Fifty years of plasma analysis and imaging, recollections and reflections
Freddy Adams*
Department of Chemistry, University of Antwerp, B-2610 Wilrijk, Belgium. E-mail: freddy.adams@ua.ac.be
Abstract
Spectroscopic analytical methodologies are reviewed from a personal perspective, with special emphasis on plasma analytical techniques and imaging analysis. Selected examples from the laboratory experience of the author over his career illustrate the constantly increasing sophistication (sensitivity, specificity, spatial discrimination…) of analytical techniques over the last 4–5 decades, with an unfaltering evolution with ongoing scientific and technological progress, and, at particular occasions, unexpected and abrupt swerves in this uninterrupted development process.
Introduction
In a recent paper Sydney Brenner, 2002 Nobel laureate for physiology and medicine mentioned “Students divide history into two epochs: the past 2 years and everything else before that, where Archimedes, Newton, Darwin, Mendel, Watson & Crick, inhabit a time-compressed universe as uneasy contemporaries”.1In what follows we will follow up on these comments and review briefly the recent history of some aspects of analytical chemistry with special emphasis on techniques based on plasma spectroscopy and its applications in imaging analysis. We will try to identify, from a personal viewpoint, a few of the watershed moments in the historic development process over the last 50 years, while thus banishing everything that occurred before that time to Brenner's time-compressed universe. Doing this, we will try to define some interesting areas for future development in the discipline.
The reason why we begin this account in the early 1960s, is not because it was then that I started my own scientific career as an apprentice preparing a PhD, but rather because of the appearance of a major event in the history of analytical chemistry, the transition of the discipline from the analog world centered on wet chemical analysis to the digital world using analytical instrumentation. Indeed, over a time span of only a few years, between 1960 and 1965, analog devices producing graphs and pictures, were rapidly superseded by digital devices, producing numerical analytical data with increasing abundance; these could also be manipulated with computers of increasing sophistication.
This transition produced wide-ranging changes, not only in analytical chemistry; it swept through all major scientific fields and profoundly affected our daily life. In what follows we will examine briefly some aspects of the transition as it took place in our discipline.
Black Swans and Big Waves
In his popular book, “The Black Swan: the impact of the highly improbable”, first published in 2007 and then revised and completed in 2010, Nicholas Taleb epitomizes uncommon, unexpected, unforeseeable events with lasting impact on society as Black Swans.2 Black Swans occur in the financial markets, his specialization area, but also in history, artistic accomplishments, and in science. According to Taleb, Black Swans explain about everything significant about our world. Yet, he considers—rather pessimistically—that we are hardwired to be blind to them. So, according to him, the Black Swans never factors into our planning, our economics, our politics, our business models and our lives.As far as it is applied to scientific development, the Black Swan metaphor reverberates on the basic ideas in the seminal and highly influential book (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 citations at present!) of Thomas Kuhn, first published in 1962, “The Structure of Scientific Revolutions” which was recently re-edited on the occasion of the 50th anniversary of its first edition.3 The most basic idea conveyed in this book is, that over the course of history, periods of continuity were sometimes interrupted by periods of revolutionary science, idea-driven revolutions. Progress occurs in revolutionary steps by the introduction of new paradigms, which may be new theories—new ways of looking at the world—or new technical methods that enhance observation and analysis. Since its introduction by Kuhn, the paradigm shift concept for the sciences has been used in numerous non-scientific contexts to describe abrupt changes in historic development. The iconic Black Swan concept of Nicholas Taleb (represented in Fig. 1a) is one recent vulgarization of the concept. Kuhn uses another illustration, shown in Fig. 1b, to represent a major change in a thought-pattern (paradigm shift). The duck-rabbit optical illusion illustrates how, in a paradigm shift, one can interpret the same information in two entirely different ways, until eventually, one conceptual model is replaced by another. For some period of time, the two explanations exist, then one viewpoint wins the competition.
000 citations at present!) of Thomas Kuhn, first published in 1962, “The Structure of Scientific Revolutions” which was recently re-edited on the occasion of the 50th anniversary of its first edition.3 The most basic idea conveyed in this book is, that over the course of history, periods of continuity were sometimes interrupted by periods of revolutionary science, idea-driven revolutions. Progress occurs in revolutionary steps by the introduction of new paradigms, which may be new theories—new ways of looking at the world—or new technical methods that enhance observation and analysis. Since its introduction by Kuhn, the paradigm shift concept for the sciences has been used in numerous non-scientific contexts to describe abrupt changes in historic development. The iconic Black Swan concept of Nicholas Taleb (represented in Fig. 1a) is one recent vulgarization of the concept. Kuhn uses another illustration, shown in Fig. 1b, to represent a major change in a thought-pattern (paradigm shift). The duck-rabbit optical illusion illustrates how, in a paradigm shift, one can interpret the same information in two entirely different ways, until eventually, one conceptual model is replaced by another. For some period of time, the two explanations exist, then one viewpoint wins the competition.
 | ||
| Fig. 1 The two phases in scientific revolutions. The paradigm shift to represent occurrence of new paradigms in science with (a) The Black Swan concept of Nicholas Taleb2 and (b) the duck-rabbit optical illusion of Thomas Kuhn.3 The rapid progress that follows a paradigm shift is illustrated with (c) the Big Wave as conceived by Katsushika Hokusai. | ||
When a scientific paradigm is replaced with a new one by the scientific community, it eventually gives rise to rapid succession of new scientific developments. Let us, for simplicity's sake illustrate the result of such rapid development processes as a Big Wave, using one of the editions of the well-known Japanese woodblock prints of Katsushika Hokusai (1760–1831) as a visual representation (Fig. 1c).
Examples of such scientific revolutions are well documented and were commonly identified in the past, with the scientists with whom they originate: the Copernican revolution, the Newtonian revolution, the Darwinian revolution and so on, culminating in the second half of the 20th century in the microelectronics/very large scale integration revolution (VLSI). The VLSI roadmap, also commonly identified with Moore's Law, is undoubtedly, the most prominent and lasting soliton-type Big Wave of the last century. In this particular Big Wave, analytical instrumentation based on spectroscopy and other physical principles, became the building base for progress in analytical chemistry, with development based on two correlated technologies, microelectronics with integrated circuit (IC) technology and information technology (IT).4
The rationalization principle in analytical chemistry
Analytical chemistry is governed by two opposing principles that, in a balancing act, impose choices; they can be described with the two following quotes.The first quote is from Karl Marx in his Economic and Philosophic Manuscripts (1844) and reads as “Mankind always takes up only those problems as it can solve; …we will always find that the problem itself arises only when the material conditions necessary for its solution already exist or are at least in the process of formation”. The opposite of this statement is also true: when a material condition is possible it will be offered for exploitation. In the context of our topic, it means that, when it is possible to construct a triple-quadrupole inductively coupled plasma mass spectrometer (triple Q-ICP-MS or ICP-QQQ), then someone will produce it.
The other quote expresses the rationalization principle. It comes from Albert Einstein and says “Any intelligent fool can make things bigger, more complex, and more violent. It takes a touch of genius – and a lot of courage – to move in the opposite direction.” We can also express this particular idea more concisely; it is expressed by the famous architect Mies van der Rohe as “less is more” or it can be defined as: the principle of parsimony (the simplest solutions are the best, Ockham's razor). In our example, the resulting simplicity means that when triple Q-ICP-MS is becoming available, it is therefore not necessary to apply this instrument to any problem where a less complicated instrument would be sufficient.
Plasma and imaging analysis, analytical chemistry since 1960
In the late 1950s, there were only a few instrumental techniques available for elemental analysis. Spectroscopic tools, such as spark or arc emission spectroscopy or early wavelength dispersive X-ray fluorescence spectrometry, existed already but they were not widely available in analytical laboratories. At that time, the analytical instrumentation market was also still in its infancy.Neutron activation analysis (NAA) was considered to be the most sensitive elemental analytical tool for elemental analysis with detection limits down to the nanogram per gram level for a considerable number of elements, while remaining crually insensitive to a large number of others. In fact, elemental coverage and sensitivity depended on how nature had decided on the nuclear constants of the different nuclear reactions, nuclide decay processes and gamma spectrometric emission conditions. This is why an element such as dysprosium got such a disproportionate interest in the scientific literature of those days. An alternative elemental analytical technique, based on high-resolution spark source MS was being developed for sensitive panoramic elemental analysis of solid samples at the lower ppb concentration level.
In these conditions, I started my PhD in 1961, with the late Professor Julien Hoste (1923–2011), one of the European pioneers in radiochemistry and instrumental analytical chemistry.5 The radiochemical techniques were quite popular in those days of the “Atoms for Peace” programme; they got more than adequate funding. An unexpected and unforeseen bonus was that radioanalytical techniques are based on pulse counting, thus gamma-ray spectrometry and instrumental NAA was the first analytical technique that made the transfer from analog to digital with the analog-to-digital converter and the multichannel analyzer. My first contact with the Big Wave thus occurred sometime in 1962 when I became—without any realization of the importance of the event—an early immigrant in the digital world.
The ensuing rapid development from the 1960s is illustrated in Table 1 for the range of techniques that were of most concern to me, those involving mass spectrometry. The table is quite incomplete in its coverage of the field but illustrates the importance of the Big Wave in this particular field and the appearance, in rapid succession, of a range of different techniques for elemental, organic, surface and imaging analysis.6 The dates are not more than indicative, e.g., ICP-MS instrumentation started with Alan Gray's work with a capillary direct current (DC) arc plasma coupled to a quadrupole mass spectrometer, that was published in 1975.7 This work stimulated research into the use of ICP plasmas with the first papers appearing in 1978 onwards8 and a first commercial ICP-MS instrument in 1983. The technique matured over the years and, at present, detection limits of ICP-MS far exceed those of NAA, even for the detection of radioactive nuclides.
| Ion sources | Instrumentation |
|---|---|
| Inorganic | Double focusing magnetic sector instrument (1934), time-of-flight (1946), ion cyclotron resonance (1949), quadrupole (1946), ion trap, orbitrap, double and triple combination instruments, gas chromatography and liquid chromatography coupled to mass spectrometry (1959), ion mobility mass spectrometry (1960s) |
| Spark discharge (1960s), glow discharge (1970s), inductively coupled plasma (1980s) | Detection |
| Organic | Electron multiplier tube, charge coupled device (CCD)…, instrument automation and integration, database searching techniques |
| Electron impact, collision induced dissociation (1964), chemical ionization (1968), electron spray (1968), fast atom bombardment (1981), electron capture dissociation (1998), matrix assisted laser desorption (1988) | |
| Surface and imaging | |
| Primary ion bombardment (SIMS, 1960s), glow discharge (1980s), laser ablation mass spectrometry (1990s), matrix assisted laser desorption… | |
| Biological imaging analysis, MALDI, DESI, etc. |
It is impossible to cover in any detail the evolution of analytical techniques with its unfaltering evolution with ongoing scientific and technological progress, and, at particular occasions, unexpected and abrupt swerves into Big Waves. In what follows I will give a few examples concerned with plasma techniques from my personal experience.
Examples of particular developments
The first plasma-like technique that I encountered was coincidental, when during a postdoctoral stay in the Cyclotron Institute of Texas A&M University, I was able to observe early experiments that eventually led to the discovery of Califormium-252 plasma desorption mass spectrometry. The technique evolved from experiments in which fission fragments from spontaneous or radiation induced fission were transported with a laminar ultrasonic helium jet to a measurement position away from the radiation source.9 In the course of these experiments, while experimenting with organic compounds, Macfarlane and Torgerson measured mass spectra with a time-of-flight (TOF) MS, that were representative of quasi-molecular ions for a wide variety of compounds, including amino acids, moderately large peptides, nucleotides, and natural products.10 Apparently, at the end of its energy deposition process in a sample, the heavy fission fragments are able to act as a soft ionization tool for numerous organic molecules. We will come back later on this as an early manifestation of phenomena that we now learned to master as soft plasma ionization techniques.The first mass spectrometer for elemental analysis that I worked with was based on the Mattauch–Herzog double focusing design in which the ion beam—generated by a spark or arc ionization source—is energy focused and mass separated and brought to a focus on a plane. The main advantage of this particular mass spectrometer, over alternative existing designs based on point-focusing, is that the ions of different masses that are produced at one single moment in time, are all focused onto a flat surface, which led to the use of an ion sensitive photographic plate as a detector. Compared to scanning type mass spectrometers, this configuration has a non-negligible advantage for the measurement of heterogeneous samples. Drawbacks of the technique resided with the erratic spark ion source, the bulky instrumentation, but especially with the cumbersome detection procedure involving photographic emulsions and their development and reading, their reduced dynamic range and erratic non-linear response. The Mattauch–Herzog geometry is now on the brink of a rebirth with the ICP as an ionization source and a 4800 position sensitive detector in the Spectro GmbH instrument.11
Another early plasma technique in which I became involved was glow discharge (GD) MS based on the reverse Nier–Johnson geometry with the VG-9000 instrument (Thermo Instruments). GD plasmas are frequently used as sources of atomization and excitation in spectrometric determination of trace elements in which the material's constituents may be detected either by optical emission spectroscopy (OES) or MS.12 One advantage is that the sputtering process is separated from the excitation/ionization processes. This results in minor variations in sensitivity and weak matrix dependence, thus allowing a relatively straightforward calibration.13 Until now, GD-OES and GD-MS instrumentation is still the industrial standard for depth profiling of multi-layered materials and coatings.
At present, GD-OES and GD-MS are re-emerging in radiofrequency (RF) GD instruments. Coupling radiofrequency pulsed GD to a TOF mass analyzer, quasi-simultaneous extraction of all ions is possible, and consequently, rapid depth profiling of nm-thick layers of atomic and molecular ions, thus providing information about the chemical species.14 Pulse processing GD-OES is also being proposed as a new commercial instrument by Horiba Jobin-Yvon. A third-generation Faraday-strip array detector has also been coupled to a Mattauch–Herzog mass spectrometer and dc glow-discharge ionization source.15 This provides possibilities for the simultaneous determination of many elements with good resolving power and high sensitivity.
The microwave helium-induced plasma light source and an emission spectrometer (Hewlett Packard) became popular as a chromatographic detector for speciation analysis in the late 1980s-early 1990s. In this instrument a microwave helium-induced plasma light source is generated in a water-cooled tube contained in a re-entrant cavity that is waveguide-coupled to a magnetron supplying microwave power.16 This arrangement provides a stable atomic-emission source that does not require tuning of microwaves. An elliptical mirror in the spectrometer focuses light from the plasma onto a slit, which is then dispersed by a holographic diffraction grating. A photodiode array (PDA) detector simultaneously detects the elements that have emission lines within a given wavelength span. The main advantage of this system is its very high sensitivity, which is due to the miniaturized source of radiation. It is out of service now but a similar instrument is, at present, coming back with the magnetically excited microwave plasma source of Agilent (4100 MP-AES) which is reported to provide high sensitivity, linear dynamic range, detection limits and analysis speed and works without flammable or expensive gas supply, thus significantly reducing operating costs.
ICP-MS and dynamic and static SIMS developed steadily with the availability of new scientific and technological advances to the instrumentation we know today.
These examples might give the impression that instruments are constantly refined and updated to keep them in-line with technological and scientific developments, but this does not always occur. In the early 1980s laser microprobe mass analysis with the Leyboldt-Heraeus LAMMA-500 instrument was a welcomed addition to the tools available for microscopic elemental and molecular analysis.17,18 This instrument delivers the ions produced by a focused laser beam in a reflectron-TOF system. The major drawback of the technique was the insufficient resolution for the complex fragmentation spectra that were produced. Indeed, this now looks obvious, without proper focusing methods, the mass resolution of TOF MS with gaseous ion sources is limited to ca. one atomic mass.19 With up to date instruments, this shortcoming could be easily overcome, but this was never realized, apparently, there was no willingness to optimize the design.
Analytical instrument development is an open innovation process. The necessity for teamwork in developing analytical instrumentation requires a dynamic environment where instrument makers, theorists, and experimentalists share knowledge to coordinate the renewal of instrumental development. Similar dynamic “trading zones” also exist in other areas of experimental science, such as physics20 and present-day biological research. In an article in this journal on the reunion of natural philosophy and science Christian Enke19 remarks that science (also analytical chemistry) is a human endeavor and perhaps not as objective as we might think.
Black Swans and Big Waves in analytical chemistry
It is possible to define several areas of development in analytical chemistry where paradigm shifts, and soon after that, rapid development, occur. In the discussion that follows, I define some that were of special concern for me.X-rays for analysis and microscopic imaging
From immediately after their discovery by Wilhelm Conrad Röntgen in 1895, X-rays were used for imaging. From the 1950s onwards, X-ray fluorescence (XRF) analysis with X-ray tube excitation developed as a standard multi-element technique in scientific and industrial laboratories. X-ray tubes provide isotropic diverging X-rays of too low intensities to be of much use in microscopic applications. A breakthrough in brilliance (directional radiation flux) occurred when it was realized that the spurious production of X-rays in particle accelerators could be exploited in specially designed electron storage rings for the production of intense X-ray beams. This was a major paradigm shift.The construction and use of extremely bright sources of X-rays in synchrotron radiation (SR) sources has been one of the most significant success stories of science and technology over the past few decades; their rate of improvement since the early 1960s is unmatched by other developments.21 Indeed, with a doubling time of ca. 10 months over the last 30 years the rate of increase of synchrotron radiation brilliance has been evolving faster than Moore's Law.22,23 This paradigm shift (or Black Swan) increased the radiation flux tremendously; SR sources were optimized for the production of X-ray beams of increasing brilliance.
The resulting Big Wave produced a tsunami-like impact. The unique properties of SR generated by third generation sources such as high degree of polarization and energy tunability, coupled with state-of-the-art X-ray optical elements/detectors, have provided new possibilities in various X-ray microscopy and microprobe methods both in terms of achievable spatial resolution and sensitivity. SR sources now provide sensitive elemental analysis with XRF, structural analysis with X-ray diffraction, speciation analysis with X-ray absorption spectrometry. Besides this, micro/nano-analysis, 2-D imaging analysis and 3-D tomographic imaging became possible with a spatial resolution now reaching a lower limit of 20 nm. Due to their high penetration power, X-rays also allow the study of bulk regions of thick samples to be investigated in their natural environment (air, water…). Applications are numerous throughout the literature.
From bulk analysis to microscopic analysis
Imaging is one of the most pervasive and fundamental practices in modern science.24A brief history of imaging is given in Table 2. Observation beyond the potential of the naked eye appeared as a scientific revolution with the development of the compound lens microscope (or telescope) which appeared late in the 16th century. This paradigm shift had an enormous impact, it became one of the main contributors to scientific development. Before the early 1960s, microscopy was based on conventional observational tools (optical microscopy, electron microscopy…) for the measurement of morphology and shape through the observation of interaction processes of radiation with matter. It provided little or no compositional analytical information.
| 1. ca. 1600–1960 | 2. 1960–2000 | 3. 2000– |
|---|---|---|
| Conventional imaging | Imaging analysis | Nanoscopic imaging |
| Optical microscope | Electron microscopy | Super-resolution techniques |
| Morphology, shape | Beam techniques | Hyperspectral analysis |
| Probe techniques (X-ray analysis, mass spectrometry) | Full data analysis, data management | |
| Spectral range limited (Vis, IR, UV) | Extended spectral range, spatial resolution (micron scale) | Spatial resolution (nanoscale) 4-D analysis (time) |
| Limited spatial resolution (diffraction) | 3D analysis (tomography, depth profiling) | Hyper-data cube, single atom/molecule detection |
A second paradigm shift, illustrated in Fig. 2, occurred in the early 1960s when interest in chemical analysis changed from bulk analysis, with emphasis on the average composition of a sample, to imaging analysis revealing the local compositional differences. This change issued from the development of a number of specialized analytical techniques that, with time, matured into powerful tools for visualizing structural and compositional heterogeneity. These techniques evolved first at the microscopic, then the mesoscopic level (the range between 100 and 1000 nm) and later for increasingly smaller dimensions, now down to a few nm.25 This brings us to a third paradigm shift.
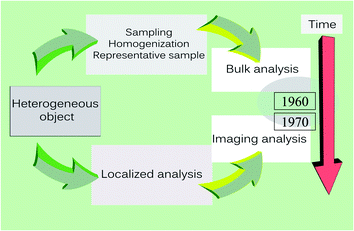 | ||
| Fig. 2 The paradigm shift in imaging, the sudden shift of attention from bulk analysis to localized analysis and the birth of imaging analysis in the early 1960s. | ||
Imaging has limitations in spatial resolution and conventionally cannot go beyond a given limit of lateral resolution, the diffraction limit of the interacting light or radiation. Diffraction fundamentally limits the resolution that a microscope is able to achieve to ca. half the wavelength of the light used. It is only since the beginning of this century that super-resolution microscopy techniques (localization microscopy) were developed. Through this third paradigm shift, such techniques have been optimized to obtain a spatial resolution down to a few nm now for visible light. Scanning near-field optical microscopy (SNOM) uses an optical probe with a sub-wavelength-sized opening, within a distance comparable to the aperture's radius, r, to a sample. Progress in the methodology led to the development of “nano-optics” able to manipulate light in sub-wavelength dimensions. Nano-local spectroscopy became a common tool for sub-100 nm spatial resolution optical imaging.26 A number of linear and non-linear spectroscopic techniques are now able to determine specific molecular components in individual cells and can even follow their fate as a function of time.
Simultaneously with this development, the ability for observation and analysis expanded from a narrow wavelength range to large portions of the electromagnetic spectrum and complex higher-order interactions in multi-spectral and hyperspectral imaging.
Hyperspectral imaging now extends also to the observation and analysis of macroscopic objects, from applications in earth observation and remote sensing to a number of new applications in art and archaeology that extend the available possibilities in conservation and restoration. Established spectroscopic techniques, such as X-ray radiography, infrared reflectography and neutron activation autoradiography, are now complemented with multi-element XRF, full spectrum infrared imaging and Raman imaging, to investigate paintings, or other art objects. Recent applications of a combination of macroscopic imaging tools, for instance, show potential for the study of paintings.27 Bruker Nano GmbH (Berlin, Germany) decided to develop the ‘M6 Jetstream’ a commercial XRF macroscanning instrument on the basis of such work.
Mass spectrometry, mass spectrometry imaging
Elemental and molecular mass spectrometric imaging is presently in full development as an instrumental technology. There is increasing emphasis on speed of analysis, robust data processing and normalization strategies28 means for increasing spatial and mass spectrometric resolution,29 atmospheric pressure measurements30 and extension of the mass range to higher masses.Recent advances in ion probe technologies have increased the dynamic range and sensitivity of SIMS, allowing routine 2-D and 3-D localization of analytes with a spatial resolution well below the submicron level.31,32 Major development areas concern the manipulation of ions and the increasing possibilities for soft ionization cluster projectiles in SIMS (SF5, Aun/Bin (n = 2, 3), C60, gas cluster ion beams) provide non-linear enhancement of ion yield, reduced chemical damage and reduced damage depth. Development in mass spectrometry imaging, such as matrix-assisted laser desorption imaging (MALDI) and dynamic electrospray ionization (DESI) are driven by applications for the analysis at the single cell level for cells, sub-cellular structures and tissues.33 Other approaches for mass spectrometry imaging are those based on near-field laser ablation with ICP-MS.34,35
In his Nobel lecture of 2002, with as a title “Electrospray wings for molecular elephants”, John Fenn described the birth and early development of methods for producing intact ions from complex and non-volatile molecules in “energy sudden” approaches. He describes how in the 1970s techniques were developed that ultimately matured into ESI and MALDI.36 Macfarlane's Cf-252 plasma desorption MS mentioned earlier was nothing else than an early manifestation of such soft ionization techniques. There, the elephant with wings can undoubtedly be identified as the emergence of a Black Swan.
Nanoscience and nanotechnology
Nanoscience and nanotechnology currently provide several promising possibilities for development in analytical chemistry. Spectroscopic techniques capable of compositional mapping on the spatial scale of a few nm became a topic of increasing interest in recent years.One of the areas in which the Big Wave nature of the impact of nanotechnology on analytical chemistry manifests itself quite clearly, is in the application of nanosize particles and fibers.37 Modern allotropes of carbon such as fullerenes, single and multi-walled nanotubes (SWNT, MWNT) and graphene are featuring actors in the nanotechnology revolution and play an important role in analytical chemistry. Nanosize particles and clusters of silica, gold, silver, or are more complex as oxides, or semiconductors such as, for instance, quantum nano-dots (QD) also account for many uses in analytical chemistry.
Metallic nanoparticles and engineered nanoplasmonic hot spots38 arise when metal nanostructures interact with light in such a way that conduction electrons are collectively excited, in resonance with the frequency of the incident radiation. They can be used to increase the sensitivity of fluorescent detection because they generate a phenomenon known as metal-enhanced fluorescence, increasing fluorescence lifetime and quantum yields. They also result in localized surface plasmon resonance (LSPR).39 Quantum dots have the potential to solve many of the problems associated with organic fluorophores in near-infrared spectroscopy. The use of nano-structured metal surfaces increased also the potential of surface-enhanced Raman scattering (SERS), tip-enhanced Raman spectroscopy (TERS) and coherent anti-Stokes Raman spectroscopy (CARS) in sensitivity and localized specificity. All this has led to many applications, including those for single molecule detection.40 Future issues of plasmonics, including those in metamaterials, and its extension to the UV and the terahertz region, have been recently reviewed by Kawata.41
Fig. 3 summarizes some of the present areas of development in this area.
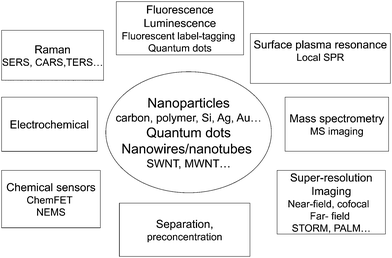 | ||
| Fig. 3 The many different uses of nanoparticles, nanofibers and nanotubes in separation and chemical analysis. | ||
Conclusion
We can now go back to where I started, with the comments of Sydney Brenner1 in which he also mentions “Historians have the luxury of looking back at human endeavor over long periods of time, but most scientists are too busy working in the present and thinking anxiously about the future and have no time to view their work in the context of what has gone before”.What are the new developments in analytical chemistry stemming from fast developing 21st century science and new enabling technologies? What are the coming killer applications that will have a great impact in the near future? The answer to such questions is far from obvious. From my undoubtedly biased personal viewpoint, I see several areas of quick and exciting development for the coming years, potential oncoming Big Waves.
Imaging analysis
New tools for imaging extend the range of wavelengths to the large parts of the electromagnetic spectrum, or high resolution mass spectra, while expanding the magnification to the atomic level. As such goals of visualization evolve, increasingly detailed 2-D pixel and 3-D voxel maps, containing datasets of increasing complexity, provide single or multiple spectral data representing composition and structure, together with other information such as density. In high-throughput mass spectrometric imaging (MSI), for example, it becomes now possible to collect the impact of the entire ion load from an ionization event as a high resolution MS spectrum, measuring the molecular ion distributions of sub-micron pixel sizes with a position-sensitive detection system.28 There will be increasing needs for the development and use of multi-technique correlations for various combinations of imaging techniques, e.g., combinations of SERS and nanoscale probe techniques, or combinations of X-ray, electron and scanning probe techniques.With hyperspectral imaging techniques covering large parts of the electromagnetic spectrum, including now the terahertz region, and various types of interaction modes, it is possible to go back with visual imagery to the pre-Darwinian times for the study of nature and to learn from a number of evolutionary tricks that evolved over billions of years of evolution.
Martin Kemp42 expresses it as follows in a recent article concerning the new applications of imaging in biology: “fundamental work done now once again involves seeing shapes and forms”, (and I would personally add, … composition and structure).
Chemometrics
The rapid growth in the size of databases creates needs for tools and techniques for intelligent data understanding. Chemometrics principles will need to evolve from the use of statistical tools, to more complex techniques in order to find answers that are hidden in overwhelmingly detailed datasets. This will require mastering knowledge discovery tools including inductive learning, pattern recognition tools, Bayesian statistics, knowledge acquisition for expert systems, information theory. In these searches for the needle in a haystack, data fishing, data mining, even data dredging techniques will be necessary.Analytical chemistry and biology
Living species are highly complex and their spatial and temporal heterogeneity plays at the single molecule level. Molecular imaging is developing rapidly, mainly as a result of its applications in the biosciences. Continuous method development and instrumentation advances provide a diverse set of analytical tools for sensitive analysis of cells and sub-cellular features with high sensitivity, spatial and even temporal resolution.43 Super-resolution microscopy techniques will revolutionize the understanding of the living cell by localizing the precise position of fluorescent molecules using probe based techniques, such as photoactivation localization microscopy (PALM) or stochastic optical reconstruction microscopy (STORM).44,45Analytical chemistry and also plasma spectrometric techniques will play a crucial role in the “-omics” revolution. Rapid progress was realized during the last decade, to promote hyphenated ICP-MS to one of the most versatile tools for the analysis of biological compounds containing covalently bound heteroatoms such as phosphorus, sulphur, selenium, or halogens in various biological samples.46,47
Biomimetics (bio-inspiration, bio-mimicry) is the study of the structure and function of biological systems as models for the design and engineering of materials and machines. The detailed study of nature eventually might provide an integration of analytical chemistry with nanotechnology and biology. This can result in sensors (ChemFET, nanoelectromechanical systems or NEMS), each of these is a paragon of parsimony, combining extreme biological specificity with nano-engineered structures. All this might provide simple tools with a specificity and sensitivity that matches massive spectroscopic instruments. Eventually, they should provide personalized DNA sequencing, high-throughput screening for pathogens and tools for characterizing proteins, all this at affordable cost.48
The fourth dimension, time
Up to the present, time is an incommensurable parameter in analysis. Now, it increasingly appears as a variable, on a par with the three spatial dimensions. The integration of time, down to the atomic scale in the analytical process can transform static imaging into the realm of dynamics in both space and time. Opportunities for imaging nanomaterials (and particularly, biostructures) in controlled conditions are numerous. Visualization of the sub-cellular localization of proteins and the dynamics of their transport, trafficking, and interactions with other cellular components is now done with single-molecule spectroscopic measurements of fluorescent proteins.49,50 Time-resolved single-molecule emission spectroscopy increases significantly the understanding of biological processes in cells, particularly by indicating transitions and dynamics.Ahmed Zewail, Nobel laureate for chemistry in 1999, summarizes space-time applications, particularly in 4-D electron microscopy51 but also fluorescence tools and label-free techniques based on Raman microscopy and imaging can be exploited for such work. The time domain that needs to be considered is enormously large; it ranges from the femtosecond (chemical reactions) to seconds or longer (movement of macromolecules in cells).
Scientific methodology
Since the digital revolution, chemical analysis evolved from the exploitation of straightforward measurements providing simple data of compositional information to strategies for handling of data collections of increasing complexity. We are presently moving to applications of analytical chemistry that are discovery (shotgun) driven, instead of hypothesis driven (targeted). As such, this development illustrates the heuristic way (using experience-based techniques for problem solving and discovery) in which the presently available visualization and analysis tools explore structure and form in nature as it evolved.The evaluation of large collections of analytical data requires data evaluation tools of increasing complexity and requires chemometric tools at higher level, as we mentioned already. Besides, traditional concepts, such as those used on limited datasets (validation tools and standard metrological concepts for evaluating trueness of the analytical results) may lose their direct meaning. In imaging analysis, for instance, the utility of the dataset concerns the structural variation pattern of composition and structure, not the data's absolute trueness. Eventually, it becomes necessary to substitute validating tools based on metrology by questioning the falsifiability, the inherent testability of the scientific hypothesis using the dataset as obtained and interpreted in the experiment. Epistemological concepts become then central validating tools, with as a basic question: can these experiments be repeated and controlled? Is this a manifestation of the cradle of a new Black Swan, an upcoming paradigm shift?
As chemical analysis now moves to the measurement of single atoms and molecules, counting the atoms or molecules in a given volume of sample provides accurate analytical results within statistical limits of the measurement set. While focusing on such minute details, however, we run certain risks with such a reductionist approach of losing the context of the object of study. Is the minute sample adequate, in other words are we overlooking the relationship of the detail to the whole it is supposed to represent? Are we not affecting our sample with our measurement tools?
On the other hand, we certainly are watching the apex of the digital Big Wave that started its course in the 1960s when we conclude with David Walt:52 “It is much easier to measure the presence or absence of signal than to detect the absolute amount of signal, i.e., counting is easier than integrating. In fact, one could argue that the future of all analytical measurements will be molecule counting. It cannot get any better”.
Acknowledgements
The help of Mr Fabio Polo, Ca' Foscari University, Venice, Italy in the design of the figures is greatly appreciated.References
- S. Brenner, Science, 2012, 338, 1427–1428 CrossRef CAS.
- N. M. Taleb, The Black Swan, the Impact of the Highly Improbable, Random House, New York, 2nd edn, 2010 Search PubMed.
- T. Kuhn, The Structure of Scientific Revolutions, University of Chicago Press, Chicago, 50th Anniversary edition, 2012 Search PubMed.
- F. Adams, Talanta, 2011, 85, 1230–1232 CrossRef CAS.
- F. De Corte, J. Radioanal. Nucl. Chem., 2012, 293, 199 CrossRef.
- J. R. Yates III, Nat. Methods, 2011, 8, 633–637 CrossRef.
- A. L. Gray, Analyst, 1975, 100, 289–299 RSC.
- R. S. Houk, V. A. Fassel, G. D. Flesch, H. J. Svec, A. I. Gray and C. E. Taylor, Anal. Chem., 1980, 52, 2283–2289 CrossRef CAS.
- D. F. Torgerson, R. F. Skowronski and R. D. Macfarlane, Biochem. Biophys. Res. Commun., 1974, 60, 616–621 CrossRef CAS.
- R. D. Macfarlane and D. F. Torgerson, Science, 1976, 191, 920–925 CAS.
- M. Resano, K. S. McIntosh and F. Vanhaecke, J. Anal. At. Spectrom., 2012, 27, 165–173 RSC.
- R. Jäger, J. S. Becker, H. J. Dietze and J. A. C. Broekaert, Fresenius' J. Anal. Chem., 1997, 358, 214–217 CrossRef.
- V. Hoffmann, M. Kasik, P. K. Robinson and C. Venzago, Anal. Bioanal. Chem., 2005, 381, 173–188 CrossRef CAS.
- L. Lobo, J. Pisonero, N. Bordel, R. Pereiro, A. Tempez, P. Chapon, J. Michler, M. Hohl and A. Sanz-Medel, J. Anal. At. Spectrom., 2009, 24, 1373–1381 RSC.
- A. A. Rubinstein, J. Anal. At. Spectrom., 2010, 25, 735–738 RSC.
- R. Lobinski and F. C. Adams, Spectrochim. Acta, Part B, 1997, 52, 1865–1903 CrossRef.
- H. Vogt, H. J. Heinen, S. Meier and R. Wechsung, Fresenius' J. Anal. Chem., 1981, 308, 195–200 CrossRef CAS.
- E. Denoyer, R. Van Grieken, F. Adams and D. Natusch, Anal. Chem., 1982, 54, 26A–41A CrossRef CAS.
- C. G. Enke, J. Anal. At. Spectrom., 2012, 27, 1177–1180 RSC.
- P. Galison, Image and logic: a material culture of microphysics, University of Chicago Press, 1997 Search PubMed.
- D. H. Bilderback, P. Elleaume and E. Weckert, J. Phys. B: At., Mol. Opt. Phys., 2005, 38, S773–S797 CrossRef CAS.
- F. Adams, L. Van Vaeck and L. Barrett, Spectrochim. Acta, Part B, 2005, 60, 13–26 CrossRef.
- V. T. Yadugiri and R. Malhotra, Curr. Sci., 2010, 99, 900–907 Search PubMed.
- Histories of Scientific Observation, ed. L. Daston and E. Lunbeck, University of Chicago Press, 2011 Search PubMed.
- F. Adams and C. Barbante, Talanta, 2012, 102, 16–25 CrossRef CAS.
- D. Richards and Z. Anatoly, Philos. Trans. R. Soc., A, 2004, 362, 699–700 CrossRef.
- M. Alfeld, K. Janssens, J. Dik, W. de Nolf and G. van der Snickt, J. Anal. At. Spectrom., 2011, 26, 899–909 RSC.
- J. M. Fonville, C. Carter, O. Cloarec, J. K. Nicholson, J. C. Lindon, J. Bunch and E. Holmes, Anal. Chem., 2013, 85, 1415–1423 CrossRef CAS.
- F. Xian, C. L. Hendrickson and A. G. Marshall, Anal. Chem., 2012, 84, 708–719 CrossRef CAS.
- Y. Li, B. Shrestha and A. Vertes, Anal. Chem., 2007, 79, 523–532 CrossRef CAS.
- N. Winograd, Z. Postawa, J. Cheng, C. Szakal, J. Kozole and B. J. Garrison, Appl. Surf. Sci., 2006, 252, 6836–6843 CrossRef CAS.
- M. Senoner and W. E. S. Unger, J. Anal. At. Spectrom., 2012, 27, 1050–1068 RSC.
- E. J. Lanni, S. S. Rubakhin and J. V. Sweedler, J. Proteomics, 2012, 75, 5036–5051 CrossRef CAS.
- M. V. Zoriy and J. S. Becker, Rapid Commun. Mass Spectrom., 2009, 23, 23–30 CrossRef CAS.
- B. Wu and J. S. Becker, Int. J. Mass Spectrom., 2012, 323–324, 34–40 CAS.
- J. B. Fenn, Angew. Chem., 2003, 42, 3871–3894 CAS.
- F. Adams and C. Barbante, Spectrochim. Acta, in press Search PubMed.
- M. I. Stockman, Nature, 2010, 467, 541–542 CrossRef CAS.
- O. R. Bolduc and J. F. Masson, Anal. Chem., 2011, 83, 8057–8062 CrossRef CAS.
- N. G. Greeneltch, M. G. Blaber, A. I. Henry, G. C. Schatz and R. P. Van Duyne, Anal. Chem., 2013, 85, 2297–2303 CrossRef CAS.
- S. Kawata, Appl. Spectrosc., 2013, 67, 117–125 CAS.
- M. Kemp, Nature, 2008, 453, 596 CrossRef CAS.
- Y. Lin, R. Trouillon, G. Safina and A. G. Ewing, Anal. Chem., 2011, 83, 4369–4392 CrossRef CAS.
- J. Lippincott-Schwarz and S. Manley, Nat. Methods, 2009, 6, 21–23 CrossRef.
- B. Leung and K. Chou, Appl. Spectrosc., 2011, 65, 967–1086 CrossRef CAS.
- R. Lobinski, D. Schaumlôffel and J. Szpunar, Mass Spectrom. Rev., 2006, 25, 255–289 CrossRef CAS.
- D. Pröfrock and A. Prange, Appl. Spectrosc., 2012, 66, 843–868 CrossRef.
- R. Roy, S. Hohng and T. Ha, Nat. Methods, 2008, 5, 507–516 CrossRef CAS.
- C. Blum and V. Subramania, Anal. Bioanal. Chem., 2009, 393, 527–541 CrossRef CAS.
- S. J. Lord, H. D. Lee and W. E. Moerner, Anal. Chem., 2010, 82, 2192–2203 CrossRef CAS.
- A. Zewail, Science, 2010, 328, 187–193 CrossRef CAS.
- D. R. Walt, Anal. Chem., 2013, 85, 1258–1263 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2013 |
