Three-dimensional cell culture based on microfluidic techniques to mimic living tissues
Yuya
Morimoto
a and
Shoji
Takeuchi
*ab
aCenter for International Research on Micronano Mechatronics (CIRMM), Institute of Industrial Science (IIS), The University of Tokyo, 4-6-1 Komaba, Meguro-ku, Tokyo 153-8505, Japan
bERATO, Japan Science and Technology (JST), Komaba Open Laboratory (KOL), Room M202, 4-6-1 Komaba, Meguro-ku, Tokyo 153–8904, Japan. E-mail: takeuchi@iis.u-tokyo.ac.jp; Fax: +81-3-5452–6649; Tel: +81-3-5452-6650
First published on 20th November 2012
Abstract
This mini-review consists of microfluidic fabrication methods of cellular spheroids and cell-laden hydrogels, and their applications for tissue engineering. Using microfluidic devices, cellular spheroids and cell-laden hydrogels with controllable design are formed reproducibly. Owing to their size uniformity, they are used as building blocks for bottom–up tissue engineering to construct uniform and arbitrarily shaped tissues. Thus, cellular spheroids and cell-laden hydrogels based on microfluidic techniques are powerful tools to create tissues for human implantation and the treatment of diseases.
 Yuya Morimoto | Yuya Morimoto received his bachelor's and master's degrees from the University of Tokyo, Japan. He was a mechanical engineer in Fujifilm Corporation, Japan, and developed medical endoscopes. He is currently a PhD candidate in the University of Tokyo, Japan, and a research fellow of the Japan Society for the Promotion of Science. His research interests are in the field of microfabrication techniques for bottom–up tissue engineering. |
 Shoji Takeuchi | Shoji Takeuchi received the B.E, M.E., and Dr Eng. degrees in mechanical engineering from the University of Tokyo, Tokyo, Japan, in 1995, 1997 and 2000, respectively. He is currently an Associate Professor in the Center for International Research on Micronano Mechatronics (CIRMM), Institute of Industrial Science (IIS), University of Tokyo. Since 2008, he is a director of the Collaborative Research Center for Bio/Nano Hybrid Process at IIS. His current research interests include membrane protein chips, bottom–up tissue engineering and biohybrid MEMS. He has received several awards including the Young Scientists’ Prize, the Commendation for Science and Technology by the Minister of Education, Culture, Sports, Science and Technology in 2008, the JSPS prize from the Japan Society for the Promotion of Science in 2010, Yomiuri-Techno Forum Gold Medal Prize in 2012. |
1. Introduction
In the field of tissue engineering, the reconstruction of organs and tissues for use as living replacement parts in the body and as pharmacokinetic models is a major challenge. To achieve this goal, cells must be grown in an environment similar to that found in living tissues. Many types of cells in vivo interact with other cells and with extracellular matrices (ECMs) within three-dimensional (3D) structures.1 The cell–cell and cell–ECM interactions provide mechanical and biochemical cues that can influence cellular function and differentiation.2,3 Therefore, 3D cell culture models combining cells and ECMs should be used to mimic and analyze the cellular functions of living tissues.1–5This mini-review focuses on cellular spheroids and cell-laden hydrogels as the microsized 3D cell culture models. These microsized models provide attractive 3D microenvironments to culture cells because they consist of microstructures and cell–cell and/or cell–ECM interactions that are typical in living tissues. Microfabrication based on microfluidic techniques is well suited to the fabrication of these microsized models because such techniques can help build and manipulate 3D objects in a high-throughput manner with high reproducibility5 (Table 1). In addition, microfluidic techniques facilitate assembling them into large cellular constructs. While it is difficult to mimic dense and complex cellular morphologies in living tissues using conventional 3D cellular constructs produced using large biodegradable scaffolds, microsized 3D cell culture models and their assemblies have microstructures resembling living tissues because the engineered tissues retain specific microstructural features.4 Therefore, cellular spheroids and cell-laden hydrogels are suitable for use as standardized modules in a wide range of applications to investigate cellular functions and reconstruct complex 3D cellular structures. Here, we briefly discuss the fabrication techniques of cellular spheroids and cell-laden hydrogels and finally introduce their assembly methods.
| Type | Shape | Fabrication method | Characteristics | Supplementary | Assembly method | |||
|---|---|---|---|---|---|---|---|---|
| Size control | Fabrication | |||||||
| Simple | Rapid | Mass | ||||||
| ✓: good; —: fair. | ||||||||
| Cellular spheroids | Sphere | Culture on non-adhesive surface | Not good | ✓ | — | — |
“Spheroid assembly”
• Swelling medium11 • Microfluidic device • Molding43 • Printing45,46 |
|
| Rotary bioreactors | Not good | — | — | ✓ | Need spinner devices | |||
| Culture in confined spaces7–9 | ✓ | — | — | — | Can be scale up to array format | |||
| Culture using external force10–13 | ✓ | — | ✓ | — | Need fabrication techniques | |||
| Parallel culture in microfluidic device14–16 | ✓ | — | — | ✓ | Need fluidic techniques | |||
| Cell-laden hydrogel | Block | Micromolding20 | ✓ | ✓ | ✓ | — | Difficult to include complex designs |
“Block assembly”
• Surface tension at liquid interface39,40 • Guided fluidic device42 |
| Photolithography (with dynamic mask and variable focus)21 | ✓ | — | ✓ | — | Use only photoreactive hydrogel | |||
| Changes shape easily without molds | ||||||||
| Flow-lithography22–24 | ✓ | — | ✓ | ✓ | Use only photoreactive hydrogel | |||
| Need fluidic techniques | ||||||||
| Sphere | Drop formation at T-junction25 | ✓ | — | ✓ | ✓ | Need fluidic techniques |
“Sphere assembly”
• Swelling medium • Microfluidic device41 • Molding30 • Printing30 |
|
| 2D flow-focusing26 | ||||||||
| Axisymmetric flow-focusing27–31 | ✓ | — | ✓ | ✓ | Need fluidic techniques | |||
| Encapsulates cell easily | ||||||||
| Fiber | Coaxial flow32–37 | ✓ | — | ✓ | ✓ | Need fluidic techniques |
“Fiber, Tube assembly”
• Weaving35 |
|
| Tube | Use only rapid gelling hydrogel | |||||||
2. Three-dimensional cell culture models
2.1 Cellular spheroids
Cellular spheroids have been proposed as simple 3D cell culture models.6 Because many cell types tend to aggregate, preventing such cells from adhering to the culture substrate, it allows them to form cellular spheroids. Thus, cellular spheroids can be easily produced by various simple methods, including culturing on a non-adhesive surface, spinner culture, and NASA rotary culture. However, it is difficult to control the size of cellular spheroids and to produce them rapidly when using these simple methods. To produce uniform cellular spheroids, hanging-drop culture7,8 and culture in microwells9 are commonly used. In addition, culture under external forces such as dielectrophoresis10 and the use of swelling medium11 facilitates the rapid production of spheroids. Recent spheroid-formation methods that include microfabrication techniques combined with external forces, such as culture under micro-rotational flow12 and culture based on the magneto-Archimedes effect,13 have been reported to efficiently produce spheroids of uniform size (Fig. 1(a–d)). Moreover, various microfluidic devices have been reported to produce large numbers of spheroids of a defined size14–16 (Fig. 1(e, f)).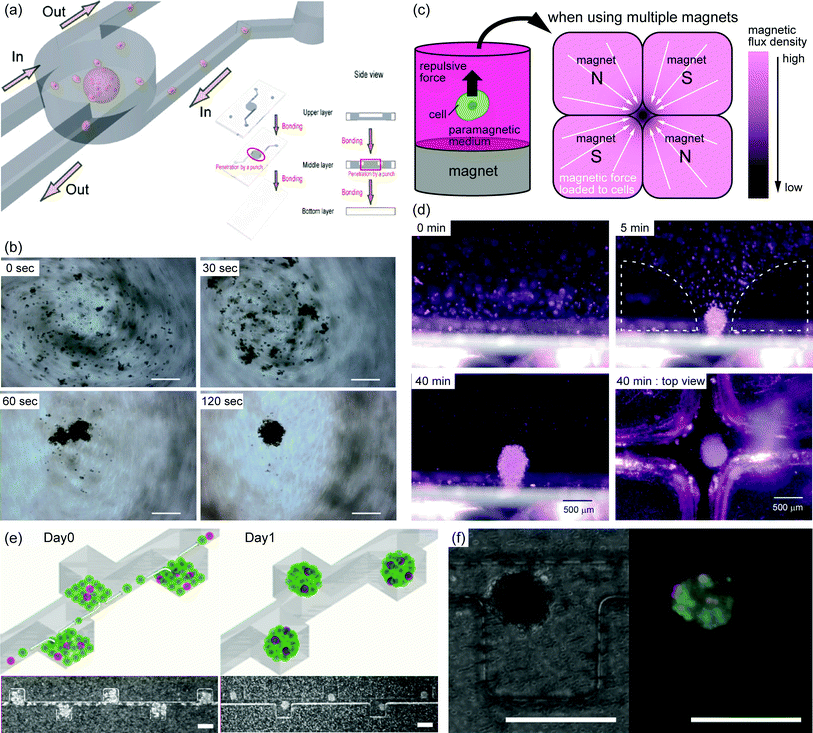 | ||
| Fig. 1 (a) Schematic diagram of spheroid formation using micro-rotational flow. When cells suspended in culture medium are introduced into the device, the cells aggregate into a spheroid due to the micro-rotational flow.12 (b) Sequential images of spheroid formation driven by micro-rotational flow. At 0 s, cell rotation occurs throughout the entire area with high-speed flow. When the flow speed is reduced, cells start aggregating on the left of the device (∼120 s). Scale bars, 200 μm.12 (c) Schematic diagram of spheroid formation using the magneto-Archimedes effect. The magnetic force acts on cells within the paramagnetic medium, and cells aggregate on the spots with the lowest magnetic flux density.13 (d) Sequential images of spheroid formation by magnetic force. Cells gradually aggregate in the gap of the magnet array.13 (e) Production of spheroids using a microfluidic device. The device has a main channel and side chambers; the surfaces of all channels are rendered resistant to cell adhesion, resulting in cell aggregation in the side chambers. Scale bars, 200 μm.16 (f) Image of a co-culture spheroid after 1 day in culture in the microfluidic device. Red areas represent PC-3DsRed cells, and green areas represent live cells. Scale bars, 200 μm.16 “(a, b) Copyright (2010) Elsevier, (d) Copyright (2011) Royal Society of Chemistry, (e, f) Copyright (2009) Royal Society of Chemistry.” | ||
Cell–cell interactions occur in spheroids, and spheroids have the ability to mimic avascular tumors with inherent oxygen metabolism and nutrient gradients.16,17 Therefore, spheroids can be used as stable artificial tissue models for pharmacokinetic and biological analyses. However, because cellular spheroids are random aggregates of different types of cells, there are problems associated with the spatial arrangements of the cells within such spheroids.
2.2 Cell-laden hydrogel
The culture of cells with biomaterials has been proposed as a method to control the configuration of 3D cell culture models and to mimic cell–ECM interactions. Biomaterial selection is important for mimicking the mechanical, physical, and chemical properties of ECMs in vivo. In the field of tissue engineering, hydrogels are commonly used for cell culture under 3D culture conditions because they are mostly biocompatible, biodegradable, and mechanically processable.18,19 Hydrogels based on both natural polymers (i.e., collagen, hyaluronate, fibrin, alginate, and agarose) and on synthetic polymers (i.e., poly(ethylene glycol) (PEG) and polypeptides) are potentially attractive for tissue engineering. Such hydrogels are created using simple methods such as chemical reactions, temperature transition, and exposure to ultraviolet (UV) light. Pore sizes of the hydrogels are determined by gelling conditions such as the size and the concentration of monomers and the concentration of their cross-linkers.In the field of tissue engineering, hydrogels need to have microsized structures because microsized structures promote the circulation of oxygen and nutrients and mimic the intricate shapes of living tissues. Recently, microfabrication techniques have been used to form microsized hydrogel structures with controlled shapes, allowing cells to attach to their surfaces and/or to be encapsulated.5,18,19 Micromolding and photolithography in particular are used to produce cell-laden hydrogel microsized blocks with designed shapes.20,21 In addition, photolithography combined with microfluidic devices, that is, the so-called “flow-lithography,” can be used to fabricate cell-laden hydrogel blocks in a high-throughput manner.22–24 Other microfluidic devices are used mainly to fabricate large numbers of cell-laden hydrogel structures in bead and fiber shapes with high uniformity and design flexibility. Microfluidic devices such as T-junction microchannels,25 2D microfluidic flow-focusing devices,26 and axisymmetric flow-focusing devices (AFFDs)27–31 (Fig. 2(a)) produce monodisperse droplets that form monodisperse hydrogel beads after gelation (Fig. 2(b, c)). Moreover, microfluidic devices can form monodisperse hydrogel beads that can reproducibly encapsulate cells. Using AFFDs to produce collagen beads that encapsulate specific cells and that are surrounded by another type of cell, cell-laden collagen beads with a hierarchical 3D co-culture systems that promote cell–cell and cell–ECM interactions can be produced as microscopic tissue models30,31 (Fig. 2(d, e)). As cellular functions within living tissues are more likely to be reproduced in 3D co-culture than in conventional 2D culture, cell-laden hydrogel beads successfully promoted the secretion of proteins.30,31 Thus, cell-laden hydrogel beads will be useful tools for high-throughput studies of in vivo pathological and physiological phenomena.
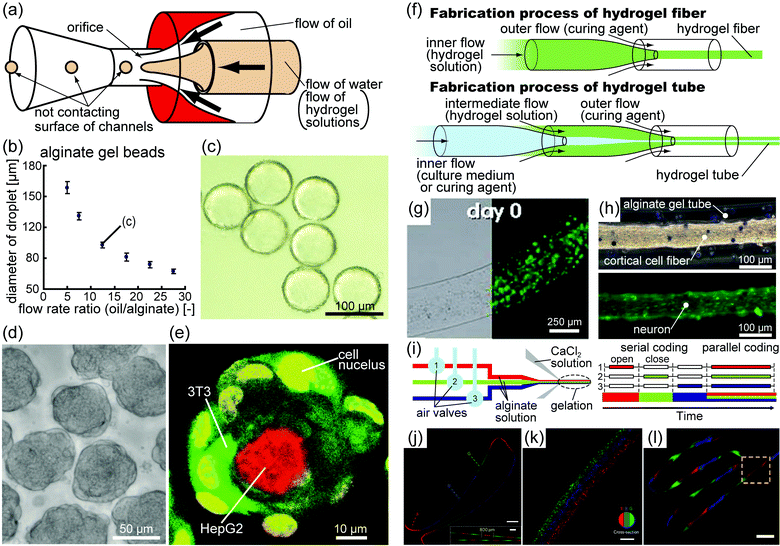 | ||
| Fig. 2 (a) Schematic diagram of an AFFD used to produce monodisperse droplets. AFFDs prevent droplets from adhering to the surfaces of channels because the droplets are always surrounded by the outer fluid. (b) Plot of the sizes of the alginate gel beads in the oil versus the flow rate ratio (outer flow rate/inner flow rate). The coefficient of variation for every point is <5%, indicating that the beads produced by the AFFD are monodisperse.27 (c) Image of alginate gel beads in oil corresponding to point (c) in figure (b).27 (d) Image of cell beads seeded with 3T3 cells based on monodisperse collagen beads.30 (e) Fluorescence confocal microscopy of hierarchical co-cultured cell beads. Collagen gel beads encapsulating HepG2 cells (red) were seeded with 3T3 cells (green) after 17 h of incubation.30 (f) Schematic diagram of the fabrication procedure for hydrogel fibers and tubes. (g) Optical bright field and fluorescence images of an alginate fiber containing Wharton's jelly mesenchymal stem cells (green).31 (h) Optical bright field and fluorescence images of alginate tubes containing cortical cell fibers. Green indicates neurons.34 (i) Schematic diagram of alginate fibers in which the enclosed material can be altered using air valves.36 (j) Image of a serially coded fiber and a magnified image (inset). Scale bars, 1 mm and 400 μm (inset). (k) Image of a parallel coded fiber. Scale bar, 200 μm. (l) Image of a fiber with spatiotemporal variations in morphology and chemical composition. Scale bar, 1 mm. (j–l) Different types of fluorescent polystyrene beads emit different colors: red, blue, and green.36 “(b, c) Copyright (2009) Royal Society of Chemistry, (d, e) Copyright (2011) John Wiley and Sons, (g) Copyright (2009) Royal Society of Chemistry, (j–l) Copyright (2011) Nature Publishing Group.” | ||
In addition, cell-laden hydrogel fibers and tubes can be generated by the coaxial cylindrical flow of solutions inside microfluidic devices without length limitations32–36 (Fig. 2(f–h)), and the fluid flow rate and channel dimension can control the diameter of the fiber. Furthermore, by embedding air valves into the microfluidic devices, hydrogel fibers of varying compositions and topography can be generated37 (Fig. 2(i–l)). Cell-laden hydrogel tubes encapsulating human endothelial cells co-cultured with smooth muscle cells were able to mimic the structure of human vascular vessels.36 Although these tubes do not currently mimic the cellular functions of living vascular vessels, such as providing barrier function and neovessel formation,38 it is anticipated that further developments, such as using appropriate biomaterials instead of alginate gel, will lead to the construction of blood vessel equivalents.
2.3 Assembly of 3D cell culture models to produce complex tissue-like structures
The assembly of cellular spheroids and cell-laden hydrogels has received attention as a method for engineering complex tissue-like 3D cell structures. Most assembly techniques are categorized into two types: (i) assembly of a small number (< approximately 102) of cellular spheroids or cell-laden hydrogels to precisely control their arrangement, (ii) and assembly of a large number of these structures to form macroscopic structures.To assemble small numbers of cellular spheroids and cell-laden hydrogels, the various physical properties of liquids are manipulated. The swelling properties of a solution including a high-molecular-weight material (methylcellulose) allow cellular spheroids to rapidly aggregate without cell modification.11 This method also facilitates the construction of multicellular aggregates such as hepatocyte cellular spheroids embedded in larger aggregates including mesothelioma cells. Although this method cannot control the arrangement of cellular spheroids and other cells, cellular spheroids and other materials are assembled in a short time without cumbersome procedures. Khademhosseini and coworkers proposed another method using surface tension at the water–oil or the air–liquid interface to assemble cell-laden hydrogel blocks constructed from photocrosslinkable PEG-methacrylate (PEGmA)39,40 (Fig. 3(a)). The cell-laden PEGmA gel blocks were transferred into mineral oil, where the blocks were grouped together using manual mechanical agitation39 or placed on the surface of carbon tetrachloride or perfluorodecalin, where the blocks self-grouped to minimize surface free energy.40 Further exposure to UV light facilitates block assembly, and the final shape of the assemblies is controlled by the properties of agitation and the configuration of the blocks (Fig. 3(b)). Thus, the method allowed the production of complex 3D structures, such as lock-and-key assemblies, containing multiple cell types (Fig. 3(c)), indicating that it enables the reproducible assembly of cell-laden gel blocks while controlling the locations of cells and the shapes of the structures.
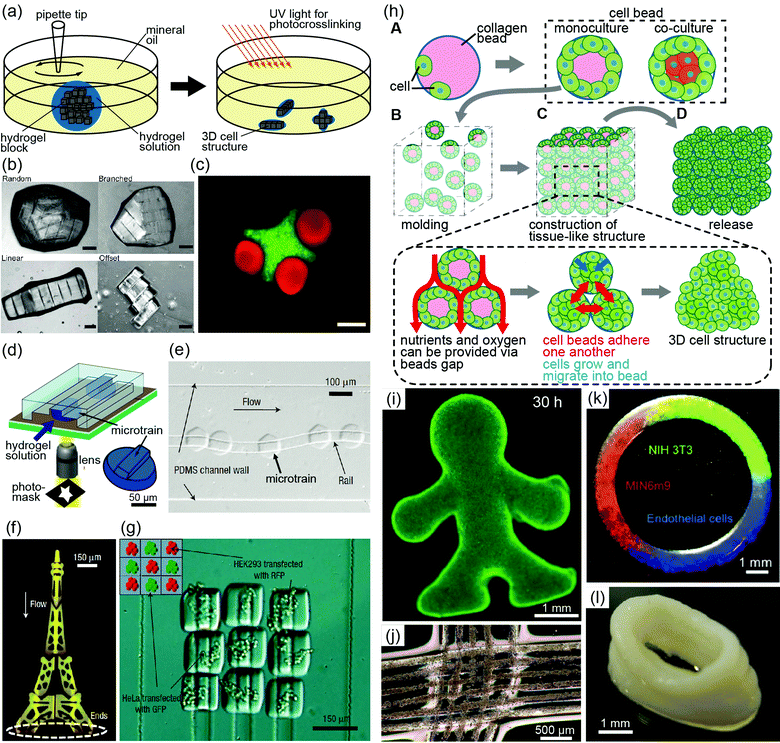 | ||
| Fig. 3 (a) Schematic diagram showing the assembly of cell-laden photocrosslinkable hydrogel blocks in oil. Mechanical agitation is performed using a pipette tip, and secondary cross-linking forms diverse gel assembly shapes.38 (b) Assembly of PEGmA blocks in random, branched, linear, and offset shapes. Scale bar, 200 μm.38 (c) Images of the lock-and-key-shaped gel assembly containing three rods per cross. The cross-shaped gel and rod-shaped gel encapsulate red-labeled and green-labeled 3T3 cells, respectively. Scale bars, 200 μm.38 (d) Schematic diagram of the fabrication process of microtrains. PEG is gelated into convex structures by UV exposure in microfluidic channels.41 (e) Guided movement of a microtrain along a reentrant rail.41 (f) Image of the Eiffel tower produced by the guided assembly method.41 (g) Image of rail-guided assembly using two different types of living cells. Green and red indicate HeLa and HEK293 cells, respectively.41 (h) Concept of bead-based tissue engineering: (A, B) monodisperse cell beads are molded into a macroscopic cell structure and poured into a designed PDMS mold. (C) During tissue formation, the medium diffuses into the 3D cell structures via cavities in the cell beads, thus supplying nutrients to all cells. (D) Macroscopic 3D cell structures are released from the PDMS mold.30 (i) Fluorescent image of a macroscopic structure with a complex shape. Live cell staining indicates that almost all cells within the structure are alive.30 (j) 3D cell structures formed by knitting cell-laden hydrogel fibers. The fibers are composed of collagen and alginate gel encapsulating HeLa cells.34 (k) Ring-shaped cell structure fabricated by printing cell beads. Various cell types are spatially coded within the structure.30 (l) Tube-shaped structure prepared by printing cell beads.30 “(b, c) Copyright (2008) National Academy of Sciences, U.S.A., (e–g) Copyright (2008) Nature Publishing Group, (i, k, l) John Wiley and Sons.” | ||
The assembly of a small number of cell-laden hydrogels with a detailed arrangement can be achieved using microfluidic systems.41 By controlling the configurations of the microfluidic channel and cell-laden hydrogels, cell-laden hydrogels are assembled with arbitrary shapes and detailed arrangements of multiple cells. Furthermore, to assemble these structures in a more precise manner, Kwon's group proposed the use of convex PEG gel blocks termed “microtrains” and a concave “rail”; the rails would guide the microtrains through the microfluidic channels in an error-free manner42 (Fig. 3(d, e)). By adjusting the combinations of rail networks and microtrain shapes, microfluidic force can assemble the microtrains to construct complex 3D structures (Fig. 3(f)). In addition, heterogeneous structures can be produced using different types of cell-laden microtrains (Fig. 3(g)). Although the guided fluidic method is limited to planar hydrogel assembly, the flexibility of this method provides the potential to produce heterogeneous and complex-shaped cell-laden structures.
Using large numbers of cellular spheroids and cell-laden hydrogel beads, the bead-based assembly approach with molds can be used to construct millimetre-thick complex 3D cell structures. In the case of cellular spheroids, they are assembled in non-adherent agarose molds with arbitrary shapes.43 By sequentially aggregating the cells, free-standing, biomaterial-free and millimetre-sized 3D structures are obtained. As an example of using cell-laden hydrogels, monodisperse collagen gel beads fabricated by an AFFD can be covered with cells to produce “cell beads”, and the beads can then be loaded into a designed poly(dimethylsiloxane) (PDMS) mold30 (Fig. 3(h)). After a 30 h culture of the beads in the molds, millimetre-thick complex 3D cell structures are obtained (Fig. 3(i)). This method has been used to produce structures from various single cell types and from mixtures of different cell types by pouring different types of cell beads into a single PDMS mold. Although approximately 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 monodisperse cell beads are required to fabricate millimetre-scale 3D tissue, this high number of cell beads is not a problem because AFFDs can rapidly produce collagen droplets (approximately 200 per second) of defined size and uniformity.27 The primary advantages of this method are rapid production of millimetre-thick 3D cell structures, homogeneous cell density, and tissue formation without necrosis in a period of less than a week because of the supply of the cell culture medium through cavities between cell beads. In addition, when the millimetre-thick 3D cell structure was composed of HepG2 cells and NIH 3T3 cells, albumin secretion increased daily from the HepG2 cells in the 3D tissue, but not in the 2D culture system. However, the construction of the vessel network in the 3D cell structures is required to maintain cell viability over a long period. Adaptations of this method will allow the formation of capillary networks in the structure because methods using the mold can integrate cell-laden fibers as capillaries into bead-based cell structures.
000 monodisperse cell beads are required to fabricate millimetre-scale 3D tissue, this high number of cell beads is not a problem because AFFDs can rapidly produce collagen droplets (approximately 200 per second) of defined size and uniformity.27 The primary advantages of this method are rapid production of millimetre-thick 3D cell structures, homogeneous cell density, and tissue formation without necrosis in a period of less than a week because of the supply of the cell culture medium through cavities between cell beads. In addition, when the millimetre-thick 3D cell structure was composed of HepG2 cells and NIH 3T3 cells, albumin secretion increased daily from the HepG2 cells in the 3D tissue, but not in the 2D culture system. However, the construction of the vessel network in the 3D cell structures is required to maintain cell viability over a long period. Adaptations of this method will allow the formation of capillary networks in the structure because methods using the mold can integrate cell-laden fibers as capillaries into bead-based cell structures.
As another method of a bead-based assembly, the printing of cellular spheroids or cell-laden hydrogel beads can be used to sequentially stack these materials layer-by-layer.30,44–46 Combined with computer-aided printing systems, the printing approach enables the rapid construction of complex 3D structures with different types of cells. For example, the printing of cellular spheroids or cell-laden collagen beads has been used to reproducibly construct ring-shaped structures containing multiple cell-laden beads in specific designed locations (Fig. 3(k)) and hollow tubes (Fig. 3(l)).30,45,46
A fiber-based assembly method was recently proposed for the construction of 3D cell structures with complex shapes. Assemblies of cell-laden hydrogel fibers can be formed by weaving these fibers together, much as threads are woven to form cloth35 (Fig. 3(j)). When using this method to weave together various types of cell-laden fibers, detailed arrangements of different types of cells in 3D cell structures can be formed.
3. Conclusion
By selecting the appropriate fabrication techniques, 3D cell structures containing a variety of cell types can be easily fabricated in a high-throughput manner with a high degree of uniformity and design flexibility. Moreover, by assembling 3D cell structures, complex tissue equivalents that reconstitute cell–cell and ECM–cell interactions can be constructed. These 3D cell structures are therefore useful tools for mimicking cell properties under in vivo-like environments. Furthermore, the use of microfabrication techniques, including those based on microfluidic systems, enables easy handling, analysis, and collection for cell property analysis for 3D cell structures. Such 3D cell structures have applications in various fields ranging from basic biology to tissue engineering.Acknowledgements
The authors thank Shigenori Miura and Hiroaki Onoe for their valuable comments. Y. Morimoto is supported by a Research Fellowship from the Japan Society for the Promotion of Science (JSPS) for Young Scientists, Japan.References
- D. R. Albrecht, G. H. Underhill, T. B. Wassermann, R. L. Sah and S. N. Bhatia, Probing the role of multicellular organization in three-dimensional microenvironments, Nat. Methods, 2006, 3(5), 369–375 CrossRef CAS.
- F. Pampaloni, E. G. Reynaud and E. H. K. Stelzer, The third dimension bridges the gap between cell culture and live tissue, Nat. Rev. Mol. Cell Biol., 2007, 8(10), 839–845 CrossRef.
- K. M. Yamada and E. Cukierman, Modeling tissue morphogenesis and cancer in 3D, Cell, 2007, 130(4), 601–610 CrossRef CAS.
- T. Taguchi, Assembly of cells and vesicles for organ engineering, Sci. Technol. Adv. Mater., 2011, 12(6), 064703 Search PubMed.
- B. G. Chung, K.-H. Lee, A. Khademhosseini and S.-H. Lee, Microfluidic fabrication of microengineered hydrogels and their application in tissue engineering, Lab Chip, 2011, 12(1), 45–59 Search PubMed.
- R.-Z. Lin and H. Y. Chang, Recent advances in three-dimensional multicellular spheroid culture for biomedical research, Biotechnol. J., 2008, 3(9–10), 1172–1184 Search PubMed.
- Y. C. Tung, A. Y. Hsiao, S. G. Allen, Y. S. Torisawa, M. Ho and S. Takayama, High-throughput 3D spheroid culture and drug testing using a 384 hanging drop array, Analyst, 2011, 136(3), 473–478 RSC.
- W. G. Lee, D. Ortmann, M. J. Hancock, H. Bae and A. Khademhosseini, A hollow sphere soft lithography approach for long-term hanging drop methods, Tissue Eng., Part C, 2010, 16(2), 249–259 Search PubMed.
- M. Kato-Negishi, Y. Tsuda, H. Onoe and S. Takeuchi, A neurospheroid network-stamping method for neural transplantation to the brain, Biomaterials, 2010, 31(34), 8939–8945 Search PubMed.
- A. Sebastian, A. G. Venkatesh and G. H. Markx, Tissue engineering with electric fields: investigation of the shape of mammalian cell aggregates formed at interdigitated oppositely castellated electrodes, Electrophoresis, 2007, 28(21), 3821–3828 Search PubMed.
- N. Kojima, S. Takeuchi and Y. Sakai, Rapid aggregation of heterogeneous cells and multiple-sized microspheres in methylcellulose medium, Biomaterials, 2012, 33(18), 4508–4514 Search PubMed.
- H. Ota, R. Yamamoto, K. Deguchi, Y. Tanaka, Y. Kazoe, Y. Sato and N. Miki, Three-dimensional spheroid-forming lab-on-a-chip using micro-rotational flow, Sens. Actuators, B, 2010, 147(1), 359–365 CrossRef.
- Y. Akiyama and K. Morishima, Label-free cell aggregate formation based on the magneto-Archimedes effect, Appl. Phys. Lett., 2011, 98(16), 163702 Search PubMed.
- L. Y. Wu, D. Di Carlo and L. P. Lee, Microfluidic self-assembly of tumor spheroids for anticancer drug discovery, Biomed. Microdevices, 2008, 10(2), 197–202 CrossRef CAS.
- Y. Torisawa, A. Takagi, Y. Nashimoto, T. Yasukawa, H. Shiku and T. Matsue, A multicellular spheroid array to realize spheroid formation, culture, and viability assay on a chip, Biomaterials, 2007, 28(3), 559–566 CrossRef CAS.
- A. Y. Hsiao, Y. S. Torisawa, Y. C. Tung, S. Sud, R. S. Taichman, K. J. Pienta and S. Takayama, Microfluidic system for formation of PC-3 prostate cancer co-culture spheroids, Biomaterials, 2009, 30(16), 3020–3027 CrossRef CAS.
- J. Friedrich, C. Seidel, R. Ebner and A. Kunz-Schughart, Spheroid-based drug screen: considerations and practical approach, Nat. Protoc., 2009, 4(3), 309–324 Search PubMed.
- A. Khademhosseini and R. Langer, Microengineered hydrogels for tissue engineering, Biomaterials, 2007, 28(34), 5087–5092 CrossRef CAS.
- A. Khademhosseini and J. W. Nichol, Modular tissue engineering: engineering biological tissues from the bottom up, Soft Matter, 2009, 5(7), 1312–1319 RSC.
- A. P. McGuigan, D. A. Bruzewicz, A. Giavan, M. Butte and G. Whitesides, Cell encapsulation in sub-mm sized gel modules using replica molding, PLoS One, 2008, 3(5), e2258 CrossRef.
- L. N. Kim, S.-E. Choi, J. Kim, H. Kim and S. Kwon, Single exposure fabrication and manipulation of 3D hydrogel cell microcarriers, Lab Chip, 2011, 11(1), 48–51 RSC.
- P. Panda, S. Ali, E. Lo, B. G. Chung, T. A. Hatton, A. Khademhosseini and P. S. Doyle, Stop-flow lithography to generate cell-laden microgel particles, Lab Chip, 2008, 8(7), 1056–1061 RSC.
- D. Dendukuri, D. C. Pregibon, J. Collins, T. A. Hatton and P. S. Doyle, Continuous-flow lithography for high-throughput microparticle synthesis, Nat. Mater., 2006, 5(5), 365–369 CrossRef CAS.
- H. Lee, J. Kim, H. Kim, J. Kim and S. Kwon, Colour-barcoded magnetic microparticles for multiplexed bioassays, Nat. Mater., 2010, 9(9), 745–749 CrossRef CAS.
- W. H. Tan and S. Takeuchi, Monodisperse alginate hydrogel microbeads for cell encapsulation, Adv. Mater., 2007, 19, 2696–2701 CrossRef CAS.
- E. Um, D.-S. Lee, H.-B. Ryo and J.-K. Park, Continuous generation of hydrogel beads and encapsulation of biological materials using a microfluidic droplet-merging channel, Microfluid. Nanofluid., 2008, 5(4), 541–549 CrossRef.
- Y. Morimoto, W. H. Tan and S. Takeuchi, Three-dimensional axisymmetric flow-focusing device using stereolithography, Biomed. Microdevices, 2009, 11(2), 369–377 CrossRef.
- Y. Morimoto, K. Kuribayashi-Shigetomi and S. Takeuchi, A hybrid axisymmetric flow-focusing device for monodisperse picoliter droplets, J. Micromech. Microeng., 2011, 21(5), 054031 Search PubMed.
- S. Takeuchi, P. Garstecki, D. B. Weibel and G. M. Whitesides, An axisymmetric flow-focusing microfluidic device, Adv. Mater., 2005, 17(8), 1067–1072 CrossRef CAS.
- Y. T. Matsunaga, Y. Morimoto and S. Takeuchi, Molding cell beads for rapid construction of macroscopic 3D tissue architecture, Adv. Mater., 2011, 23(12), H90–H94 CrossRef CAS.
- Y. Morimoto, R. Tanaka and S. Takeuchi, Construction of 3D, layered skin, microsized tissues by using cell beads for cellular function analysis, Adv. Healthcare Mater., 2012 DOI:10.1002/adhm.201200189.
- S. Mazzitelli, L. Capretto, D. Carugo, X. Zhang, R. Piva and C. Nastruzzi, Optimised production of multifunctional microfibres by microfluidic chip technology for tissue engineering applications, Lab Chip, 2011, 11(10), 1776–1785 RSC.
- M. Yamada, S. Sugaya, Y. Naganuma and M. Seki, Microfluidic synthesis of chemically and physically anisotropic hydrogel microfibers for guided cell growth and networking, Soft Matter, 2012, 8(11), 3122–3130 RSC.
- D. Kiriya, M. Ikeda, H. Onoe, M. Takinoue, H. Komatsu, Y. Shimoyama, I. Hamachi and S. Takeuchi, Meter-long and robust supramolecular strands encapsulated in hydrogel jackets, Angew. Chem., Int. Ed., 2011, 51(7), 1553–1537 Search PubMed.
- H. Onoe, R. Gojo, Y. Tsuda, D. Kiriya, M. Kato-Negishi and S. Takeuchi, Cell fibers: construction of centimeter-scale 3D tissues by weaving, 14th International Conference on Miniaturized Systems for Chemistry and Life Sciences, 2010, pp. 629–631.
- K. H. Lee, S. J. Shin, Y. Park and S. H. Lee, Synthesis of cell-laden alginate hollow fibers using microfluidic chips and microvascularized tissue-engineering applications, Small, 2009, 5(11), 1264–1268 CrossRef CAS.
- E. Kang, G. S. Jeong, Y. Y. Choi, K. H. Lee, A. Khademhosseini and S. H. Lee, Digitally tunable physicochemical coding of material composition and topography in continuous microfibres, Nat. Mater., 2011, 10(11), 877–883 CrossRef CAS.
- S. P. Herbert and D. Y. R. Stainier, Molecular control of endothelial cell behaviour during blood vessel morphogenesis, Nat. Rev. Mol. Cell Biol., 2011, 12(9), 551–564 Search PubMed.
- Y. Du, E. Lo, S. Ali and A. Khademhosseini, Directed assembly of cell-laden microgels for fabrication of 3D tissue constructs, Proc. Natl. Acad. Sci. U. S. A., 2008, 105(28), 9522–9527 CrossRef CAS.
- B. Zamanian, M. Masaeli, J. W. Nichol, M. Khabiry, M. J. Hancock, H. Bae and A. Khademhosseini, Interface-directed self-assembly of cell-laden microgels, Small, 2010, 6(8), 937–944 CrossRef CAS.
- D. A. Bruzewicz, A. P. McGuigan and G. M. Whitesides, Fabrication of a modular tissue construct in a microfluidic chip, Lab Chip, 2008, 8(5), 663–671 RSC.
- S. E. Chung, W. Park, S. Shin, S. A. Lee and S. Kwon, Guided and fluidic self-assembly of microstructures using railed microfluidic channels, Nat. Mater., 2008, 7(7), 581–587 CrossRef CAS.
- N. C. Rivron, E. J. Vrij, J. Rouwkema, S. Le Gac, A. van den Berg, R. K. Truckenmuller and C. A. van Blitterswijk, Tissue deformation spatially modulates VEGF signaling and angiogenesis, Proc. Natl. Acad. Sci. U. S. A., 2012, 109(18), 6886–6891 Search PubMed.
- P. Calvert, Printing cells, Science, 2007, 318(5848), 208–209 CrossRef CAS.
- C. Norotte, F. S. Marga, L. E. Niklason and G. Forgacs, Scaffold-free vascular tissue engineering using bioprinting, Biomaterials, 2009, 30(30), 5910–5917 CrossRef CAS.
- V. Mironov, R. P. Visconti, V. Kasyanov, G. Forgacs, C. J. Drake and R. R. Markwald, Organ printing: tissue spheroids as building blocks, Biomaterials, 2009, 30(12), 2164–2174 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2013 |
