Advanced nanoparticle generation and excitation by lasers in liquids†
Stephan Barcikowski*a and Giuseppe Compagninib
aTechnical Chemistry I, University of Duisburg-Essen and Center for Nanointegration Duisburg-Essen CENIDE, Universitaetsstrasse 7, 45141 Essen, Germany. E-mail: stephan.barcikowski@uni-due.de
bDipartimento di Scienze Chimiche, Università di Catania, Viale A.Doria 6, Catania 95125, Italy
Abstract
Today, nanoparticles are widely implemented as functional elements onto surfaces, into volumes and as nano-hybrids, resulting for example in bioactive composites and biomolecule conjugates. However, only limited varieties of materials compatible for integration into advanced functional materials are available: nanoparticles synthesized using conventional gas phase processes are often agglomerated into micro powders that are hard to re-disperse into functional matrices. Chemical synthesis methods often lead to impurities of the nanoparticle colloids caused by additives and precursor reaction products. In the last decade, laser ablation and nanoparticle generation in liquids has proven to be a unique and efficient technique to generate, excite, fragment, and conjugate a large variety of nanostructures in a scalable and clean manner. This editorial briefly highlights selected recent advancements and critical aspects in the field of pulsed laser-based nanoparticle generation and manipulation, including exemplary strategies to harvest the unique properties of the laser-generated nanomaterials in the field of biomedicine and catalysis. The presented critical aspects address future assignments such as size control and scale-up.
1. Motivation for applying laser ablation and laser excitation in liquids
In emerging sectors such as medical technology, biotechnology and energy technology, nanoparticles are playing an increasingly important role. They are embedded into materials or attached to molecules in order to achieve specific effects. In the billion-dollar market of nanotechnology, the share of such nanoparticles shows above-average growth rates, with metal nanoparticle colloids having a market volume of 300 bill. EUR in 2010.1However, most of the above-mentioned applications require nanomaterials with specific surface activities and this frequently has to be accomplished independently with respect to the production methods. In this respect, nanoparticles without residual chemical precursors and without the addition of any stabilizing ligands verifiably increase the efficiency, which applies to nano-applications in medical technology, catalysis, biotechnology, and other nanotechnology markets. In addition, expensive follow-up treatments and cleaning2 become unnecessary (e.g., calcination of catalyst supports or filtration of bio-conjugates). Pulsed Laser Ablation and excitation of nanoparticles in Liquids (PLAL) has been proposed as an alternative synthesis method, addressing some of these drawbacks of the current fabrication methods. By this method, laser radiation is used to ablate a solid target in a liquid environment, resulting in the formation of a nanoparticle colloid. A huge advantage of this synthesis route is its independence from chemical precursors (such as metal-organic substances), avoiding the use of toxic substances or by-products that possibly adsorb onto the nanoparticle surface. Those adsorbates may impose a toxicology issue in biological applications,3 or block the surface against further functionalization. In contrast, ligand-free nanoparticles, for example, benefit from a higher therapeutic window during cell transfection4 and the nanoparticle surface activity measured by conjugation efficiency and adsorption capacity is significantly higher.5–7
Driven by the novel colloidal properties that are achieved not only by laser ablation in liquid, but also by laser fragmentation and laser melting, a vivid community is currently building up, also stimulated during the biannual ANGEL conference†, to which this editorial is dedicated. The number of publications on laser ablation and nanoparticle generation in liquids has increased by a factor of 15 in the last decade,8 where journals from physical chemistry or chemical physics are consistently dominant. As can be seen in Fig. 1, this trend of increased publication activity in the field of PLAL is ongoing, with an increased number of publications and an even higher increased rate of citations, with currently more than 700 citations per year.
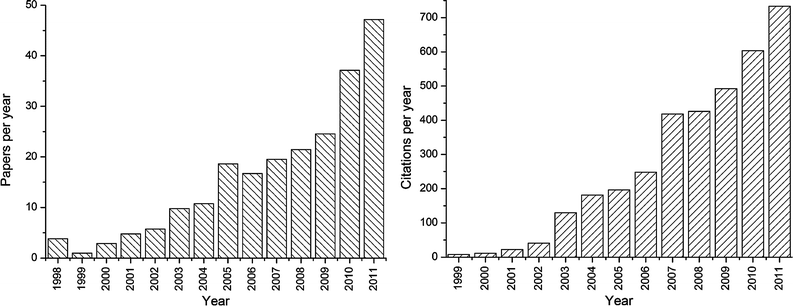 | ||
| Fig. 1 Publications in the field of nanoparticle generation and excitation by lasers in liquids. Top: number of publications per year (not cumulative). Bottom: number of citations per year (not cumulative). Database: Web of Science, using refined search term published in ref. 8. | ||
It would be beyond the scope of this editorial to comprehensively introduce all the new exciting research findings that were recently presented and published, however, we aim to highlight current trends and topics. After trying to shortly point out central characteristics of laser-generated colloids, we give some examples where these characteristics are exploited and finally draw conclusions on the problems on the road that are still unsolved.
2. What makes the difference?
Laser ablation is a physical process that is fundamentally different from chemical nanoparticle synthesis routes. In the past, this process has been widely performed in a vacuum chamber (UHV or moderate gas pressure) and only recently systematic studies of laser ablation in liquids have been conducted. Such laser-generated colloidal nanoparticles are characterized by the following potential advantages:(a) versatility: compared to common chemical reduction or precipitation routes which rely on the availability of the respective precursors, this physico-chemical laser ablation method allows for the production of nanoparticles from any base material (metal, alloy, semiconductor, ceramic) and in numerous liquids, including polymer-dissolving organic liquids or even ionic liquids.
(b) availability of precursors: the solid raw material for laser-based nanoparticle production is easily available and often 5 to 10 times cheaper than commonly used metal-organic precursor compounds.
(c) purity: the ligand-free synthesis method gives access to highly pure colloids resulting in a high nanoparticle surface activity – the particle surface is not blocked by the chemical ligands or residues of the reducing agents, which leads to significant advantages for quality-demanding nanotechnology applications in biomedicine and catalysis.
(d) electroaffinity: laser-generated (noble) metal colloids are electron acceptors because of surface atom oxidation9,10 resulting in a relatively high particle surface charge. If this charge is not screened by impurities, the particles attract oxygen species and the resulting surface charge triggers electrostatic repulsion. At the same time, the Lewis-acidity of the inorganic nanoparticle makes it possible to achieve efficient electron-donative ligand adsorption.11,12
(e) defects: under controlled conditions, defect-rich materials and suboxides13–15 can be sythesized, potentially broadening the range of optical, semiconducting, or catalytic properties.
In the last few years, more and more examples of synthesis strategies that fully harvest at least one of the described unique features have been published (e.g., see recent review of Zheng et al.16). In the following, we exemplarily present model strategies which consequently benefit from one of these characteristics, or even a combination of the unique features.
3. Examples of strategies harvesting the unique properties of PLAL-derived nanomaterials
3.1 Submicron spheres
Under appropriate conditions, in particular with a carefully selected wavelength and laser fluence, laser ablation of solids or post-irradiation of nanoparticle aggregate dispersions lead to the formation of hollow or solid submicron spheres. It recently has been discovered that pulsed UV laser ablation benefits from bubble-associated condensation or ripening, which results in assemblies of nanoparticles forming hollow submicron spheres, mostly of oxidic materials.17–19Under more gentle conditions, in particular with a low fluence of only several hundreds of mJ cm−2, pulsed laser melting can be employed for the production of mostly spherical particles of several hundreds of nm in size.20,21 This was initially reported for B4C spherical particles, and the wide applicability of the method has recently been demonstrated by Koshizaki et al. for various kinds of materials, such as metals (Au, Ag, etc.), oxides (ZnO, TiO2, etc.) and semiconductors (Si, GaP, etc.).20,21
3.2 Bioconjugated nanoparticles
The conjugation of laser-generated gold nanoparticles with biomolecules allows for their use in nano-gold applications that have been demonstrated in biotechnology research. The use of laser-generated particles often leads to significantly better results in medical technology because they are more reactive,22 as well as less toxic,4 than chemically synthesized materials, meaning that they open up a larger applicability window. The nanoparticle surface coverage with bio-functional ligands is 5 times higher than for chemically synthesized nano-gold,6 which is advantageous for applications that benefit from a high specificity, for example in the case when antibody, DNA or aptamer-targeting is used.23 Hence, the purity of the gold colloids implies three advantages for biological applications: a more efficient binding to biomolecules (higher yield), a higher ligand load (relevant to specificity of targeting), and less cleaning effort (no interfering chemical residuals).3.3 Catalysis
The large variety of nanoparticles that can be produced using PLAL and the purity of the colloids implies two main advantages for catalytic applications, namely a significant increase of the particles’ sorption efficiency as well as the fact that ligand-free heterogeneous catalysts do not require cleaning.Because of surface defects, laser-generated particles exhibit an electrical surface charge that stabilizes them electrostatically without the use of ligands. The absence of a ligand layer has a positive effect on the affinity of nanoparticles to the carrier surface and this increases the long-term stability and activity of these materials, since no catalytic centers are blocked by ligands. When comparing laser-generated nanoparticles with chemically synthesized nanoparticles (containing residual citrate), the deposition efficiency of laser-generated nanoparticles is 20 times higher in the ligand-free state compared to the citrate-stabilized nanoparticle surface.7
Another example in the field of catalysis is the use of metal nanoparticles obtained by laser ablation in liquids for the catalytic reduction of CO24 and photocatalytic hydrogen production. Moreover, if TiO2 is mixed with graphite silica (GS) in methanol, it has been observed that such a system has an enhanced activity when the particles are generated using lasers in the liquid phase.25 The improvement may be attributed to the combined effect of the fineness of GS particles, leading to an increased reaction surface area and the aggregation of laser generated GS and raw TiO2.
3.4 Example: electrophoresis and electrodeposition
The combination of electrophoresis, in particular dielectrophoresis, with PLAL benefits greatly from the plasma-based, liquid-confined process that releases charged species into the medium where an electric field can be applied in situ or ex situ. In situ electric-field assisted laser PLAL aids the anisotropic assembly of charged precursors to form various nonspherical structures in the vicinity of the electrodes, such as oxidic nanorods26 and graphene multiwalls,27 possibly by orientation-directed ripening of precursors with a lower mass than the final nanostructure collected at the electrodes. Ex situ application of electric field in the PLAL media is used for the dielectrophoretic deposition of the colloidal nanoparticles. Since the colloidal nanoparticles created using PLAL are highly charged, electrophoretic nanoparticle velocities higher than 50 μm s−1 can be achieved, in particular when electrophoresis is carried out in an organic liquid.28Using electrophoresis of gold nanoparticles produced using laser ablation in liquids, substrates for the surface-assisted laser desorption ionization (SALDI) technique were prepared, with better analytical results because of an increased uniformity and a higher control of thickness.29 Three-dimensional objects were also coated using this method, at the example of neutral electrodes coated with platinum nanoparticles.30 Even more sophisticated, hierarchical structures are accessible using this method, e.g. surface decoration of ZnO nanorod arrays with gold nanoparticles prepared using PLAL.31
3.5 Nanocomposites – nanoparticle polymer composites
The purity of laser-generated colloids allows the binding of the particles to the polymer without the need for additional matrix binders that could contaminate and influence an approved polymer. Direct laser ablation in a solvent of the final polymer utilizes this polymer as a “binder” for the nanoparticles,32 and a homogeneous, aggregation-free dispersion in the solid composite is achieved. Changing the solid target immersed in the polymer solution gives access to multi-material metal nanoparticle polymer composites, where an appropriate nanoparticle material combination of copper and silver allows for the controlled release of bioactive metal ions.333.6 Metastable phases
Several parameters influence the properties of the obtained particles: the duration of the laser pulse, the solvent used, the laser fluence, and the beam wavelength.16 Nevertheless, the intimate mechanisms involved in the nucleation and phase transition of nanocrystals during PLAL are not yet well understood. It has to be considered that quenching times in PLAL are so short that the metastable phases which form during the intermediate stage of the conversion can be frozen into this metastable state, and form the synthesized final products. Moreover the use of a liquid medium confines the expansion of the plasma. This confinement effect has been used to synthesize diamond34 and BN35 particles as well as exotic nanostructure aggregations such as inorganic fullerene-like nanoparticles and metallic nanowires.36–38Shock wave and cavitation bubble phenomena are currently extensively studied through a sophisticated series of experiments to understand the physical and the chemical mechanisms of particle generation and transformation in great detail.39 Frequently these investigations are combined with modelling studies where a number of different irradiation parameters and material properties are considered.40
4. Critical future assignments
The exploitation of the prospects of nanoparticle generation and excitation using lasers in liquids requires overcoming the limitations in size control and productivity. Despite the fact that some physico-chemical and technical problems are still unsolved, strategies have been presented to target these issues, including size control as well as refinement of temporal and spatial parameters for scale-up.4.1 Size control
For many applications, it is necessary that particle sizes can be adjusted by using appropriate laser and process parameters. There are various approaches to control the nanoparticle size during PLAL, of which four important tracks are exemplarily summarized in the following:(a) Quenching: by adjusting the concentration of a biomolecule quencher (e.g. a DNA or peptide) dissolved in water, defined particle sizes especially around 5–10 nm can be tuned, as has been investigated intensively e.g. for gold nanoparticles.22
(b) Residence time: PLAL in a flow reactor allows the adjustment of nanoparticle sizes in a range of about 20 to 50 nm.41 Further optimization of the fluid dynamics parameters could extend this tunable size range.
(c) Fragmentation: By means of laser fragmentation of organic and inorganic microparticle suspensions and colloids, different particle sizes become available.9,11,42–44 However, it is often a challenge to separate the starting material from the product during the dynamic process. Recently, it was demonstrated that the use of high-pressure chambers provide a significant advancement of the laser fragmentation method.45
(d) Selective melting: monodisperse submicron spheres are accessible by using pulsed laser melting of nanoparticle aggregates.21,46,47 The size selectivity of this method may benefit from sedimentative separation of educt and product.
While immense effort is being invested in minimizing the colloid's polydispersity by employing physical methods, it is still difficult to create nanoparticle colloids using PLAL that are simultaneously ligand-free, monomodal, and monodisperse.
4.2 Productivity: non-linearity of scale-up
Although the gram scale productivity is already accessibly using a few tens of watt output power during PLAL,48 there are several main obstacles that need to be overcome during scale-up. The reason is, that above a certain threshold, the process does scale non-linearly. Of course, the extent of non-linearity depends on the material, process, and laser beam parameters so that a general route for production at a larger scale cannot be drawn. However, examples of good practice may help to identify possible tracks that allow the harvest of as much material as possible, ideally without reduction of colloidal quality. The basic idea is to couple in as much laser pulse energy and fluence as possible. Principally, this can be achieved by using tighter focusing (higher fluence), extended processing time, or increased laser energy.First, focusing is limited by the vaporization and breakdown threshold of the liquid, with maximum productivity at a defined set of parameters such as the focal distance, liquid layer thickness and lens offset.49 Second, prolonged processing time allows the increase of the ablated mass. However, in the case of batch ablation chambers, the nanoparticle concentration gradually increases, so that post-irradiation of the nanoparticles comes into play and it is possible that shielding of the laser beam by the colloids occurs before the laser beam can reach the target. Here, processing time interval optimization may help to find the optimum production conditions. For strong absorbers such as plasmon-resonant silver or aggregating ZnO tetrahydrofuran colloids, it is notable that cumulation of shorter processing time intervals may yield more nanomaterials than a longer interval. For example, 60 minutes PLAL of Ag with second harmonic picosecond pulses saturates at a productivity of <2.5 mg h−1, whereas cumulation of 4 × 15 min processing intervals allows one to fabricate >4 mg h−1.50 When longer wavelengths are employed there is less sensitivity against such saturation effects, but on the other hand, shorter wavelengths may be desired in order to benefit from fragmentation and size reduction during ablation. Third, up-scaling may be achieved simply by increasing the laser output power, which is proportional to the pulse energy and repetition rate. Since the laser pulse energy is limited by the damage threshold of the optical components of the experimental setup, higher laser power is often achieved by increasing the repetition rate. For currently available high-power ultrashort-pulsed systems, repetition rates typically scale towards the 100 kHz to MHz regime, thereby reaching ≫50 W with only several hundred μJ pulse energy. Unfortunately, the ablation efficiency (ablated mass per pulse) may drastically decrease with repetition rates above ca. 5–10 kHz. The reason for this effect is shielding of the cavitation bubble during its lifetime, which has been characterized by Sasaki et al. to cover several hundred microseconds with lateral expansion up to millimetres depending on the properties of the liquid used.51 Strategies for temporal and spatial bypassing of the cavitation bubble have been reported recently. For example, Sasaki et al. have shown that application of external pressure to the ambient liquid can decrease the lifetime from 200 μs at 0.1 GPa to around 10 μs at higher liquid pressures (3 GPa). Alternatively, an optimal kHz repetition rate combined with fast scanning allows for temporal and spatial bypassing of the cavitation bubble increasing productivity.52
In addition to the above mentioned strategies, simple technical measures may contribute to a more robust setup, which often is a prerequisite for processing on the scale of hours and may already lead to increased productivity. After several minutes of PLAL, static bubbles occasionally stick to the target surface, which may easily be removed by liquid flow. Accordingly, ablation in liquid flow improves the reproducibility and increases the nanoparticle productivity by a factor of 3.8 compared to when ablation is performed in a stationary liquid.53
5. Summary and conclusion
Pulsed laser ablation in liquids and laser excitation of colloidal nanoparticles allows the fabrication of advanced materials with integrated nanostructures bearing unique properties. Such properties benefit from the initially ligand-free, charged, often defect-rich state of the synthesized nanoparticles. In addition, novel structures such as bivalent nanoparticle conjugates, hollow particles, and spherical submicron nanoparticles are accessible often in a single synthesis step. Currently, the prospective advantages are being exploited, e.g. the area of biomedicine and catalysis, harvesting higher conjugation or adsorption efficiency, higher biocompatibility and less cleaning efforts. Further development of the method may benefit from better size control and consequent use of optimal parameters to increase ablation efficiency and productivity. It also has to be pointed out that even two decades after first publication of the PLAL method for nanoparticle generation, the exact nanoparticle formation mechanism during and after cavitation in liquid confinement is still not fully understood.Every year, novel variations of the method are presented and significant achievements have been made during the application of PLAL-generated nanoparticles. Obviously, in this emerging research field, many white spots are still waiting to be discovered.
Acknowledgement
We gratefully acknowledge the European Optical Society (EOS), Silke Kramprich and Julia Dalichow for organizing the conference on laser ablation and nanoparticle generation in liquids ‘ANGEL 2012’, and D. D. van't Zand for help during manuscript revision.Notes and references
- R. N. W. Bundesministerium für Bildung and Forschung (BMBF und Nanotechnologie:, Nano.DE-Report 2011 - Status quo der Nanotechnologie in Deutschland, Bonn. Berlin, 2011.
- J. A. Lopez-Sanchez, N. Dimitratos, C. Hammond, G. L. Brett, L. Kesavan, S. White, P. Miedzak, R. Tiruvalam, R. L. Jenkins, A. F. Carley, D. Knight, C. J. Kiely and G. J. Hutchings, Nat. Chem., 2011, 3, 551 CrossRef CAS.
- C. Uboldi, D. Bonacchi, G. Lorenzi, M. I. Hermanns, C. Pohll, G. Baldi, R. E. Unger and C. J. Kirkpatrick, Part. Fibre Toxicol., 2009, 6, 18 CrossRef.
- M. C. Durán, S. Willenbrock, A. Barchanski, J. M. Müller, A. Maiolini, J. A. Soller, S. Barcikowski, I. Nolte, K. Feige and H. M. Escobar, J. Nanobiotechnol., 2011, 9, 47 CrossRef.
- W. Qian, M. Murakami, Y. Ichikawa and Y. Che, J. Phys. Chem. C, 2011, 115, 23293 CAS.
- S. Petersen and S. Barcikowski, J. Phys. Chem. C, 2009, 113, 19830 CAS.
- P. Wagener, A. Schwenke and S. Barcikowski, Langmuir, 2012, 28, 6132 CrossRef CAS.
- S. Barcikowski, F. Devesa and K. Moldenhauer, J. Nanopart. Res., 2009, 11, 1883 CrossRef.
- H. Muto, K. Yamada, K. Miyajima and F. Mafuné, J. Phys. Chem. C, 2007, 111, 17221 CAS.
- J.-P. Sylvestre, S. Poulin, A. V. Kabashin, E. Sacher, M. Meunier and J. T. H. Luong, J. Phys. Chem. B, 2004, 108, 16864 CrossRef CAS.
- K. Yamada, Y. Tokumoto, T. Nagata and F. Mafuné, J. Phys. Chem. B, 2006, 110, 11751 CrossRef CAS.
- F. Mafuné, Chem. Phys. Lett., 2004, 397, 133 CrossRef.
- N. G. Semaltianos, S. Logothetitis, N. Frangis, I. Tsiaoussis, W. Perrie, G. Dearden and K. G. Watkins, Chem. Phys. Lett., 2010, 496, 113 CrossRef CAS.
- J. S. Golightly and A. W. Castleman Jr., J. Phys. Chem. B, 2006, 110, 19979 CrossRef CAS.
- H. Zeng, W. Cai, Y. Li and P. Liu, J. Phys. Chem. B, 2005, 109, 18260 CrossRef CAS.
- H. B. Zeng, X. W. Du, S. C. Singh, S. A. Kulinich, S. K. Yang, J. P. He and W. P. Cai, Adv. Funct. Mater., 2012, 22, 1333 CrossRef CAS.
- Z. Yan, Q. Zhao and D. B. Chrisey, Mater. Chem. Phys., 2011, 130, 403 CrossRef CAS.
- Z. J. Yan, R. Q. Bao, C. M. Busta and D. B. Chrisey, Nanotechnology, 2011, 22, 265610 CrossRef.
- Z. Yan, R. Bao, R. N. Wright and D. B. Chrisey, Appl. Phys. Lett., 2010, 97, 124106 CrossRef.
- H. Wang, A. Pyatenko, K. Kawaguchi, X. Li, Z. Swiatkowska-Warkocka and N. Koshizaki, Angew. Chem., Int. Ed., 2010, 49, 6361 CrossRef CAS.
- H. Wang, N. Koshizaki, L. Li, L. Jia, K. Kawaguchi, X. Li, A. Pyatenko, Z. Swiatkowska-Warkocka, Y. Bando and D. Golberg, Adv. Mater., 2011, 23, 1865 CrossRef CAS.
- S. Petersen and S. Barcikowski, Adv. Funct. Mater., 2009, 19, 1167 CrossRef CAS.
- J. G. Walter, S. Petersen, F. Stahl, T. Scheper and S. Barcikowski, J. Nanobiotechnol., 2010, 8, 21 CrossRef , Nr. 21.
- F. Lin, J. Yang, S.-H. Lu, K.-Y. Niu, Y. Liu, J. Sun and X.-W. Du, J. Mater. Chem., 2010, 20, 1103 RSC.
- M. Ikeda, Y. Kusumoto, H. Yang, S. Somekawa, H. Uenjyo, M. Abdulla-Al-Mamun and Y. Horie, Catal. Commun., 2008, 9, 1329 CrossRef CAS.
- P. Liu, Y. Liang, X. Lin, C. Wang and G. Yang, ACS Nano, 2011, 5, 4748 CrossRef CAS.
- G. Compagnini, M. Sinatra, P. Russo, G. C. Messina, O. Puglisi and S. Scalese, Carbon, 2012, 50, 2362 CrossRef CAS.
- A. Menéndez-Manjón, J. Jakobi, K. Schwabe, J. K. Krauss and S. Barcikowski, J. Laser Micro/Nanoeng., 2009, 4, 95 CrossRef.
- T. Tsuji, T. Mikuzi, M. Yasutomo, M. Tsuji, H. Kawasaki, T. Yonezawa and F. Mafuné, Appl. Surf. Sci., 2011, 257, 2046 CrossRef CAS.
- J. Jakobi, A. Menéndez-Manjón, V. S. K. Chakravadhanula, L. Kienle, P. Wagener and S. Barcikowski, Nanotechnology, 2011, 22, 145601 CrossRef.
- H. He, W. Cai, Y. Lin and B. Chen, Langmuir, 2010, 26, 8925 CrossRef CAS.
- G. Compagnini, A. A. Scalisi and O. Puglisi, Phys. Chem. Chem. Phys., 2002, 4, 2787 RSC.
- A. Hahn, S. Günther, P. Wagener and S. Barcikowski, J. Mater. Chem., 2011, 21, 10287 RSC.
- S. R. J. Pearce, S. J. Henley, F. Claeyssens, P. W. May, K. R. Hallam, J. A. Smith and K. N. Rosser, Diamond Relat. Mater., 2004, 13, 661 CrossRef CAS.
- Q. X. Liu, C. X. Wang and G. W. Yang, Phys. Rev. B, 2004, 71, 15542 Search PubMed.
- G. Compagnini, M. G. Sinatra, G. C. Messina, G. Patanè, S. Scalese and O. Puglisi, Appl. Surf. Sci., 2012, 258, 5672 CrossRef CAS.
- E. T. Y. Lee, Y. Shimotsuma, M. Sakakura, M. Nishi, K. Miura and K. Hirao, J. Nanosci. Nanotechnol., 2009, 9, 918 Search PubMed.
- V. Lebedev, P. Moroshkin, B. Grobety, E. Gordon and A. Weis, J. Low Temp. Phys., 2011, 165, 166 CrossRef CAS.
- A. De Giacomo, M. Dell'Aglio, O. De Pascale and M. Capitelli, Spectrochim. Acta, Part B, 2007, 62, 721 CrossRef.
- T. E. Itina, J. Phys. Chem. C, 2011, 115, 5044 CAS.
- C. L. Sajti, A. Barchanski, P. Wagener, S. Klein and S. Barcikowski, J. Phys. Chem. C, 2011, 115, 5094 CAS.
- R. Yasukuni, T. Asahi, T. Sugiyama, H. Masuhura, M. Sliwa, J. Hofkens, F. C. De Schryver, M. Van der Auweraer, A. Herrmann and K. Müllen, Appl. Phys. A: Mater. Sci. Process., 2008, 93, 5 CrossRef CAS.
- S. Besner, A. V. Kabashin, F. M. Winnik and M. Meunier, Appl. Phys. A: Mater. Sci. Process., 2008, 93, 955 CrossRef CAS.
- J.-P. Sylvestre, M.-C. Tang, A. Furtos, G. Leclair and M. Meunier, J. Controlled Release, 2011, 149, 273 CrossRef CAS.
- S. Hashimoto, D. Werner and T. Uwada, J. Photochem. Photobiol., C, 2012, 13, 28 CrossRef CAS.
- Y. Ishikawa, S. Takeshi and N. Koshizaki, J. Nanosci. Nanotechnol., 2010, 10, 5467 CrossRef CAS.
- Y. Ishikawa, Q. Feng and N. Koshizaki, Appl. Phys. A: Mater. Sci. Process., 2010, 99, 797 CrossRef CAS.
- C. L. Sajti, R. Sattari, B. N. Chichkov and S. Barcikowski, J. Phys. Chem. C, 2010, 114, 2421 CAS.
- A. Menéndez-Manjón, P. Wagener and S. Barcikowski, J. Phys. Chem. C, 2011, 115, 5108 Search PubMed.
- A. Schwenke, P. Wagener, S. Nolte and S. Barcikowski, Appl. Phys. A: Mater. Sci. Process., 2011, 104, 77 CrossRef CAS.
- K. Sasaki, T. Nakano, W. Soliman and N. Takada, Appl. Phys. Express, 2009, 2, 046501 CrossRef.
- P. Wagener, A. Schwenke, B. N. Chichkov and S. Barcikowski, J. Phys. Chem. C, 2010, 114, 7618 CAS.
- S. Barcikowski, A. Menéndez-Manjón, B. N. Chichkov, M. Brikas and G. Račiukaitis, Appl. Phys. Lett., 2007, 91, 083113 CrossRef.
Footnote |
| † Conference series on Laser Ablation and Nanoparticle Generation in Liquids (http://www.angel-conference.org). |
| This journal is © the Owner Societies 2013 |
