect it from contamination. In use, the dynamic sampler could be switched on, and then the analyst could move around the dwelling and, if desired, locate the inlet of the sampler in regions of particular interest.
When the dynamic SPME field sampler was initially constructed, all but two38,39 of the existing dynamic SPME arrangements placed the SPME fibres perpendicular to the airflow. The axial position of the SPME fibre was selected to promote laminar airflow and to reduce the likelihood of the fibre flexing at high air flows. This parallel arrangement has also been used in two recent studies40,41 and the flow dynamics of both perpendicular and parallel arrangements of SPME fibres in an airflow have been investigated.42
Sampling of both airborne methamphetamine and surface wipe sampling at nine suspected former clandestine methamphetamine laboratories showed a correlation between the observed surface contamination and the ability to detect airborne methamphetamine, with sites at which surface contamination exceeded 40 μg/100 cm2 having measurable airborne methamphetamine, Fig. 4 and Table 1. These observations lead to two corollaries: first, that short-term (5–20 min) dynamic SPME sampling as reported here was not sufficiently sensitive to detect airborne methamphetamine contamination when surface wipe concentrations are near the remediation level (typically 0.1 to 1.5 μg/100 cm2), and second, that if airborne methamphetamine is detected using short-term dynamic SPME as described here it strongly suggests that there is significant methamphetamine contamination somewhere within the dwelling. This latter point may mean that a repeated visit for surface sampling is required if this contamination was not detected in the initial surface wipe sampling.
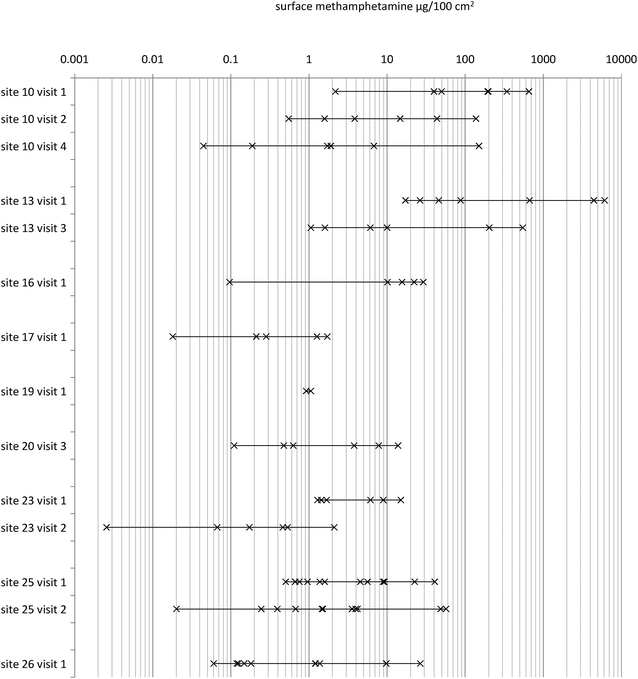 | ||
| Fig. 4 Diagram showing the surface wipe methamphetamine concentrations from nine suspected former clandestine laboratories. Airborne methamphetamine was detected from sites 10, 13 and 25 using the dynamic SPME sampler combined with GC-MS. | ||
Other compounds
The SPME chromatograms from the three sites that were found to have detectable methamphetamine in air were inspected to identify what other compounds were present. SPME field blanks were also analysed. Compounds were tentatively identified using NIST08 and SWGDRUG mass spectral libraries. A total of 65 compounds were identified, however only methamphetamine, 1-phenyl-2-propanone and N-formylmethamphetamine were clearly related to drug use or manufacture. The other compounds identified derive from cleaning compounds, insecticides, insect repellents, air fresheners, plant and food volatiles, cosmetics, perfumes, fuels, textile surface treatments and plastics.Conclusion
A dynamic SPME sampler has been constructed that allows detection of airborne methamphetamine at former clandestine laboratory sites even when sampling times are restricted to 5–20 min. When combined with GC-MS analysis, the sampler has given positive detection of airborne methamphetamine when surface wipe samples showed concentrations of >40 μg/100 cm2. The method requires less sampling time in the field than traditional exhaustive extraction methods, and no sample processing or derivatisation is required. The method does not require expensive state-of the art equipment and is suitable not only for indoor air, but may be used in shipping containers, ambient air, exhaled breath, storage facilities, and in vehicles. It could also be used to aid detection of active clandestine laboratories when limited time is available for sampling suspected contaminated air.Acknowledgements
The authors would like to thank the property owners of the sites used in this study and N. Powell and N. Hosted (Forensic & Industrial Science Ltd), for assistance with gaining access to these sites. We also thank C. Hughes (The University of Auckland) for fabrication of apparatus.References
- H. Pickmere, New Zealand Police National Drug Intelligence Bureau, New Zealand, 2012, personal communication.
- United Nations Office on Drugs and Crime, United Nations, Vienna, 2011.
- United Nations Office on Drugs and Crime, United Nations, Vienna, 2012.
- B. Lazarus, J. Clan. Lab. Investig. Chem. Assoc., 2000, 10, 21–32 Search PubMed.
- C. B. Salocks, Assessment of Children's Exposure to Surface Methamphetamine Residues in Former Clandestine Methamphetamine Labs, and Identification of a Risk-Based Cleanup Standard for Surface Methamphetamine Contamination, OEHHA, 2009, http://oehha.ca.gov/public_info/public/kids/pdf/ExposureAnalysis022709.pdf, accessed 4 Sept 2013 Search PubMed.
- Washington State Department of Health, Guidelines for Environmental Sampling at Illegal Drug Manufacturing Sites, 2005, http://www.doh.wa.gov/portals/1/Documents/Pubs/334-106.pdf, accessed 4 Sept 2013 Search PubMed.
- Minnesota Department of Health and Minnesota Pollution Control Agency, Clandestine Drug Lab General Cleanup Guidance, Minnesota Department of Health and Minnesota Pollution Control Agency, 2007, http://proteus.pca.state.mn.us/cleanup/meth.html, accessed 29 April 2008 Search PubMed.
- J. W. Martyny, S. L. Arbuckle, C. S. McCammon Jr, E. J. Esswein, N. Erb and M. Van Dyke, J. Chem. Health Saf., 2007, 14, 40–52 CAS.
- M. Vona, ed. D. Ziarkowski, Initial Evaluation of Emission from Methamphetamine Manufacturing via the Ephedrine/Red Phosphorous/Hydriodic Acid Method, Department of Toxic Substances Control, Sacramento, 2003, http://www.dtsc.ca.gov/sitecleanup/erp/upload/smbrb_memo_initial-eval.pdf, accessed 4 Sept 2013.
- M. Van Dyke, N. Erb, S. Arbuckle and J. Martyny, J. Occup. Environ. Hyg., 2009, 6, 82–89 CAS.
- Minnesota Pollution Control Agency, Data Review – Delavan Meth in Air Sampling, Minnesota Pollution Control Agency, 2007, http://proteus.pca.state.mn.us/cleanup/meth.html, accessed 29 April 2008 Search PubMed.
- Minnesota Pollution Control Agency, Personal Air Sampling, Minnesota Pollution Control Agency, 2007, http://proteus.pca.state.mn.us/cleanup/meth.html, accessed 29 April 2008 Search PubMed.
- Minnesota Pollution Control Agency, Data Review – St. Peter Meth in Air Sampling, Minnesota Pollution Control Agency, 2007, http://proteus.pca.state.mn.us/cleanup/meth.html, accessed 29 April 2008 Search PubMed.
- C. J. Banta-Green, J. A. Field, A. C. Chiaia, D. L. Sudakin, L. Power and L. De Montigny, Addiction, 2009, 104, 1874–1880 Search PubMed.
- T. H. Boles and M. J. M. Wells, J. Chromatogr., A, 2010, 1217, 2561–2568 CAS.
- M. Huerta-Fontela, M. T. Galceran and F. Ventura, Environ. Sci. Technol., 2008, 42, 6809–6816 CrossRef CAS.
- A. Janusz, K. P. Kirkbride, T. L. Scott, R. Naidu, M. V. Perkins and M. Megharaj, Forensic Sci. Int., 2003, 134, 62–71 CAS.
- C. Postigo, M. J. Lapez de Alda and D. Barcela, Environ. Int., 2010, 36, 75–84 CAS.
- P. Vazquez-Roig, V. Andreu, C. Blasco and Y. Picó, Anal. Bioanal. Chem., 2010, 397, 2851–2864 CAS.
- E. Zuccato and S. Castiglioni, Philos. Trans. R. Soc., A, 2009, 367, 3965–3978 CAS.
- P. Tayler, MSc thesis University of Auckland, 2003.
- M. L. Ochoa and P. B. Harrington, Anal. Chem., 2004, 76, 985–991 CAS.
- E. S. Reese and P. D. B. Harrington, J. Forensic Sci., 1999, 44, 68–76 CAS.
- N. Alizadeh, A. Mohammadi and M. Tabrizchi, J. Chromatogr., A, 2008, 1183, 21–28 CAS.
- P. Guerra-Diaz, S. Gura and J. R. Almirall, Anal. Chem., 2010, 82, 2826–2835 CAS.
- O. Beck, S. Sandqvist, P. Eriksen, J. Franck and G. Palmskog, J. Anal. Toxicol., 2011, 35, 129–133 CAS.
- P. C. Raynor and T. Carmody, Final Report: Meth Labs Sampling: Air and HVAC Systems. Minnesota Pollution Control Agency CFMS No. A-79651, University of Minnesota, Minneapolis, 2006 Search PubMed.
- C. L. Arthur and J. Pawliszyn, Anal. Chem., 1990, 62, 2145–2148 CAS.
- J. Pawliszyn, Solid Phase Microextraction: Theory and Practice, Wiley-VCH, New York, 1997 Search PubMed.
- R. J. Casey, MSc thesis, University of Auckland, 2007.
- N. D. Tung, MSc thesis, University of Auckland, Auckland, 2006.
- S. Gentili, M. Cornetta and T. Macchia, J. Chromatogr., B: Anal. Technol. Biomed. Life Sci., 2004, 801, 289–296 CAS.
- C. J. Koester, B. D. Andresen and P. M. Grant, J. Forensic Sci., 2002, 47, 1–6 Search PubMed.
- D.-T. T. Vu, J. Forensic Sci., 2001, 46, 1014–1024 CAS.
- V. Larroque, V. Desauziers and P. Mocho, J. Chromatogr., A, 2006, 1124, 106–111 CAS.
- J. J. Langenfeld, S. B. Hawthorne and D. J. Miller, J. Chromatogr., A, 1996, 740, 139–145 CAS.
- A. F. L. Abdullah, PhD thesis, University of Auckland, 2007.
- R. J. Bartelt and B. W. Zilkowski, Anal. Chem., 2000, 72, 3949–3955 CAS.
- C. M. Bravo-Linares and S. M. Mudge, J. Environ. Monit., 2007, 9, 411–418 CAS.
- B. Baker and M. Sinnott, J. Chromatogr., A, 2009, 1216, 8442–8451 CAS.
- S. Shu and G. Morrison, Talanta, 2010, 82, 1884–1891 CAS.
- E. Gómez Alvarez, M. V. Moreno, S. Gligorovski, H. Wortham and M. V. Cases, Talanta, 2012, 88, 252–258 Search PubMed.
Footnote |
| † Electronic supplementary information available: GC-MS chromatogram from an SPME fibre exposed during cleaning of a clandestine laboratory. See DOI: 10.1039/c3ay40537k |
| This journal is © The Royal Society of Chemistry 2013 |
