Microwave heating for rapid conversion of sugars and polysaccharides to 5-chloromethyl furfural†
S. W.
Breeden
a,
J. H.
Clark
a,
T. J.
Farmer
a,
D. J.
Macquarrie
*a,
J. S.
Meimoun
b,
Y.
Nonne
c and
J. E. S. J.
Reid
a
aGreen Chemistry Centre of Excellence, Department of Chemistry, University of York, Heslington, York, YO10 5DD, UK. E-mail: duncan.macquarrie@york.ac.uk; Fax: +44 (0)1904 322705; Tel: +44 (0)1904 322568
bENSICAEN, 6 boulevard Maréchal Juin, 14050 CAEN Cedex 04, France
cESCOM, 1 allée du réseau Jean-Marie Buckmaster, 60200 Compiegne, France
First published on 18th October 2012
Abstract
A range of carbohydrates has been rapidly and selectively converted to 5-chloromethyl furfural using microwave heating in a biphasic reaction system with a range of organic solvents. Fructose and inulin were especially effective for production of this valuable bio-platform molecule, with yields of >70% obtained in 15 minutes under microwave heating. Yields from cellulose were dramatically increased with ball mill pre-treatment, this being associated with a reduction in polysaccharide crystallinity.
The need for the chemical and associated industries to move away from unsustainable fossil derived building block molecules to those sourced from biomass is well documented and extensively discussed.1,2 A key step in this progression from fossil to bio-derived chemicals and materials is the establishment of a range of simple, yet functional, bio-platform molecules, from which more complex compounds and materials can be produced.3 These bio-platform molecules must be sustainably and cheaply produced from biomass, avoiding competition with food production and ideally be sourced from waste streams (e.g. orange peel, waste paper and bagasse), while their production must also incorporate green chemistry techniques.4 Both pyrolysis and fermentations are currently utilised for the production of chemicals from biomass, though both have disadvantages. Pyrolysis is limited due to the complexity of the chemical mixture produced, while fermentation requires biomass pre-treatment, the addition of nutrients and difficult recovery of the molecules from the broth.5 Recent research has demonstrated the potential to selectively produce 5-chloromethyl furfural (CMF) from a range of carbohydrate sources, opening the door to a new multifunctional bio-platform molecule of great potential value.6–8 CMF can be converted to numerous valuable furan moieties by means of oxidation, reduction and aldol additions, while synthesis of various levulinate esters has also been demonstrated (Fig. 1).9–11
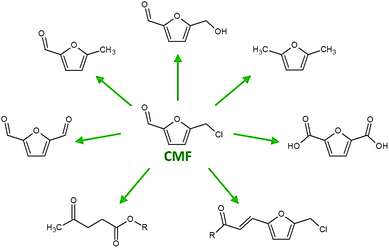 | ||
| Fig. 1 5-Chloromethyl furfural (CMF) as a bio-platform molecule. | ||
Typically, production of CMF utilises a conventionally heated biphasic reaction medium, where concentrated HCl is used for dissolution, hydrolysis, dehydration and halogenation of the carbohydrate, yielding CMF. The CMF rapidly passes into the organic layer of the biphasic system. Evident from this reaction protocol described above is the desire to selectively heat the concentrated acid layer where the reactions takes place, while avoiding heating of the organic layer and as such reducing side-product formation. While recent research has demonstrated the effective application of flow reactors for the production of CMF from sugars, it is likely that use of non-food polysaccharides such as cellulose and inulin would lead to viscosity issues with a reactor of this kind.12 Herein we report a biphasic reaction system, heated by microwave energy, which is capable of yields of CMF over 70%, but in a shorter time and with reduced by-product formation than those previously presented using conventional heating. We also show applications of greener solvents, replacing the currently favoured 1,2-dichloroethane.
During the study we investigated the potential to utilise a range of carbohydrate sources, both sugars and polysaccharides (Fig. 2), for production of CMF. When using fructose under the standard reaction protocol (entry 1a, Table 1) a yield of 71% and a selectivity of 98% was achieved in just 15 minutes microwave heating. Doubling the loading of fructose (entry 1b) resulted in only a slight reduction in yield (66%) while maintaining the excellent selectivity of the reaction (98%). This yield was further increased by lowering the reaction temperature and time (entry 1c), with a yield of 85% obtained in 10 minutes at 70 °C. Conventional heating for the same loading (entry 1d) gave both a drop in yield (75%) and selectivity (96%). When using glucose (entry 2) under microwave activation the yield of CMF dropped, in comparison to fructose under the same conditions (entry 1a, 15 minutes, 70 °C), to 37%, though the excellent selectivity remained. We believed this difference in yield was a result of the 6-membered ring pyranose configuration of the glucose requiring conversion to the 5-membered furanose before dehydration to the furan could occur, while the fructose, already in the furanose form, could undergo rapid conversion to the furan.
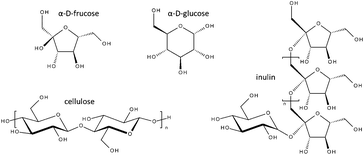 | ||
| Fig. 2 Carbohydrates used for CMF production. | ||
| Entry | Carbohydrate | % Isolated yield | % Selectivity |
|---|---|---|---|
| 1a | Fructose | 71 | 98 |
| 1b | Fructose | 66 | 98 |
| 1c | Fructose | 85 | 98 |
| 1d | Fructose | 75 | 96 |
| 2 | Glucose | 39 | >99 |
| 3 | Inulin | 70 | 98 |
| 4a | Cellulose | 5 | >99 |
| 4b | Cellulose | 12 | 98 |
| 4c | Cellulose | 24 | >99 |
| 5 | HMF | 87 | >99 |
Mascal highlighted in his research the importance of utilising non-food carbohydrates for the production of CMF, thus we wished to demonstrate the use of polysaccharides in our microwave assisted synthesis. When using the polyfructan inulin (entry 3) a 70% yield, with 98% selectivity, was achieved under standard reaction conditions, this being almost identical to that achieved for fructose under the same conditions (71%). When using microcrystalline cellulose, under the same conditions, the yield dropped significantly to just 5%. Longer reaction times (45 minutes, 4b) and fresh DCE used at 15 minute intervals (4c) increased isolated yields of CMF from cellulose, though only up to 24%. Extending the number of washes further increased yields, with a maximum of 58% achieved after 90 minutes (6 × 15 minutes, Fig. 3).
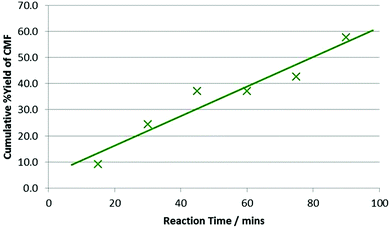 | ||
| Fig. 3 Effect of extended reaction duration on the recovered %yield of CMF from microcrystalline cellulose. 250 mg cellulose, 5 ml conc. HCl, 6 × 10 ml DCE, MW 80 °C, 6 × 15 minutes. | ||
It is known that the crystalline nature of cellulose can impact greatly on its reactivity as high crystallinity limits penetration of solvents and reactants, and that the level of crystallinity can be reduced by pre-treatment such as ball milling (Table 2).13–16 Ball mill pre-treatment of the microcrystalline cellulose prior to reaction in the microwave significantly improved CMF yield, up to 71% with 110 minutes milling pre-treatment, while maintaining selectivities >90%. Evidently, decreasing crystallinity of the cellulose allowed greater access into the polysaccharide structure, increasing dissolution and resulting in a significant increase in the rate of conversion to CMF. During Mascal's initial work using conventional heating a comparable reaction set-up (65 °C, 5 %wt cellulose loading) produced a similar yield, 71%, to our ball mill pre-treated microwave reaction, but with a reaction time of 30 hours (18 + 12 hours) compared to just 155 minutes for our combined ball milling and microwave methodology.6 A later iteration of Mascal's conventional thermal heating method produced CMF in an 84% yield from microcrystalline cellulose in a reaction time of 3 hours, this approaching the time required for the microwave methodology. 5-Hydroxymethyl furfural (HMF) was also investigated as a starting material, and was found to rapidly convert to CMF (87%, entry 5), with only CMF isolated from the organic layer.
| Entry | Ball milling/min | % Isolated yield | % Selectivity |
|---|---|---|---|
| 6a | 0 | 24 | >99 |
| 6b | 20 | 35 | 91 |
| 6c | 50 | 43 | 95 |
| 6d | 110 | 71 | 93 |
The above reactions, and those discussed in the literature, all use 1,2-dichloroethane, a solvent with serious health and safety concerns. As such we investigated the potential to use alternative solvents for our microwave assisted methodology, with the results for a range of solvents shown in Table 3. Using the lower boiling point halogenated solvents, chloroform and DCM, led to a drop in yield, though the high selectivity remained. Cyclopentyl methyl ether (CPME), toluene and cyclohexane (entries 10–12) all gave good isolated yields, but in all cases residual solvent lowered the final CMF purity. Of the alternative solvents investigated cyclohexane (entry 12a) showed the greatest promise and is our current solvent of choice, and showed a significant improvement in yield under microwave heating compared to conventional heating (entry 12b, 42%). Para-cymene, α-pinene, tbutylacetate and 2,5-dimethylfuran were also investigated as alternative solvents, though in all cases yields were low (<50%) with extensive by-product formation, typically from solvent degradation.
| Entry | Solvent | % Isolated yield | % Purity (GC) |
|---|---|---|---|
| 7 | DCE | 85 | 98 |
| 8 | Chloroform | 58 | 98 |
| 9 | DCM | 61 | 98 |
| 10 | CPME | 70 | 88 |
| 11 | Toluene | 68 | 74 |
| 12a | Cyclohexane | 75 | 91 |
| 12b | Cyclohexane | 42 | 98 |
The excellent selectivity observed throughout this investigation was likely a result of the microwave energy selectively heating the acid layer of the biphasic layer based on the difference in dissipation factors for this and the organic layer. In previous investigations into formation of CMF using conventional heating levulinic acid is formed as a by-product typically in 5–10% yields, however we did not observe this in any cases during our investigation of microwave heating.
Conclusions
We have demonstrated the simple, rapid and selective synthesis of CMF from a range of carbohydrates using a biphasic reaction mixture and microwave heating. The excellent selectivity observed in comparison to earlier syntheses of this molecule is attributed to the selective microwave heating of the acidic aqueous layer while avoiding excessive heating of the relatively microwave transparent DCE layer. The impressive yield obtained from inulin is a particular highlight as this demonstrates the ability to use a non-food polysaccharide for formation of an interesting and valuable bio-platform molecule in extremely high yields and selectivities. Ball mill pre-treatment of microcrystalline cellulose resulted in a three-fold increase of CMF yield, demonstrating the detrimental effect high crystallinity of cellulose can have for its application in CMF syntheses.Experimental procedure
α-D-Glucose, α-D-fructose, microcrystalline cellulose and inulin were purchased from Sigma-Aldrich. 1,2-Dichloroethane was purchased from Fluka Chemicals. Concentrated hydrochloric acid (S.G. 1.19, ∼37%) was purchased from Fisher Scientific. All chemicals were used as supplied, with the exception of the experiments using ball milling pre-treatment of the microcrystalline cellulose. Typical experimental procedure for microwave reaction: 250 mg of fructose was charged into a 35 ml CEM microwave reactor vessel with a magnetic stirrer bar. To this 10 ml of 1,2-dichloroethane and 5 ml concentrated HCl were added. The reaction vessel was sealed with a rubber lid (CEM) and placed in a CEM Discover SP Microwave Synthesiser. A fixed temperature method protocol with 15 minute hold time at 80 °C and high stirring speed was used, with a 200 W max power setting and a typical ramp time of 2 minutes. After microwave heating the reaction mixture was passed into a separating funnel and the lower organic layer collected, filtered and the DCE removed in vacuo yielding a light brown oil (143 mg, 71% yield, 98% purity by GC). 1H NMR (CDCl3, 400 MHz): 4.59 (s, 2 H, CH2), 6.55 (d, J = 3.6 Hz, 1 H, Ar–H), 7.16 (d, J = 3.6 Hz, 1 H, Ar–H), 9.58 (s, 1 H, CHO) ppm. 13C NMR (CDCl3, 100 MHz): 36.6 (CH2), 112.1, 121.9 (Ar), 152.9 (Ar), 156.1 (Ar), 177.8 (CHO) ppm. Spectroscopic data are in agreement with the literature.17 Purity of the isolated product was determined by GC-FID and peaks assigned using GC-MS. Ball mill pre-treatment was performed in a Retsch PM-400. Conventional heating studies were performed in 35 ml CEM microwave tubes, charged with reagents, and placed into a pre-heated water-bath for the allotted time. See ESI† for further experimental details.Notes and references
- J. J. Bozell and G. R. Petersen, Green Chem., 2010, 12, 539 RSC.
- F. Cherubini and A. H. Stromman, Biofuels, Bioprod. Biorefin., 2011, 5, 548 Search PubMed.
- T. Werpy and G. Pedersen, Top Value Added Chemicals from Biomass – Vol. 1, US Department of Energy Report, 2005.
- J. H. Clark, F. E. I. Deswarte and T. J. Farmer, Biofuels, Bioprod. Biorefin., 2009, 3, 72 Search PubMed.
- P. Gallezot, Green Chem., 2007, 9, 295 RSC.
- M. Mascal and E. B. Nikitin, Angew. Chem., Int. Ed., 2008, 47, 7924 CrossRef CAS.
- M. Mascal and E. B. Nikitin, ChemSusChem, 2009, 2, 859 CrossRef CAS.
- M. Mascal, Pat., US007829732 B2, 2010 Search PubMed.
- M. Mascal and E. B. Nikitin, Green Chem., 2010, 12, 370 RSC.
- M. Mascal and S. Dutta, Green Chem., 2011, 13, 40 RSC.
- L. A. Silks, J. C. Gordon, R. Wu and S. K. Hanson, Pat., US 2011/0040109 A1, 2011 Search PubMed.
- M. Brasholz, K. von Kanel, C. H. Hornung, S. Saubern and J. Tsanaktsidis, Green Chem., 2011, 13, 1114 RSC.
- G. Maier, P. Zipper, M. Stubicar and J. Schurz, Cellul. Chem. Technol., 2005, 39, 167 Search PubMed.
- N. Mosier, C. Wyman, B. Dale, R. Elander, Y. Y. Lee, M. Holtzapple and M. Ladisch, Bioresour. Technol., 2005, 96, 673 CrossRef CAS.
- P. Alvira, E. Tomas-Pejo, M. Ballesteros and M. J. Negro, Bioresour. Technol., 2010, 101, 4851 CrossRef CAS.
- Z. D. Liao, Z. Q. Huang, H. Y. Hu, Y. J. Zhang and Y. F. Tan, Bioresour. Technol., 2011, 102, 7953 Search PubMed.
- K. Sanda, L. Rigal, M. Delmas and A. Gaset, Synthesis, 1992, 541 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Further information regarding experimental procedures including ball-mill pretreatment, conventional heating and sample analysis. See DOI: 10.1039/c2gc36290b |
| This journal is © The Royal Society of Chemistry 2013 |
