Nano–bio effects: interaction of nanomaterials with cells
Liang-Chien
Cheng†
a,
Xiumei
Jiang†
b,
Jing
Wang
b,
Chunying
Chen
*b and
Ru-Shi
Liu
*ac
aDepartment of Chemistry, National Taiwan University, Taipei 106, Taiwan. E-mail: rsliu@ntu.edu.tw; Fax: +886-2-33668671; Tel: +886-2-33661169
bCAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety, National Center for Nanoscience and Technology, Beijing 100190, China. E-mail: chenchy@nanoctr.cn; Fax: +86-10-62656765; Tel: +86-10-82545560
cGenomics Research Center, Academia Sinica, Taipei 115, Taiwan
First published on 12th March 2013
Abstract
With the advancements in nanotechnology, studies on the synthesis, modification, application, and toxicology evaluation of nanomaterials are gaining increased attention. In particular, the applications of nanomaterials in biological systems are attracting considerable interest because of their unique, tunable, and versatile physicochemical properties. Artificially engineered nanomaterials can be well controlled for appropriate usage, and the tuned physicochemical properties directly influence the interactions between nanomaterials and cells. This review summarizes recently synthesized major nanomaterials that have potential biomedical applications. Focus is given on the interactions, including cellular uptake, intracellular trafficking, and toxic response, while changing the physicochemical properties of versatile materials. The importance of physicochemical properties such as the size, shape, and surface modifications of the nanomaterials in their biological effects is also highlighted in detail. The challenges of recent studies and future prospects are presented as well. This review benefits relatively new researchers in this area and gives them a systematic overview of nano–bio interaction, hopefully for further experimental design.
 Liang-Chien Cheng | Liang-Chien Cheng is currently a PhD student at the Department of Chemistry, National Taiwan University, supervised by Prof. Ru-Shi Liu. He received his MS degree in Chemistry from National Sun Yat-sen University in 2008. His current research interests include the synthesis of metallic NPs, nanodiamonds and the upconversion NMs for bio-applications. |
 Xiumei Jiang | Xiumei Jiang is a PhD student at the CAS Key Laboratory for Biomedical Effects of NMs and Nanosafety, National Center for Nanoscience and Technology of China. She received her Bachelor's Degree in Biology from Northeast Normal University, China (2010). Her research interests include the biomedical application and potential toxicity of NPs in cancer therapy. |
 Jing Wang | Jing Wang is a masters student at the CAS Key Laboratory for Biomedical Effects of NMs and Nanosafety, National Center for Nanoscience and Technology of China. She obtained her Bachelor's Degree in Chemistry at the China Agricultural University in 2011. Her research field mainly involves NPs as a multifunctional theranostic platform for cancer therapy. |
 Chunying Chen | Chunying Chen is a principal investigator at the CAS Key Laboratory for Biomedical Effects of NMs and Nanosafety, National Center for Nanoscience and Technology of China. Dr Chen received her Bachelor's Degree in Chemistry (1991) and obtained her PhD in Biomedical Engineering (1996) from Huazhong University of Science and Technology of China. Her research interests include the potential toxicity of NPs, therapies for malignant tumors using theranostic nanomedicine systems that carry chemotherapeutics and imaging tags, and vaccine nanoadjuvants using NMs as a potential nonviral adjuvant. Her research is supported by the China MOST 973 Program, Natural Science Foundation of China, EU-FP6 and FP7, and IAEA. She has authored/co-authored over 100 peer-reviewed papers, two books, and ten book chapters. She is an Editorial Board Member at Current Drug Metabolism. |
 Ru-Shi Liu | Ru-Shi Liu is currently a professor at the Department of Chemistry, National Taiwan University. He received his Bachelor's Degree in Chemistry from Shoochow University (Taiwan) in 1981. He received his Master's Degree in Nuclear Science from the National TsingHua University (Taiwan) in 1983. He obtained two PhDs in Chemistry: one from National TsingHua University in 1990 and another from the University of Cambridge in 1992. He worked at Materials Research Laboratories at the Industrial Technology Research Institute from 1983 to 1985. He was an associate professor at the Department of Chemistry of National Taiwan University from 1995 to 1999, and was promoted to professorship in 1999. His research concerns the field of Materials Chemistry. He is an author or coauthor of more than 400 studies published in scientific international journals. He has also been granted more than 80 patents. |
1 Introduction
Nanotechnology has become a research hotspot because of its extremely small size that enables potential use in wide-ranging applications.1 Nanomaterials (NMs) are a combination of a group of atoms and molecules possessing advantageous chemical and physical properties that significantly vary from their bulk counterparts. NMs are the transition state between bulk materials and molecular clusters. Thus, NMs remarkably diverge from traditional macro- or micro-perspectives, showing unique optical, magnetic, electrical, chemical, and mechanical properties.1–3Recently, NMs have been widely investigated because of their potential applications in biomedicine, surface enhanced Raman spectroscopy, and catalysis. The outstanding properties of NMs make them appropriate for utilization in various biological and medical systems, including cancer and gene therapies, photothermal and photodynamic therapy, bio-imaging, diagnostics, bioanalytical methods, and pharmacokinetics studies.1,4–6 Thus, interactions between NMs and cells present the most primitive fundamental phenomenon, and highlight the importance of basic research. Most bio-applications, including drug delivery, bioimaging, or therapeutic treatments, begin from the attachment of NMs onto targeting cells. The versatile behaviors are highly dependent on the physical and chemical properties of NMs. In this review, we discuss the influences of the morphology, size, surface charge, surface modifications, and chemical compositions of NMs on cells based on recent studies. We also summarize the cellular uptake, intracellular trafficking, and cytotoxicity of different kinds of NMs. Strategies on how to improve the interactions between NMs and cells, as well as their future applications in biological systems are discussed in detail.
2 Nanostructured materials and their fabrication
NMs are of great scientific interest because their properties are a bridge between their molecular and bulk species. Their remarkable changes in the nanoscale can be divided into three main physical effects.I Surface effect
Compared with bulk materials, nanoparticles (NPs) have a small size that ensures a high percentage of surface atoms at or near interfaces and a high surface energy. The specific surface area is defined as the ratio of the surface area to volume.7 With decreased particle size, the surface atoms greatly increase and the atomic coordination numbers decrease. Consequently, the surface energy is high, leading to high reactivity of these surface atoms and ease in combining with the other atoms. In catalysis, the surface energy is directly proportional to the size effect. To increase catalysis and reactivity, the NP size must be decreased.II Small scale effect
With decreased particle size to the nanoscale or even smaller, the regular boundary condition of crystals becomes destructive, and the surface molecular density of amorphous NPs decreases. This phenomenon changes their physical properties, such as optical, thermal, magnetic, and mechanical. The small scale effect is defined as macroscopic changes in their physical properties with decreased particle size. The main properties are presented as follows.III Quantum size effect
As aforementioned, the size effect is important because the electron band gap of the Fermi level changes from continuous to discrete with decreased size. Semi-conductor NPs with sizes below the Bohr radius lead to an increase in the band gap energy, and cause the highest occupied molecular orbital and lowest unoccupied molecular orbital to break into quantized energy levels. The particle sizes of quantum dots (QDs) directly influence their emission through the quantum confinement effect.12As previously discussed, the small size effect, surface effect, and quantum effect directly influence the physical and chemical properties of nanostructured materials. This phenomenon is verified from their bulk formations and widely observed in several materials. For example, conductive bulk metals become non-conductive metals with decreased size; ferromagnetic substances change their multiple magnetic domain into a single magnetic domain in the nanoscale; and the inert metal platinum can become highly reactive catalyst NPs.13 Considering the specific interaction and novel effects of these NMs that are between bulk and molecular materials, the science of NMs becomes a new focus of materials science research. Most of these materials have different characteristics with decreased sizes down to nanometers. The next section discusses metal-based, carbon-based, and semiconductor NMs.
2.1 Noble metal-based NMs
Colloidal gold (Au) was first produced by Michael Faraday, who found that fine particles are produced by the reduction of Au chloride and stabilization of carbon disulfide. Nowadays, colloidal NP syntheses mostly take a similar route, i.e., the reduction of Au salt precursors and subsequent surface protection by stabilizers. To generate different morphologies or sizes for tuning chemical or physical properties, the synthesis routes have evolved into versatile methods.5The most commonly used method for fabricating NPs is the citrate method. Turkevich et al. first applied a citrate method that uses citrate as a reduction agent and stabilizer, and Au NPs ranging from 9 nm to 120 nm in size are obtained.14 Lee and Meisel used a citrate method to synthesize colloidal silver (Ag) NPs.15 The procedures of both citrate methods are the same. The metal precursors are reduced by citrate in a boiling aqueous solution and then stabilized by citrate. However, this simple synthesis process tends to yield NPs with random morphologies. To control these morphologies, the method is modified under specific conditions. Such morphology control approaches are widely discussed. The concentration of metal precursors directly influences the NP sizes. The pH of the reaction solution influences the protonation states of citrate ion and changes the reduction rate, thereby yielding NPs with different sizes and morphologies. The synthesis temperature also affects the NP shapes. The citrate method has evolved into mixing external metal precursors for morphology sculpture. This method is attracting increased attention because citrate can be easily substituted by other biological thiol terminal ligands.16 Thiol terminal proteins, DNA, and siRNA capping agents can easily replace citrate because of the strong conjugation between Au and sulfur. For further bio-applications, the replacement of the citrate ligand with a bio-friendly, targeting, or fluorescent ligand can lead to increased applications in biological systems.
In addition, the seed-growth method is gaining considerable interest because of its remarkable size and morphology-controlled process.17 As the technique name suggests, metal salts are reduced by a strong reducing agent to fabricate small, spherical, seed-mediated particles in an aqueous solution. The nucleation and growth processes are separated for better morphology control. NP surfaces are surrounded by surfactants that are used to stabilize the materials during the reactions. To generate different morphologies or NP sizes, a growth solution (containing abundant metal salt, a weak reducing agent, and structure-decorating reagents) is added to a previously fabricated seed-mediated solution to produce rod-like shapes,18 star shapes,19 urchin-like shape,20 tetrahedra,21 nanoprisms,22 obtuse triangular bipyramids,23etc. The selection of these NP structures depends on their applications. Thus, shape-controlled syntheses have become important.
The shape-controlled synthesis of metal NPs can be traced back to as early as the 1990s. Masuda et al. demonstrated Au nanorod (NR) synthesis using nanoporous aluminum oxide templates to reduce Au shaping.24 Yu et al. developed a simple and high-quality Au NR synthesis route using quaternary ammonium surfactants by electrochemical oxidation.25 Moreover, a well-known seed-mediated growth method was developed by Jana et al. to produce Au NRs with a controllable aspect ratio in high yield.26 Au NPs with versatile morphologies can also be fabricated by a seeded-growth method.27,28 Several approaches to the decoration of material morphologies have been proposed. The seed/Au salt ratio or other impurity ions in the solution direct the aspect ratio of Au NRs.29 Addition of excess Au(I) to the solution tailors the optical properties and structures of the materials through the disproportionation reaction of Au(I) to generate Au(III) or Au(0).30 Changing seed-mediated particles such as NRs also generates different morphologies.31 Galvanic replacement is another well-known method for synthesizing hollow, skeleton, or cage structures of metal NPs.32Fig. 1 shows various shape-controlled metal nanomaterials.
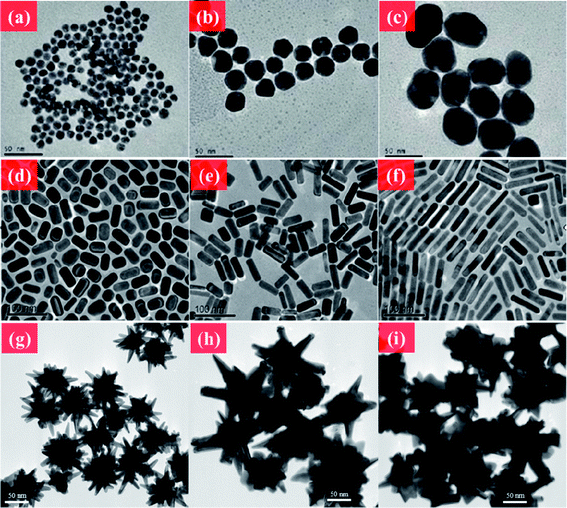 | ||
| Fig. 1 TEM images of Au NMs. (a–c) Au NPs in different sizes. (d–f) CTAB-coated Au NRs with different aspect ratios. (g–i) Au nanourchin with different morphologies. Reprinted from ref. 176 with permission. Copyright (2011) American Chemical Society. Reprinted from ref. 122 with permission. Copyright (2010) Elsevier. Reprinted from ref. 20 with permission. Copyright (2012) Royal Society of Chemistry. | ||
2.2 Carbon-based NMs
Various NMs have been extensively investigated because of their special properties different from their bulk state.33 Unlike other metal NPs, carbon-based materials in nanoscience are a special and new category in chemistry, physics, electronics, and materials. Members of the versatile allotrope of the carbon family include the fullerenes, carbon nanotubes (CNTs), graphene, graphite, nanodiamond, amorphous carbon, onions, horns, rods, cones, bells, foam, platelets, and peapods.34 Nanodiamonds mainly have an sp3-tetrahedral bond network confined in the nanoscale, and replacing the π electron network in another sp2 plate forms carbon materials, such as tubes, graphene, graphite, and fullerenes, as shown in Fig. 2.35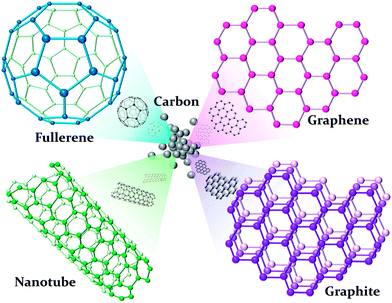 | ||
| Fig. 2 Carbon allotropes in different structures. | ||
Fullerenes (C60) have an sp2 carbon-based truncated icosahedral structure, as first reported by Kroto et al. in 1985. C60 has 12 pentagons and 20 hexagons, and an average diameter of around 7 Å.36 Controlling the self-organization of pristine C60 by altering their dimensionality to adjust their structural,37 magnetic,38 electrochemical,39 or photophysical40 properties has drawn interest.
Several shape-controlled synthesis procedures are available for C60,41 such as template-assisted, dip-drying, solution-driven, self-assembled, and vapor-driven crystalline methods.42,43 Among them, the solvent-based, morphology-controlled synthesis of C60 has been widely investigated recently, and the shape of C60 can be tuned to NRs,44 nanowhiskers,45 nanosheets,46 microtubes,47 spheres, nanoballs,48etc. The different synthesis routes of various C60 morphologies are controlled by tailoring the crystalline structures.
CNTs were first documented in 1976 by Oberlin et al. CNTs are formed by rolling up graphene sheets of a single sp2 layer and densely packing them such that diameters in the nanoscale and lengths in the microscale are achieved.49 In 1991, Iijima observed multi-walled CNTs (MWCNTs), and became interested in CNT research over the next two decades.50 Depending on the covering layer, CNTs can be classified into MWCNTs and single-walled CNTs (SWCNTs). Currently, the three main synthesis methods of CNTs are arc discharge, laser ablation, and chemical vapor deposition.51 Compared with CNTs, SWCNTs are attracting more attention because of their outstanding electrical, mechanical, thermal, sensing, and optical properties that enable them to be utilized in many applications, such as chemistry, energy, electronics, optoelectronics, biomaterials, etc.52
The shape-controlled synthesis of NMs determines the physical or chemical properties of the materials. Diameter- or length-controlled SWCNT syntheses influence the physical and electronic properties of the resulting materials, as well as their optical and electronic applications. In different synthesis routes, the diameter-controlled synthesis conditions of SWCNTs are determined. In the arc discharge method, an alternative metal catalyst or chamber pressure controls the diameter range from 1.0 nm to 1.4 nm.53 The change in furnace temperature between 780 and 1200 °C alters the diameter between 1.0 nm and 1.3 nm.54 Additionally, the SWCNT diameter always increases with increased growth temperature. Sonication power is another controlling factor for changing the SWCNT length.55 Controlling the diameter, length, concentration, and density of SWCNTs is important in a wide variety of applications.56,57
Graphene is another sp2 carbon-based material with sheets arranged in a single-layer, hexagonal network. The discovery of monolayer graphene can be traced back to the 1960s and 1970s. In 2004, Novoselov et al. demonstrated the transfer of graphene onto silicon substrates and found an interesting electronic property.58 The methods of synthesizing graphene are rapidly developing, including mechanical exfoliation,58 nanotube unzipping,59,60 chemical vapor deposition,61,62 and oxidative exfoliation.63,64 The properties of graphene are directed by different synthesis methods and determine the selection of applications in energy storage,65 nanoelectronics,66–68 or bio-applications.69
2.3 Semiconductor NMs
Fluorescent semiconductor NMs, also known as QDs, are powerful nanoscale light sources applied in various technologies. QDs are often made up of groups II and VI elements (e.g., CdSe, CdTe, or ZnO) or group III and V elements (e.g., InP). Nanosized semiconductors are gaining attention because of their interesting electronic and optical properties. The fluorescence range of QDs can be well tuned by changing their size or morphology based on the quantum confinement effect.70 The band gap of QDs is inversely proportional to their size, i.e., the fluorescence red shifts with decreased size. The tunable fluorescence property can be utilized in different applications, such as light-emitting diodes or bio-labeling.71The synthesis of colloidal QDs can be traced back to the work of Murray et al. in 1993.72 They demonstrated a high-temperature, organometallic system that processed nucleation and growth at high temperatures, and capped NPs with phosphor-based stabilizers. However, the unstable organometallic precursor they used, dimethyl cadmium, limited the synthesis of QDs with uniform morphology. To improve the quality of QDs, metal precursors have been tested, such as cadmium precursors (cadmium perchlorate, cadmium oxide, cadmium chloride, and cadmium carbonate).73–75 Simple synthesis routes for uniform-sized or aqua-synthesized QDs are now available, and high-quality QDs can be obtained by altering the synthesis conditions, such as temperature, metal precursors, or capping ligands (Fig. 3).76
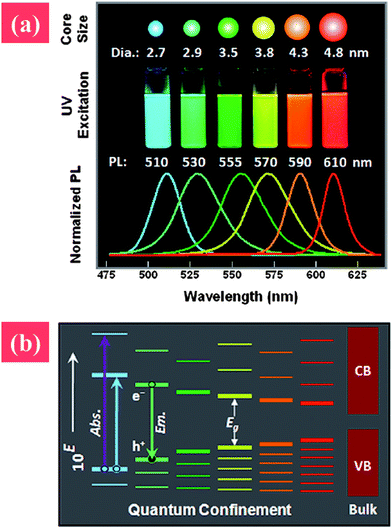 | ||
| Fig. 3 (a) Photographs and photoluminescence spectra of quantum dots with various particle sizes, and (b) qualitative alternatives of quantum dots with increased particle size. Reprinted from ref. 12 with permission. Copyright (2011) John Wiley & Sons. | ||
The shape-controlled synthesis of QDs has also been explored in recent decades. The anisotropic growth of QDs was discussed in 2000 by Peng et al.77 A change in the capping ligand from pure trioctyl phosphine oxide (TOPO) to a mixture of TOPO and hexyl phosphonic acid (HPA) can adjust the growth rate and shape of nanostructures. At high concentrations of HPA, the reaction kinetics is altered and tends to produce rod-like QDs. The aspect ratio, size, and growth kinetics of these QDs can be systematically controlled by changing the reaction times, growth temperature, and number of injections. The seed-mediated growth of QDs has also been studied to produce rod-like or tetrapod structures.78 This method can enable the selection of different materials from the seed and growth solution to fabricate core–shell structures.
3 Cellular uptake and intracellular trafficking of NMs
Given the size, unique optical property, and flexible surface modifications of NMs, they show great potential for various biomedical applications, including gene/drug delivery, biosensing, bioimaging, and photothermal cancer therapy. For safety consideration in biomedical applications, a comprehensive understanding of the interactions between NMs and biological systems is needed, leading to the following questions. Do NMs enter cells? If they do, what kind of uptake pathway is involved? What intracellular organelles do NMs penetrate? What is the final fate of both NMs and cells? To answer these questions, different uptake inhibitors and state-of-the-art techniques such as transmission electron microscopy or confocal microscopy are used to study the internalization and cellular trafficking of NMs. Thus, to design NMs for specific biomedical purposes and elucidate possible hazardous effects, the mechanisms underlying the cellular uptake and intracellular trafficking process of NMs must be understood.The uptake pathways include the clathrin-mediated, caveolae-mediated, and lipid raft-mediated endocytosis and phagocytosis, as well as pinocytosis and macropinocytosis. Phagocytosis is normally for specialized cells such as monocytes and macrophage. As shown in Fig. 4, these internalization pathways are involved in the cellular uptake of NMs.79 Given their small size and protein adsorption in cell culture media, NMs are mostly consumed by cells through endocytosis, trapped into endosomes, transferred to lysosomes, and then excluded out of cells. However, some NMs can get out of endosomes and enter other organelles such as cytosol, mitochondrion, and even nucleus. In the following section, we discuss the most widely studied NMs as examples to show their typical cellular uptake and intracellular trafficking.
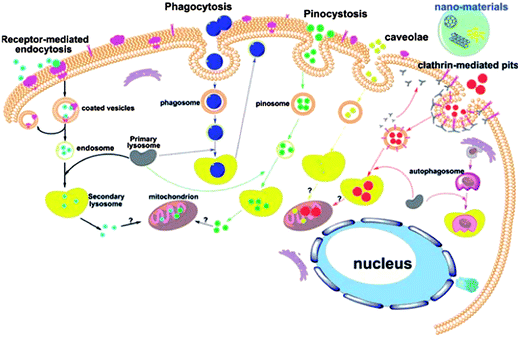 | ||
| Fig. 4 Schematic of the known pathways for the intracellular uptake of NPs. Reprinted from ref. 79 with permission. Copyright (2011) John Wiley & Sons. | ||
3.1 Noble metal-based NMs
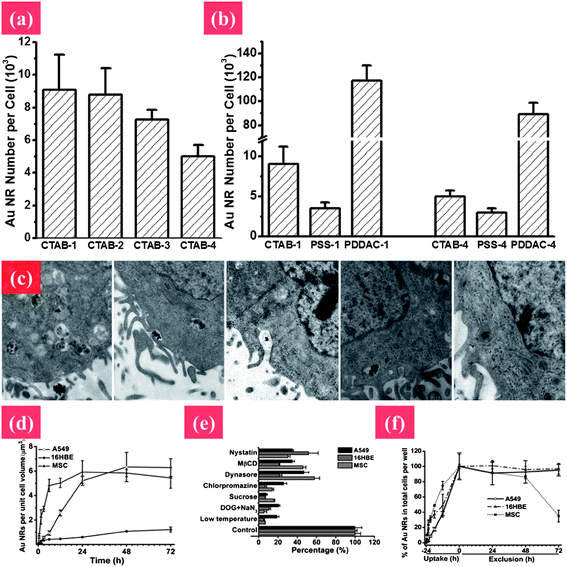 | ||
| Fig. 5 Elucidating the mechanism of endocytosis and exocytosis of Au NRs with different surface chemistry and aspect ratios. (a) Cellular uptake of Au NRs by MCF-7 cells with different aspect ratios from 1 to 4: CTAB-1, CTAB-2, CTAB-3, and CTAB-4. (b) The shape and surface coating influencing cellular uptake of Au NRs. (c) TEM images showing the process of cellular uptake. The Au NRs form aggregates, enter into vesicles and further get into lysosomes. Uptake pathways and the quantitative process of internalization and removal of Au NRs in A549, 16HBE, and MSC cells by ICP-MS after treatment with 50 μM Au NRs. (d and e) Process of cellular internalization and exclusion of Au NRs, respectively. (f) Uptake pathways for Au NRs in three types of cells using specific endocytosis inhibitors. Reprinted from ref. 122 with permission. Copyright (2010) Elsevier. Reprinted from ref. 86 with permission. Copyright (2010) American Chemical Society. | ||
In addition, the cellular uptake and trafficking of Au NMs are highly related to their size, shape, surface modification, and surface charge. Au NMs have high affinity for thiols, which provide a good chance for various surface modifications to facilitate their biomedical applications. For instance, targeted gene/drug delivery can be achieved by modifying Au NMs with a nuclear localization signal (NLS).87,88
3.2 Carbon-based NMs
3.3 Semiconductor NMs
QDs show promising properties as an alternative fluorophore to organic dyes for biological labeling and bioimaging. With their strong fluorescence intensity, photostability, small size, and flexible surface modifications, QDs are ideal agents for intracellular tracking based on in vitro and in vivo studies. Therefore, concern on the fate of QDs in biological systems is growing. For example, the cellular uptake of QDs into human epidermal keratinocytes has been explored.111 Results indicate that carboxylic acid-coated QDs composed of a cadmium/selenide core and a zinc sulfide shell are taken up by cells through the G-protein/coupled-receptor-mediated pathway and low-density lipoprotein receptor/scavenger receptor-mediated endocytosis. These QDs are subsequently internalized into early endosomes and then transferred to late endosomes/lysosomes without entering the cell nucleus. Tat-QDs were found to be trapped in vesicles and remain tethered to the inner vesicle membranes inside the cytoplasm, then the QDs-loaded vesicles were transported to a perinuclear region called the microtubule organizing center (MTOC).112 Interestingly, vesicles containing large QDs pinch off from the tips of filopodia, resulting in free vesicles with Tat-QDs bound on the outside.112 A study has shown that cell nucleus localization may be size dependent.113 Red QDs are distributed throughout the cytoplasm of N9 cells but do not enter the nucleus, whereas green QDs predominantly localize in the nucleus. Thus, the size of pores in the nucleus plays an important role in the nucleus localization of NMs. Another study has shown that the cellular uptake of QDs depends on the cell type and cell differentiation;114 the ability for QD655-COOH cellular uptake is found in monocytes but not in lymphocytes. However, monocyte differentiation into dendritic cells increases the cellular uptake of QD655-COOH by sixfold. In addition, different surface modifications can be used to facilitate QDs uptake for intracellular tracking application.112,115 For example, QD surfaces modified with dihydrolipoic acid or PEG attached onto a polyethylenimine coating can be taken up into human cells by endocytosis and then translocated into the cytoplasm.116,117 Overall, the cellular uptake and intracellular localization of various NMs somewhat depend on the size, shape, surface modification, and cell type.4 Potential toxicity induced by nanostructured materials
Recent advances in engineering and technology have led to the development of many new NMs. Gathering information on the potential hazardous effects of these NMs on human health and environmental safety is becoming urgent. Given their size and large surface area, NMs are much more active than their bulk counterparts. Upon exposure, NMs can easily enter cells by direct penetration or receptor-mediated endocytosis, and are then translocated into different organelles. The NMs may then interact with intracellular components such as proteins, lipids, or nucleic acids. Production of increased reactive oxygen species (ROS) is considered as the most common pathway for NMs induced toxicity. High ROS levels are indicative of oxidative stress, and can damage cells by peroxidizing lipids, inducing inflammation, altering proteins and DNA, as well as interfering with signaling and gene functions.4.1 Noble metal-based NMs
The mechanism underlying the toxicity of Ag NMs has been investigated. Binding of Ag ions to SH groups in proteins is suggested to be the mechanism behind the well-known antibacterial effect of Ag.129 The toxic effects of Ag NPs can theoretically be related to the release of free Ag ions. There had been a discussion about which component contributes most to the toxicity of Ag NMs, the Ag NMs themselves or Ag ions being released. Two recent studies tested the content of free Ag ions in Ag NP solutions and found low levels of Ag+ (0–1%). Furthermore, both studies concluded that the toxicity of Ag NPs exposure cannot be explained solely by the presence of Ag ions in the NP solution.127,130 Christiane Beer also suggested that both Ag NPs and Ag ions contribute to the toxicity of Ag NP solution.131 Consequently, the question remains whether Ag NPs are intrinsically toxic or they act in a Trojan-horse like mode that enables uptake of the NPs and subsequent liberation of ions inside the cell.
4.2 Carbon-based NMs
The reported underlying mechanisms are also controversial. As shown in Fig. 6, CNT-induced oxidative stress is regarded as the main toxic mechanism.144–146 Conversely, Fenoglio et al. demonstrated that MWCNTs exhibited a remarkable scavenging capacity against an external source of hydroxyl or superoxide radicals.147 In addition, iron impurity of CNTs is considered as another important reason for CNTs' toxicity.148 Complications arise when comparing these investigations as there are often considerable variations in the methodologies used including differences in exposure protocols and duration, and length and frequency of post-exposure sampling. More importantly, pristine CNTs are highly hydrophobic, whereas surface functionalization (carboxylation, amination, or PEGylation) renders hydrophilicity and dispersibility in the aqueous phase, enabling varied interactions with biological systems.149,150 Further extensive in vitro and in vivo investigation is necessary to arrive at more definitive conclusions about the genotoxic properties of CNTs and the possible mechanisms involved in such toxicity.
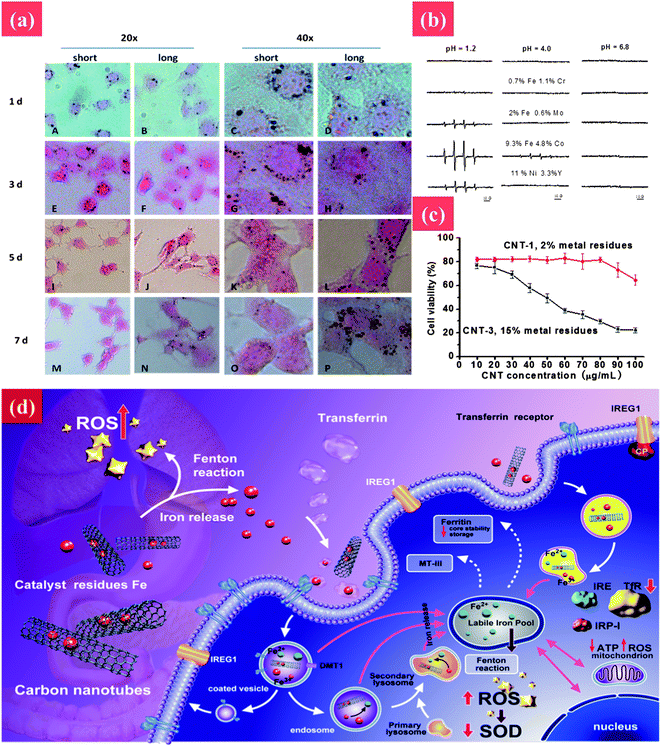 | ||
| Fig. 6 The toxic effect of CNTs to cells and underlying mechanism. (a) Rapid transport of MWCNTs in PC12 cells. The cellular uptake and rapid removal of short MWCNTs at different time points were tested by HE staining during exposure to MWCNTs (30 μg ml−1) for 2 days following culture in fresh medium in the absence of MWCNTs for 5 days. Cell images were taken at 1, 3, 5, and 7 days, respectively. (b) Hydroxyl radical formation from nanotubes having different metal contents at different pHs. (c) The cell viability after treatment with CNTs with different iron impurities. (d) Transport pathway, cellular and molecular mechanisms of toxicity associated with cellular exposure to carbon nanotubes and leached metal. Reprinted from ref. 145 with permission. Copyright (2012) Nature. Reprinted from ref. 146 with permission. Copyright (2013) John Wiley & Sons. | ||
The toxicity of graphene and its derivatives to bacteria and cells have been studied. For example, GO and reduced GO (rGO) can inhibit bacterial growth while causing minimal toxicity to human alveolar epithelial A549 cells.153 However, the cytotoxicity of graphene and its derivatives is still without consensus. For example, PG and GO induce cytotoxic effects on phaeochromocytoma (PC-12) cells and human fibroblast cells.138,154 However, two other recent reports indicated that PG and GO are quite biocompatible.155,156 For example, PG and GO substrates are highly biocompatible with improved gene transfection efficiency in NIH-3T3 fibroblasts.154 A possible explanation for this discrepancy is that the physicochemical properties of PG and GO, including size, surface charge, and surface functional groups, are not always well controlled, which may have significant influence on toxicological effects.157 For example, Sasidharan et al. compared the cytotoxicity of PG and surface functionalized graphene; they found that PG accumulates on the monkey kidney cell membrane, causing high oxidative stress and subsequent apoptosis, whereas carboxyl functionalized hydrophilic graphene taken up by cells does not result in any toxicity.158 For further toxicology study, as shown in Fig. 7, the underlying mechanism of the cytotoxicity of graphene has been studied.159,160 Li et al. reported that PG induces cytotoxicity in macrophages through the depletion of the mitochondrial membrane potential (MMP) and the increase of intracellular reactive oxygen species (ROS), then triggers apoptosis by activation of the mitochondrial pathway.159 However, at a lower concentration, graphene significantly stimulates the secretion of Th1/Th2 cytokines including IL-1α, IL-6, IL-10, TNF-α and GM-CSF as well as chemokines such as MCP-1, MIP-1α, MIP-1β and RANTES, probably by activating TLR-mediated and NF-κB-dependent transcription. This feedback of the immune response of macrophages by graphene-induced factors may play an important role in the prevention of their over-activation after graphene exposure.160
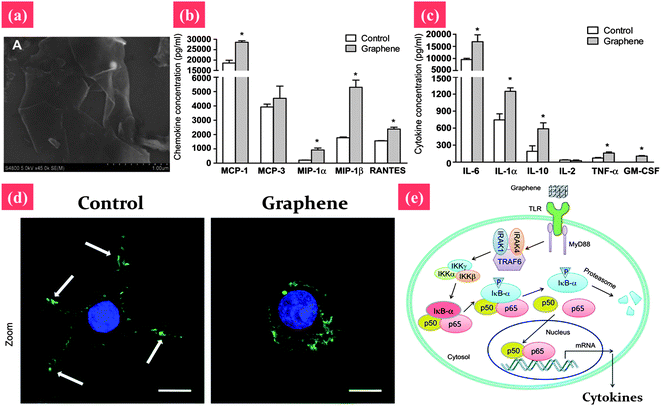 | ||
| Fig. 7 Signaling the pathway of macrophage activation stimulated by graphene nanosheets. (a) The SEM image of pristine graphene nanosheets. (b and c) Graphene nanosheets stimulate the secretion of cytokines and chemokines in macrophages. (d) Actin cytoskeleton of macrophages. The arrows indicate the F-actin foci of podosomes. (e) Graphene may be recognized by certain types of TLRs, thus activating kinase cascades by a MyD88-dependent mechanism. Activation of IKK initiates the phosphorylation and consequent degradation of IkB, resulting in the release of NF-κB subunits, and their translocation into the nucleus. NF-κB binds to the promoter regions of its effector genes, and initiates the transcription of multiple proinflammatory genes and the secretion of various proinflammatory factors, including IL-1α, IL-6, IL-10, TNF-α, CM-CSF, MCP-1, MIP-1α, MIP-1β and RANTES. These proinflammatory factors modulate the immune responses of neighboring macrophages. Reprinted from ref. 159 with permission. Copyright (2012) Elsevier. Reprinted from ref. 160 with permission. Copyright (2012) Elsevier. | ||
4.3 Semiconductor NMs
QDs are of special interest because of their potential application as fluorescent probes for bioimaging and diagnostics. However, before QDs can be used safely in vivo, more information about their interaction with and potential toxicity to biological systems is needed. The toxicity of QDs has been studied in a large number of in vitro studies.113,114,161,162 Results showed that exposure to QDs affects cell growth and viability. The extent of cytotoxicity depends on a number of factors including composition, size, and surface capping materials.113,163,164 The possible reasons for the toxicity of QDs are discussed, including desorption of free Cd (QD core degradation),163 free radical formation, and interaction with intracellular components. However, studies demonstrated that correct capping and improved surface coating of QDs can minimize cytotoxicity arising from air and photo-oxidation. Several synthesis, storage, and coating strategies have been developed for QDs to ensure stability of these NPs and minimize toxicity.74,164,165 In addition, cellular uptake plays an important role in NMs toxicity. Chang et al. evaluated the toxicity of surface coated QDs based on intracellular uptake and demonstrated that the improved biocompatibility of PEG coated QDs compared with bare ones is because of the decreased cellular uptake through endocytosis.161 Therefore, the cellular uptake of NMs directly affects their toxicity, not their surface modification.In addition, it is important that the cytotoxicity evaluation assays that are being used are appropriate for the materials being tested. For example, in the neutral red assay, carbon black has been shown to adsorb neutral red dye molecules which can give false positive results.166 Regarding the toxicology of NMs, it is difficult to draw conclusions because of the different experimental systems, including cell types, NMs compositions, exposure strategies, and toxicity evaluation systems in various laboratories. Therefore, to compare results from different labs, it is highly essential to include all the information referring to NPs characterization, exposure strategy, and toxicity evaluation systems in the published paper.
5 Major factors influencing the biological effects of nanostructured materials
The interaction between NMs and cells has been considered in recent studies because the physical and chemical properties of NMs strongly influence the biochemical properties of cells while they are in contact with each other. The physical and chemical properties include physical factors (size, shape, surface area, and surface compositions), surface chemistry (surface charge, surface functionalization and whether the NM is hydrophobic or hydrophilic), and physiological stability (aggregation, agglomeration, biodegradability, and solubility). In nature, physicochemical properties are crucial to the physiology of cells, including uptake properties (ratio, amount, and mechanism), transportation properties (accumulation location and transportation process), cytotoxicity (necrosis, apoptosis, and decreased cell viability), and exclusion. Owing to interaction between NMs and cells, these effects and behaviors should be seriously considered before being applied to any additional biological systems. Certain physicochemical parameters of NMs affect the physiological interactions of cells, as shown in Table 1.| Category | Material | Characterization | Cell type | Exposure | Uptake pathway | Intracellular localization | Method | Toxicity | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Metal | Au | Au NPs, 60 nm | Murine macrophage cells | 0.1–100 μg ml−1; 24 and 48 h | Endocytosis | Endosomal vacuoles | MTT, LDH, ELISA, flow cytometry, transmission electron microscopy (TEM), EDX | No cytotoxicity and no elevated production of pro-inflammatory mediators | 84 |
| Metal | Au | Au NRs; CTAB capped; length: 55.6 ± 7.8 nm, width: 13.3 ± 1.8 nm | Lung carcinoma cells (A549), normal bronchial epithelial cells (16HBE), and primary adult stem cells (MSC) | 25–200 μM; 24, 48, and 72 h | Clathrin and lipid raft-dependent (dynamin-mediated) endocytosis | Endosomes/lysosomes of 16HBE cells and stem cells, endosomes/lysosomes and mitochondria in A549 cells | CCK-8, confocal microscopy, flow cytometry, ICP-MS, TEM | Kill cancer cells while posing negligible impact on normal cells and stem cells | 86 |
| Metal | Au | Au NRs; CTAB, PDDAC, PSS capped; aspect ratios 1.1, 2, 3, 4 | Human breast adenocarcinoma cell line (MCF-7) | 70 pM; 6, 12, 24, and 72 h | Receptor mediated endocytosis | Endosomes/lysosomes, mitochondria | CCK-8, confocal microscopy, flow cytometry, ICP-MS, TEM | Free CTAB in CTAB coated Au NRs decreases cell viability, damages mitochondrial membrane, increases ROS level and induces apoptosis | 122 |
| Metal | Au | PEG-Au NPs, 16 nm; PEG-Au NPs modified with cell penetrating peptides (CPPs) and nuclear localization signal peptide (NLS) | Human fibroblast cells (HeLa cells) | 5 nM; 2 h | Clathrin and caveolae dependent endocytosis, cell penetration | Endosomes, cytosol, nuclei | TEM, atomic emission spectroscopy (AES) | NM | 87 |
| Metal | Au | Au NPs, 3.7 nm, Au@MPA–PEG, Au@MPA–PEG–FITC | Human cervical cancer (HeLa) cells | 0.08, 0.313, 1.25, 2.5, 5 and 10 μM; 6, 12, 24, 36, 48 and 72 h | NM | Vesicles, cytoplasm, nuclei | Confocal laser scanning microscopy (CLSM), TEM, ICP-MS | Au NP@MPA–PEG did not induce obvious cytotoxicity | 85 |
| Metal | Ag | Spherical, mean diameter 46 ± 21 nm | Human mesenchymal stem cells (hMSCs) | 0.01–10 μg ml−1; 1, 3, and 24 h | Endocytosis as well as diffusion | Vesicles, cytoplasm, and nucleus | Trypan blue exclusion test, comet assay and chromosomal aberration test, ELISA, TEM | Decrease cell viability, induce DNA damage, increase of IL-6, IL-8, and VEGF release | 90 |
| Metal | Ag | Ag NPs, 43–260 nm | Normal human lung bronchial epithelial cells (BEAS-2B) | 0.01–10 μg ml−1; 24 h | Endocytosis | Endocytic vesicle within the cytoplasm and nucleus | TEM, comet assay, micronucleus assay, flow cytometry | Increase ROS, DNA damage and chromosome aberration | 92 |
| Metal | Ag | Ag NPs, 25 nm, polysaccharide coated or not | Mouse embryonic stem (mES) cells, mouse embryonic fibroblasts (MEF) | 50 μg ml−1; 24, 48, and 72 h | NM | Uncoated Ag NPs agglomerate while the polysaccharide coated Ag NPs do not agglomerate and are distributed throughout the cell | Annexin V protein expression and MTT assay | Upregulated the cell cycle checkpoint protein p53 and DNA damage repair proteins Rad51 and phosphorylated-H2AX expression, induced cell death, coated Ag NPs exhibited more severe damage than uncoated Ag NPs | 94 |
| Metal | Ag | PVP-coated Ag NPs | A549 human lung carcinoma epithelial-like cell | 1–15 μg ml−1; 0–24 h | NM | NM | MTT, annexin V/propidium iodide assays, atomic absorption spectroscopy, flow cytometry, 32P-postlabeling | Dose-dependent cellular toxicity and genotoxicity which can be inhibited by antioxidants | 124 |
| Metal | Ag | Ag NPs, 6–20 nm, starch surface coating | Normal human lung fibroblast cells (IMR-90) and human glioblastoma cells (U251). | 0–100 μg ml−1; 24, 48, and 72 h | Endocytosis | Lysosomes, mitochondria, nucleus and nucleolus | ATP assays, DCF-DA and DHE staining, CellTiter Blue viability assay, flow cytometry, single cell gel electrophoresis (SCGE) and cytokinesis blocked micronucleus assay (CBMN), TEM | Interruption of ATP synthesis, production of ROS, cell cycle arrest in the G2/M phase, DNA damage | 215 |
| Carbon | SWCNTs | Ammonium-functionalized single-walled carbon nanotubes (SWNTs-NH3+) | Human Caucasian lung carcinoma (A549) and Chinese hamster ovary (CHO) cells | 5–500 μg; 2 h | NM | Perinuclear | Confocal laser scanning microscopy (CLSM), flow cytometry | Absence of toxic effects | 101 |
| Carbon | MWCNTs | Multi-walled carbon nano-tubes diameter of 40–100 nm, 600–800 nm in length; and three kinds of carbon blacks, PG, S160, and P90 sized 51, 20, and 14 nm, respectively | HeLa cells | 0.1–100 μg ml−1; 2 h | Endocytosis | Endosome | MTT, optical microscopy, TEM | Cytotoxicity, MDA level of lipid peroxidation product increase and SOD reactivity decrease; a greater toxicity was observed in cells exposed to CNPs in the medium without serum compared with cells in the medium with serum | 216 |
| Carbon | MWCNTs | MWCNTs, mean diameter, 67 nm; surface area, 26 m2 g−1; carbon purity, 99.79 wt% | BEAS-2B, a human bronchial epithelial cell line, transiently transfected CHO-KI cells | 1–10 μg ml−1; 0–24 h | NM | NM | WST-8, SEM, RT-PCR, western blot, reporter gene assay | MWCNT activated NF-κB, enhanced phosphorylation of MAP kinase pathway components, and increased production of pro-inflammatory cytokines in human bronchial epithelial cells | 139 |
| Carbon | MWCNTs | Carboxylated (MWCNT–COOH) and aminated (MWCNT–NH2) CNTs | Human embryonic kidney epithelial cells (HEK293) | 100 μg ml−1; 1 and 48 h | Single MWCNTs enter cells through direct penetration while MWCNT bundles enter through endocytosis | Endosomes, cytoplasm, nucleus, and lysosome | TEM, flow cytometry | NM | 108 |
| Carbon | Fullerene | C60, individual particles are 60–270 nm and the diameters of clusters are 420–1300 nm | Mature human macrophages | 0–10 μg ml−1; 24 and 48 h | Phagocytosis of larger aggregates or by endocytosis | Secondary lysosomes, along the outer and nuclear membrane and inside the nucleus | Neutral red assay, energy-filtered transmission electron microscopy (EFTEM), and scanning transmission electron microscopy (STEM)-based electron tomography | Concentrations of C60 used in this study are not toxic | 95 |
| Carbon | Fullerene | Nano-C60 | Human dermal fibroblasts, human liver carcinoma cells (HepG2), and neuronal human astrocytes | 48 h | NM | NM | MTT, live/dead staining, LDH release, fluorescence microscopy, spectro-fluorometry | Decrease cell viability, damage membrane integrity; however, DNA concentration and mitochondrial activity are not affected | 131 |
| Carbon | Fullerene | Fullerenes (C70) | Human skin, tryptase–chymase positive MC (MCTC) type cells | 5 μg ml−1 of C70–TR; 5 h, washed, and photodocumented, or placed back in the culture for up to 1 week at 37 °C in a 6% CO2 incubator | Endocytosed | Cytoplasm, lysosomes, mitochondria, and endoplasmic reticulum; no nuclear or secretory granule localization was observed | Confocal microscopy | Calcium and reactive oxygen species production | 99 |
| Carbon | Fullerene | C60, C3, Na2–3+[C60O7–9(OH)12–15](2−3), and C60(OH)24 | Human skin (HDF) and liver carcinoma (HepG2) cells | 0.24–2400 ppb; 48 h | NM | NM | Live/dead viability/cytotoxicity kit, LDH, MTT | Oxidative damage to the cell membranes and subsequent cell death. C60 was substantially more toxic than the other three derivatives | 135 |
| Carbon | Fullerene | C60(C(COOH)2)2 | HeLa and Rh35 cells | 0.01, 0.1, 1, 10, 100 mg l−1; 24 h | Endocytosis or other pathways | Cytoplasm | Fluorescence microscopy, flow cytometry | Concentrations ranging from 1 × 10−2 to 1 × 102 mg l−1, C60(C(COOH)2)2 does not show any detectable cytotoxicity | 97 |
| Carbon | Graphene | Graphene oxide | Human fibroblast cells | 5, 10, 20, 50, and 100 μg ml−1; 5 days | Endocytosis | Lysosomes, mitochondrion, endoplasm, and cell nucleus | CCK8, spectrophotometry, western blot, TEM | A dose less than 20 μg ml−1 does not exhibit toxicity; a dose above 50 μg ml−1 exhibits obvious cytotoxicity, decreasing cell adhesion and induces cell apoptosis | 154 |
| Carbon | Graphene | Pristine graphene, carboxyl functionalized hydrophilic graphene | Vero cells | 0–300 mg ml−1; 24 h | NM | Pristine graphene was found to accumulate on the cell membrane, carboxyl functionalized hydrophilic graphene was internalized by the cells | Confocal and flow cytometry, cell viability and LDH leakage | Pristine graphene causes high oxidative stress leading to apoptosis, whereas carboxyl functionalized hydrophilic graphene causes no toxicity | 158 |
| Carbon | Graphene | Graphene- and carbon nanotube-coated substrates | NIH-3T3 fibroblast cells | 24 h and 48 h | NM | NM | Live/dead assay, RT-PCR, fluorescence microscopy | High biocompatibility, enhance gene transfection efficiency | 155 |
| Semi-conductor | QDs | Cadmium/selenide core with a zinc sulfide shell, carboxylic acid surface coating | Human epidermal keratinocytes (HEKs) | 2 nM; 2, 6, 12, and 24 h | Lipid rafts mediated endocytosis | Early endosomes and then transferred to late endosomes or lysosomes | TEM, confocal microscopy, flow cytometry, spectrofluorometry | The low toxicity of QDs was shown with the 20 nM dose in HEK at 48 h, but not at 24 h by the live/dead cell assay. QDs induced more actin filaments formation in the cytoplasm | 111 |
| Semi-conductor | QDs | QD 565 and QD 655, neutral (polyethylene glycol (PEG)), cationic (PEG–amine), or anionic (carboxylic acid) coatings | Human epidermal keratinocytes (HEKs) | 20 nM; 24 h, 48 h | NM | Cytoplasm, cytoplasmic vacuole, nucleus | MTT, confocal laser scanning microscopy, and TEM | Cytotoxicity was observed for QD 565 and QD 655 coated with carboxylic acids or PEG–amine in 48 hours, with little cytotoxicity observed for PEG-coated QDs. Only carboxylic acid-coated QDs significantly increased release of IL-1β, IL-6, and IL-8 | 196 |
| Semi-conductor | QDs | Tat peptide-conjugated quantum dots (Tat-QDs), emission peak wavelength – 655 nm | HeLa cells | 1 nM; 1–24 h | Macropinocytosis | Cytoplasmic organelles, asymmetric perinuclear region (outside the cell nucleus) | Spinning disk confocal microscopy | NM | 112 |
5.1 Effects of NM size
The size of NMs strongly affects their optical properties, as discussed in the previous section. However, NM size is also crucial to physiological interaction. NMs have six size-dependent pathways through which they enter cells, with sizes ranging from over 1000 nm to less than 10 nm: phagocytosis, macro-pinocytosis, clathrin-mediated endocytosis, caveolin-mediated endocytosis, clathrin/caveolin-independent endocytosis, and direct cell membrane penetration.167 Each pathway possesses its own limited size range and dynamics. The size range for phagocytosis is between 500 nm and 10 μm.168 By contrast, most ligand-modified NMs, which are less than 500 nm, enter cells through endocytosis. To penetrate the cell membrane directly, the size of the material should be less than the thickness of the membrane bilayers, which is 4 to 10 nm. However, particles less than 5 nm are rapidly removed from the cell by renal clearance.169–171The perfect size for NMs to be used in bio-applications such as drug delivery or cancer therapy has been the subject of much recent discussion. Chithrani et al. found that NMs with diameters <100 nm have strong size-dependent intracellular uptakes.172 NPs with diameters of 14, 50, and 74 nm exhibit size-dependent cell uptake numbers and uptake halflife. NMs with 50 nm diameters exhibit higher cell uptake rates and numbers than the others because of the difference in wrapping time.83 Jiang et al. found that the interaction between antibody (Herceptin)–Au NPs and SK-BR-3 breast cancer cell receptors is also size-dependent, and that NPs ranging from 25 to 50 nm in diameter exhibit the most efficient uptake.173 The most efficient size, 50 nm, has been approximated by an experiment involving hydroxyapatite NPs (45 nm), other Au NPs (50 nm), and polypyrrole NPs (60 nm).174–177 The size of the NMs also strongly influences uptake properties during phagocytosis. The highest phagocytosis occurs when the diameter ranges from 2 μm to 3 μm.178 In addition, size must be considered because of NMs' toxic properties. Pan et al. found that 1.4 nm Au NPs exhibit high toxicity, whereas 15 nm Au NPs exhibit non-toxicity at 100-fold concentrations of 1.4 nm experiments.179 The dramatic difference of the strong toxicity of Au clusters is because of the cluster size that facilitates combination with DNA and their major groove dimension.180 Park et al. demonstrated that Ag NPs with a diameter of 20 nm are more toxic than larger NPs (80 nm and 113 nm) and Ag ions.174
The size of nanostructured materials is crucial to cell physiology in numerous bio-applications. The appropriate size of NPs for therapeutic treatment ranges from 10 nm to 100 nm, with larger particles limiting the diffusion in extracellular spaces. Moreover, diameters of 50 nm are appropriate for cellular uptake, drug delivery, and therapy for the ideal optical and size properties.
5.2 Effects of shape
The NM shape is another significant factor affecting the interaction between materials and cells. Tang's group pointed out that mesoporous silica NPs (MSNs) with different aspect ratios have major effects on cellular functions, such as uptake rate, cytoskeleton formation, adhesion, migration, viability, and proliferation.181–183 The longer NR of MSNs (NLR450, aspect ratio ∼ 4) is more easily internalized by A347 human melanoma cells compared with shorter NR (NLR240, aspect ratio ∼ 2) and spherical (NS100, aspect ratio ∼ 1) MSNs. In addition, the cytotoxicity of the MSNs decreases as the aspect ratio decreases. Gratton et al. obtained similar results using cylindrical PRINT particles with varying aspect ratios.184 High aspect ratio (d = 150 nm, h = 450 nm, aspect ratio = 3) rod-like particles are internalized more efficiently by HeLa cells than 200 nm symmetric cylindrical particles. Muro et al. investigated the targeted accumulation of various sizes (100 nm to 10 μm) and shapes (spherical versus elliptical disk-like particles) in endothelial cells, and discovered that the elliptical disks, which are micro-scale, had better targeting efficiency than any other spherical NPs.185 However, the scale of NP changes at around or lower than 100 nm may present different results than those observed in previous discussions. Chan's group demonstrated that transferrin-coated spherical Au NPs (14 and 50 nm in diameter) showed higher uptake rates than transferrin-coated, rod-like Au NPs (aspect ratio = 1.5 (20 nm × 30 nm), 3.5 (14 nm × 50 nm), and 6 (7 nm × 42 nm)) for HeLa cells, with uncoated Au NPs presenting the same result.83,172 Cell uptake efficiency also decreases as the aspect ratio increases. Florez et al. showed that nonspherical polymeric NPs (191 nm × 84 nm, 279 nm × 70 nm, and 381 nm × 65 nm) exhibit lower uptake efficiency than their spherical counterpart for mesenchymal stem cells (MSC) and HeLa cells, and the uptake rate decreases as the aspect ratio increases.186 Qiu et al. compared spheres (30 nm × 33 nm) and rod-like Au NPs with various aspect ratios (40 nm × 21 nm, 50 nm × 17 nm, and 55 nm × 14 nm), resulting in the higher-aspect-ratio rod-like NPs being more slowly internalized than the lower-aspect-ratio rod-like and spherical Au NPs in MCF-7 cells.122In the previous discussion, the interaction between the shape and size is shown to have a powerful effect on the biophysical reaction of cells, and the volume of the particles also seems to determine cell uptake efficiency. Moreover, the shape of NMs has also been proven by theoretical models and experimental studies to affect the internalization and vascular dynamics.187–190 Nanostructured materials with controllable sizes and shapes, as well as their application have also been demonstrated.191
5.3 Effects of surface chemistry
The surface chemistry of NPs exerts various significant effects on cellular processes. The surface functional groups of NPs determine most of the physicochemical properties strongly related to further interaction between materials and cells. Among these physicochemical properties, the surface charge of NPs has the greatest effect on the interaction of NPs with cells. Cho et al. demonstrated that poly(vinyl alcohol) (PVA)-coated and citrate-coated Au NPs, which possess neutral and negative charges, respectively, absorb much less amounts on the negatively charged cell membranes than positively charged poly(allyamine hydrochloride) (PAA)-coated Au NPs, based on the I2/KI etchant method. This method can selectively detect particles adhering to the cell surface.192 The cellular uptake of positively charged NMs has resulted in higher uptake rates and efficiency in various cell types, as well as increased anionic particle adhesion to cell surfaces. These NMs include metal oxides,193,194 metals,195 QDs,196 polymeric NPs,197 mesoporous silica NPs,198etc. This faster cellular uptake and higher uptake efficiency can improve cellular entry in several bio-applications, including drug delivery systems or therapeutic behaviors.Whether NPs are hydrophilic or hydrophobic is mostly determined by their surface ligands, surfactants, or stabilizers, which can be modified by chemical syntheses.199 Hydrophobic NPs result in decreased dispersion in biological fluids and media.200 However, the hydrophobic property enhances the penetration ability of NPs into cell membranes and nuclear pores through hydrophobic interaction.201,202 As a result of attempts to balance dispersion property (hydrophilic) with high penetration ability (hydrophobic), amphiphilic NMs are attracting increased attention because of their excellent dispersion in both aqueous and organic phases.203–206
Surface modification is another topic for improving the bio-availability and decreasing the cytotoxicity of NMs.200,207 Our previous work found that Au NRs synthesized with CTAB surface coating exhibit significant cytotoxicity to breast cancer MCF-7 cells, while further surface modification with PSS and PDDAC significantly decreased the toxicity of Au NRs and improved their cellular internalization.122
5.4 Effects of protein corona
To study the bio-interactions between NMs and cells, NMs are always introduced into the physiological environment, in which a large amount of amino acids, peptides and proteins are contained. When a NM enters a biological fluid, it rapidly adsorbs proteins and forms a protein corona.208 The protein corona alters the size and surface composition of NMs, which further determine the physiological responses, including kinetics, transport, accumulation, exclusion and toxicity of NMs. The structure and composition of the protein corona depends on the characterization of the NMs, like size, shape and composition, the nature of the physiological environment like blood, interstitial fluid, cell cytoplasm and the duration of exposure.209Protein corona has been observed with versatile NMs, such as Au NRs,86,122 Au NPs,210 SiO2,211 CNTs,200etc. For safe biomedical applications, it is critical that the interactions between NMs and cells are understood and controlled. Studies regarding the underlying mechanism of the dynamic protein adsorption process have been performed. Interactions between single-wall carbon nanotubes (SWCNTs) and human serum proteins were found to be a competitive mode of binding of these proteins with different adsorption capacities and packing modes.200 The π–π stacking interactions between SWCNTs and aromatic residues (Trp, Phe, Tyr) are found to play a critical role in determining their adsorption capacity. Additional cellular cytotoxicity assays revealed that the competitive binding of blood proteins on the SWCNT surface can greatly alter their cellular interaction pathways and result in much reduced cytotoxicity for these protein-coated SWCNTs. In addition, the protein corona will affect the cellular internalization of NMs. Uptake of a NP–protein complex by cells depends on whether the cell membrane has receptors for the proteins, whether the proteins are presented in the correct orientation to interact with the receptor, and whether the NP-bound protein can compete effectively with the free protein for the receptor.212 A critical factor for controlling serum albumin binding is surface hydrophobicity, which in turn decreases the cellular uptake of NPs. Hydrophobic NPs bind albumin more tightly, inhibiting particle uptake, with a direct correlation observed between uptake and surface hydrophobicity.213
Despite substantial progress, detailed relationships between NMs and protein are still not clear. It is also unclear whether every protein in the corona influences the physiological response, or only a subset. Without this knowledge as a guide, it is difficult to design NMs to interact with proteins and cells in a controlled way.214
6 Summary and perspective
NMs have been investigated for several decades, and have also been applied to biological systems over the past decade. Chemical or physical synthesis can be used to alter such physical properties of NMs as morphology, size, and optical, electronic, or mechanical properties, for use in different applications. In biological systems, NMs can serve as media for the delivery of drugs, genes, or proteins for therapeutic treatments. The size of NPs enables them to enter cells by direct cell penetration or endocytosis. Moreover, the surfaces of nanostructured materials can be easily functionalized by chemical synthesis, and can be designed for more accurate treatments. Given the wide range of applications for NMs in living creatures, the interaction between NMs and cells has become a more significant research topic.In this review, we summarize the NMs most commonly used in biomedical applications in the past decade. Owing to the different compositions, physical properties, and surface properties of these NMs, their use in the treatment of cells has resulted in various phenomena. Cellular uptake behaviors are strongly dependent on the size, shape, surface charge, and chemistry. The cytotoxicity of the materials is also related to several factors, including material composition, size, and surface ligands. The physicochemical properties of NMs should be traced from their synthesis procedures, intrinsic properties, and surface chemistry. Each material offers unique properties for use in different applications, but also has its specific limitations. We hope that this review will help the reader understand the basic interaction of cells and NMs.
Although numerous studies have examined the interaction between NPs and cells, much remains to be investigated. First, there are variable factors to be studied, such as the physical or chemical properties of materials, different cell lines, and the systematic study of specific materials. Second, shape conditions of NPs have not been well investigated. Third, the variations in the cell line result in different cell uptake, toxicity, or transportation in the same materials, but systematic studies of this phenomenon are scant. Fourth, the nanotoxicity issue and the accumulation of non-degradable materials relating to biosafety are yet to be understood. Fifth, the transformation of NMs' surface chemistry in living creatures is too complicated to investigate. Understanding the interactions between NMs and cells will improve the efficiency of these interactions. With the rapid increase in studies related to nanotechnology, investigation on NMs can be more beneficial than others because of their size. We believe that an extensive understanding of NM–bio interactions can serve as a foundation for future biomedical applications.
Acknowledgements
The authors thank the National Science Council (contract no. NSC 101-2113-M-002-014-MY3 and NSC 101-3113-P-002-021) for financially supporting this research. This work was also partly supported by the National Basic Research Program (2011CB933401) and the National Natural Science Foundation (31070854).Notes and references
- L. Dykman and N. Khlebtsov, Chem. Soc. Rev., 2012, 41, 2256–2282 RSC.
- F. J. Ibanez and F. P. Zamborini, Small, 2012, 8, 174–202 CrossRef CAS.
- M. B. Cortie and A. M. McDonagh, Chem. Rev., 2011, 111, 3713–3735 CrossRef CAS.
- A. Llevot and D. Astruc, Chem. Soc. Rev., 2012, 41, 242–257 RSC.
- E. C. Dreaden, A. M. Alkilany, X. Huang, C. J. Murphy and M. A. El-Sayed, Chem. Soc. Rev., 2012, 41, 2740–2779 RSC.
- S. Rana, A. Bajaj, R. Mout and V. M. Rotello, Adv. Drug Delivery Rev., 2012, 64, 200–216 CrossRef CAS.
- G. Y. Jing, H. L. Duan, X. M. Sun, Z. S. Zhang, J. Xu, Y. D. Li, J. X. Wang and D. P. Yu, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 73, 235409 CrossRef.
- W. H. Ni, T. Ambjornsson, S. P. Apell, H. J. Chen and J. F. Wang, Nano Lett., 2010, 10, 77–84 CrossRef CAS.
- K. L. Kelly, E. Coronado, L. L. Zhao and G. C. Schatz, J. Phys. Chem. B, 2003, 107, 668–677 CrossRef CAS.
- G. Schmid and B. Corain, Eur. J. Inorg. Chem., 2003, 3081–3098 CrossRef CAS.
- P. Dutta, S. Pai, M. S. Seehra, N. Shah and G. P. Huffman, J. Appl. Phys., 2009, 105, 073104 CrossRef.
- W. R. Algar, K. Susumu, J. B. Delehanty and I. L. Medintz, Anal. Chem., 2011, 83, 8826–8837 CrossRef CAS.
- S. Schafer, S. A. Wyrzgol, R. Caterino, A. Jentys, S. J. Schoell, M. Havecker, A. Knop-Gericke, J. A. Lercher, I. D. Sharp and M. Stutzmann, J. Am. Chem. Soc., 2012, 134, 12528–12535 CrossRef.
- J. Turkevich, P. C. Stevenson and J. Hillier, Discuss. Faraday Soc., 1951, 11, 55–75 RSC.
- P. C. Lee and D. Meisel, J. Phys. Chem., 1982, 86, 3391–3395 CrossRef CAS.
- M. C. Daniel and D. Astruc, Chem. Rev., 2004, 104, 293–346 CrossRef CAS.
- Y. G. Sun and Y. N. Xia, Science, 2002, 298, 2176–2179 CrossRef CAS.
- N. R. Jana, L. Gearheart and C. J. Murphy, Adv. Mater., 2001, 13, 1389–1393 CrossRef CAS.
- H. M. Chen and R. S. Liu, J. Phys. Chem. C, 2011, 115, 3513–3527 CAS.
- L. C. Cheng, J. H. Huang, H. M. Chen, T. C. Lai, K. Y. Yang, R. S. Liu, M. Hsiao, C. H. Chen, L. J. Her and D. P. Tsai, J. Mater. Chem., 2012, 22, 2244–2253 RSC.
- T. Ming, W. Feng, Q. Tang, F. Wang, L. D. Sun, J. F. Wang and C. H. Yan, J. Am. Chem. Soc., 2009, 131, 16350–16351 CrossRef CAS.
- S. S. Shankar, A. Rai, B. Ankamwar, A. Singh, A. Ahmad and M. Sastry, Nat. Mater., 2004, 3, 482–488 CrossRef CAS.
- M. L. Personick, M. R. Langille, J. Zhang, N. Harris, G. C. Schatz and C. A. Mirkin, J. Am. Chem. Soc., 2011, 133, 6170–6173 CrossRef CAS.
- H. Masuda, H. Tanaka and N. Baba, Chem. Lett., 1990, 621–622 CrossRef CAS.
- Y. Y. Yu, S. S. Chang, C. L. Lee and C. R. C. Wang, J. Phys. Chem. B, 1997, 101, 6661–6664 CrossRef CAS.
- N. R. Jana, L. Gearheart and C. J. Murphy, J. Phys. Chem. B, 2001, 105, 4065–4067 CrossRef CAS.
- W. X. Niu, S. L. Zheng, D. W. Wang, X. Q. Liu, H. J. Li, S. A. Han, J. Chen, Z. Y. Tang and G. B. Xu, J. Am. Chem. Soc., 2009, 131, 697–703 CrossRef CAS.
- Y. Y. Ma, Q. Kuang, Z. Y. Jiang, Z. X. Xie, R. B. Huang and L. S. Zheng, Angew. Chem., Int. Ed., 2008, 47, 8901–8904 CrossRef CAS.
- Z. L. Wang, M. B. Mohamed, S. Link and M. A. El-Sayed, Surf. Sci., 1999, 440, L809–L814 CrossRef CAS.
- B. P. Khanal and E. R. Zubarev, J. Am. Chem. Soc., 2008, 130, 12634–12635 CrossRef CAS.
- E. Carbo-Argibay, B. Rodriguez-Gonzalez, J. Pacifico, I. Pastoriza-Santos, J. Perez-Juste and L. M. Liz-Marzan, Angew. Chem., Int. Ed., 2007, 46, 8983–8987 CrossRef CAS.
- S. E. Skrabalak, J. Y. Chen, Y. G. Sun, X. M. Lu, L. Au, C. M. Cobley and Y. N. Xia, Acc. Chem. Res., 2008, 41, 1587–1595 CrossRef CAS.
- T. Enoki, K. Takai, V. Osipov, M. Baidakova and A. Vul, Chem.–Asian J., 2009, 4, 796–804 CrossRef CAS.
- O. A. Shenderova, V. V. Zhirnov and D. W. Brenner, Crit. Rev. Solid State Mater. Sci., 2002, 27, 227–356 CrossRef CAS.
- A. Sinitskii and J. M. Tour, IEEE Spectrum, 2010, 47, 28–33 CrossRef.
- H. W. Kroto, J. R. Heath, S. C. Obrien, R. F. Curl and R. E. Smalley, Nature, 1985, 318, 162–163 CrossRef CAS.
- A. F. Hebard, Annu. Rev. Mater. Sci., 1993, 23, 159–191 CrossRef CAS.
- B. Narymbetov, A. Omerzu, V. V. Kabanov, M. Tokumoto, H. Kobayashi and D. Mihailovic, Nature, 2000, 407, 883–885 CrossRef CAS.
- L. Echegoyen and L. E. Echegoyen, Acc. Chem. Res., 1998, 31, 593–601 CrossRef CAS.
- D. M. Guldi and M. Prato, Acc. Chem. Res., 2000, 33, 695–703 CrossRef CAS.
- K. Miyazawa, J. Nanosci. Nanotechnol., 2009, 9, 41–50 CrossRef CAS.
- H. B. Liu, Y. L. Li, L. Jiang, H. Y. Luo, S. Q. Xiao, H. J. Fang, H. M. Li, D. B. Zhu, D. P. Yu, J. Xu and B. Xiang, J. Am. Chem. Soc., 2002, 124, 13370–13371 CrossRef CAS.
- L. Wang, B. B. Liu, S. D. Yu, M. G. Yao, D. D. Liu, Y. Y. Hou, T. Cui, G. T. Zou, B. Sundqvist, H. You, D. K. Zhang and D. G. Ma, Chem. Mater., 2006, 18, 4190–4194 CrossRef CAS.
- K. Miyazawa, K. Hamamoto, S. Nagata and T. Suga, J. Mater. Res., 2003, 18, 1096–1103 CrossRef CAS.
- M. Sathish, K. Miyazawa and T. Sasaki, Chem. Mater., 2007, 19, 2398–2400 CrossRef CAS.
- M. Sathish and K. Miyazawa, J. Am. Chem. Soc., 2007, 129, 13816–13817 CrossRef CAS.
- S. I. Cha, K. Miyazawa and J. D. Kim, Chem. Mater., 2008, 20, 1667–1669 CrossRef CAS.
- A. Masuhara, Z. Q. Tan, H. Kasai, H. Nakanishi and H. Oikawa, Jpn. J. Appl. Phys., 2009, 48, 050206 CrossRef.
- A. Oberlin, M. Endo and T. Koyama, J. Cryst. Growth, 1976, 32, 335–349 CrossRef CAS.
- S. Iijima, Nature, 1991, 354, 56–58 CrossRef CAS.
- M. Endo, Y. A. Kim, T. Hayashi, K. Nishimura, T. Matusita, K. Miyashita and M. S. Dresselhaus, Carbon, 2001, 39, 1287–1297 CrossRef CAS.
- N. Saito, Y. Usui, K. Aoki, N. Narita, M. Shimizu, K. Hara, N. Ogiwara, K. Nakamura, N. Ishigaki, H. Kato, S. Taruta and M. Endo, Chem. Soc. Rev., 2009, 38, 1897–1903 RSC.
- Y. Saito, Y. Tani and A. Kasuya, J. Phys. Chem. B, 2000, 104, 2495–2499 CrossRef CAS.
- E. Anglaret, S. Rols and J. L. Sauvajol, Phys. Rev. Lett., 1998, 81, 4780 CrossRef CAS.
- P. Vichchulada, M. A. Cauble, E. A. Abdi, E. I. Obi, Q. H. Zhang and M. D. Lay, J. Phys. Chem. C, 2010, 114, 12490–12495 CAS.
- S. A. Hodge, M. K. Bayazit, K. S. Coleman and M. S. P. Shaffer, Chem. Soc. Rev., 2012, 41, 4409–4429 RSC.
- X. Z. Zhou, F. Boey and H. Zhang, Chem. Soc. Rev., 2011, 40, 5221–5231 RSC.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS.
- D. V. Kosynkin, A. L. Higginbotham, A. Sinitskii, J. R. Lomeda, A. Dimiev, B. K. Price and J. M. Tour, Nature, 2009, 458, 872–875 CrossRef CAS.
- A. Morelos-Gomez, S. M. Vega-Diaz, V. J. Gonzalez, F. Tristan-Lopez, R. Cruz-Silva, K. Fujisawa, H. Muramatsu, T. Hayashi, X. Mi, Y. F. Shi, H. Sakamoto, F. Khoerunnisa, K. Kaneko, B. G. Sumpter, Y. A. Kim, V. Meunier, M. Endo, E. Munoz-Sandoval and M. Terrones, ACS Nano, 2012, 6, 2261–2272 CrossRef CAS.
- Y. M. A. Wu, Y. Fan, S. Speller, G. L. Creeth, J. T. Sadowski, K. He, A. W. Robertson, C. S. Allen and J. H. Warner, ACS Nano, 2012, 6, 5010–5017 CrossRef CAS.
- C. Mattevi, H. Kim and M. Chhowalla, J. Mater. Chem., 2011, 21, 3324–3334 RSC.
- S. Stankovich, D. A. Dikin, R. D. Piner, K. A. Kohlhaas, A. Kleinhammes, Y. Jia, Y. Wu, S. T. Nguyen and R. S. Ruoff, Carbon, 2007, 45, 1558–1565 CrossRef CAS.
- S. Mayavan, J. B. Sim and S. M. Choi, J. Mater. Chem., 2012, 22, 6953–6958 RSC.
- M. Pumera, Energy Environ. Sci., 2011, 4, 668–674 CAS.
- M. Freitag, Nat. Nanotechnol., 2008, 3, 455–457 CrossRef CAS.
- R. M. Westervelt, Science, 2008, 320, 324–325 CrossRef CAS.
- R. S. Sundaram, M. Steiner, H. Y. Chiu, M. Engel, A. A. Bol, R. Krupke, M. Burghard, K. Kern and P. Avouris, Nano Lett., 2011, 11, 3833–3837 CrossRef CAS.
- H. Shen, L. M. Zhang, M. Liu and Z. J. Zhang, Theranostics, 2012, 2, 283–294 CrossRef CAS.
- A. P. Alivisatos, J. Phys. Chem., 1996, 100, 13226–13239 CrossRef CAS.
- P. Zrazhevskiy, M. Sena and X. Gao, Chem. Soc. Rev., 2010, 39, 4326–4354 RSC.
- C. B. Murray, D. J. Norris and M. G. Bawendi, J. Am. Chem. Soc., 1993, 115, 8706–8715 CrossRef CAS.
- Z. A. Peng and X. G. Peng, J. Am. Chem. Soc., 2001, 123, 183–184 CrossRef CAS.
- L. H. Qu, Z. A. Peng and X. G. Peng, Nano Lett., 2001, 1, 333–337 CrossRef CAS.
- Z. A. Peng and X. G. Peng, J. Am. Chem. Soc., 2002, 124, 3343–3353 CrossRef CAS.
- V. Biju, T. Itoh and M. Ishikawa, Chem. Soc. Rev., 2010, 39, 3031–3056 RSC.
- X. G. Peng, L. Manna, W. D. Yang, J. Wickham, E. Scher, A. Kadavanich and A. P. Alivisatos, Nature, 2000, 404, 59–61 CrossRef CAS.
- D. V. Talapin, J. H. Nelson, E. V. Shevchenko, S. Aloni, B. Sadtler and A. P. Alivisatos, Nano Lett., 2007, 7, 2951–2959 CrossRef CAS.
- F. Zhao, Y. Zhao, Y. Liu, X. Chang and C. Chen, Small, 2011, 7, 1322–1337 CrossRef CAS.
- B. D. Chithrani, J. Stewart, C. Allen and D. A. Jaffray, Nanomedicine, 2009, 5, 118–127 CrossRef CAS.
- D. B. Peckys and N. de Jonge, Nano Lett., 2011, 11, 1733–1738 CrossRef CAS.
- A. Albanese and W. C. W. Chan, ACS Nano, 2011, 5, 5478–5489 CrossRef CAS.
- B. D. Chithrani and W. C. W. Chan, Nano Lett., 2007, 7, 1542–1550 CrossRef CAS.
- Q. Zhang, V. M. Hitchins, A. M. Schrand, S. M. Hussain and P. L. Goering, Nanotoxicology, 2011, 5, 284–295 CrossRef CAS.
- Y. J. Gu, J. Cheng, C. C. Lin, Y. W. Lam, S. H. Cheng and W. T. Wong, Toxicol. Appl. Pharmacol., 2009, 237, 196–204 CrossRef CAS.
- L. Wang, Y. Liu, W. Li, X. Jiang, Y. Ji, X. Wu, L. Xu, Y. Qiu, K. Zhao, T. Wei, Y. Li, Y. Zhao and C. Chen, Nano Lett., 2010, 11, 772–780 CrossRef.
- P. Nativo, I. A. Prior and M. Brust, ACS Nano, 2008, 2, 1639–1644 CrossRef CAS.
- A. K. Oyelere, P. C. Chen, X. Huang, I. H. El-Sayed and M. A. El-Sayed, Bioconjugate Chem., 2007, 18, 1490–1497 CrossRef CAS.
- S. W. P. Wijnhoven, W. J. G. M. Peijnenburg, C. A. Herberts, W. I. Hagens, A. G. Oomen, E. H. W. Heugens, B. Roszek, J. Bisschops, I. Gosens and D. Van De Meent, Nanotoxicology, 2009, 3, 109–138 CrossRef CAS.
- S. Hackenberg, A. Scherzed, M. Kessler, S. Hummel, A. Technau, K. Froelich, C. Ginzkey, C. Koehler, R. Hagen and N. Kleinsasser, Toxicol. Lett., 2011, 201, 27–33 CrossRef CAS.
- P. AshaRani, G. Low Kah Mun, M. P. Hande and S. Valiyaveettil, ACS Nano, 2008, 3, 279–290 CrossRef.
- H. R. Kim, M. J. Kim, S. Y. Lee, S. M. Oh and K. H. Chung, Mutat. Res., 2011, 726, 129–135 CrossRef CAS.
- L. Wei, J. Tang, Z. Zhang, Y. Chen, G. Zhou and T. Xi, Biomed. Mater., 2010, 5, 044103 CrossRef.
- M. Ahamed, M. Karns, M. Goodson, J. Rowe, S. M. Hussain, J. J. Schlager and Y. Hong, Toxicol. Appl. Pharmacol., 2008, 233, 404–410 CrossRef CAS.
- A. E. Porter, K. Muller, J. Skepper, P. Midgley and M. Welland, Acta Biomater., 2006, 2, 409–419 CrossRef.
- M. Raoof, Y. Mackeyev, M. A. Cheney, L. J. Wilson and S. A. Curley, Biomaterials, 2012, 33, 2952–2960 CrossRef CAS.
- F. Lao, L. Chen, W. Li, C. Ge, Y. Qu, Q. Sun, Y. Zhao, D. Han and C. Chen, ACS Nano, 2009, 3, 3358–3368 CrossRef CAS.
- W. Li, C. Chen, C. Ye, T. Wei, Y. Zhao, F. Lao, Z. Chen, H. Meng, Y. Gao and H. Yuan, Nanotechnology, 2008, 19, 145102 CrossRef.
- A. Dellinger, Z. Zhou, S. K. Norton, R. Lenk, D. Conrad and C. L. Kepley, Nanomedicine, 2010, 6, 575–582 CrossRef CAS.
- Y. Liu, Y. Zhao, B. Sun and C. Chen, Acc. Chem. Res., 2013, 46, 702–713 CrossRef CAS.
- L. Lacerda, G. Pastorin, D. Gathercole, J. Buddle, M. Prato, A. Bianco and K. Kostarelos, Adv. Mater., 2007, 19, 1480–1484 CrossRef CAS.
- B. Kang, D. Yu, S. Chang, D. Chen, Y. Dai and Y. Ding, Nanotechnology, 2008, 19, 375103 CrossRef.
- A. E. Porter, M. Gass, J. S. Bendall, K. Muller, A. Goode, J. N. Skepper, P. A. Midgley and M. Welland, ACS Nano, 2009, 3, 1485–1492 CrossRef CAS.
- D. Pantarotto, J. P. Briand, M. Prato and A. Bianco, Chem. Commun., 2004, 16–17 RSC.
- E. Mooney, P. Dockery, U. Greiser, M. Murphy and V. Barron, Nano Lett., 2008, 8, 2137–2143 CrossRef CAS.
- J. Cheng, K. A. S. Fernando, L. M. Veca, Y. P. Sun, A. I. Lamond, Y. W. Lam and S. H. Cheng, ACS Nano, 2008, 2, 2085–2094 CrossRef CAS.
- H. Jin, D. A. Heller and M. S. Strano, Nano Lett., 2008, 8, 1577–1585 CrossRef.
- Q. Mu, D. L. Broughton and B. Yan, Nano Lett., 2009, 9, 4370–4375 CrossRef CAS.
- G. Y. Chen, D. W. P. Pang, S. M. Hwang, H. Y. Tuan and Y. C. Hu, Biomaterials, 2012, 33, 418–427 CrossRef CAS.
- H. Yue, W. Wei, Z. G. Yue, B. Wang, N. N. Luo, Y. J. Gao, D. Ma, G. H. Ma and Z. G. Su, Biomaterials, 2012, 33, 4013–4021 CrossRef CAS.
- L. W. Zhang and N. A. Monteiro-Riviere, Toxicol. Sci., 2009, 110, 138–155 CrossRef CAS.
- G. Ruan, A. Agrawal, A. I. Marcus and S. Nie, J. Am. Chem. Soc., 2007, 129, 14759–14766 CrossRef CAS.
- J. Lovrić, H. S. Bazzi, Y. Cuie, G. R. A. Fortin, F. M. Winnik and D. Maysinger, J. Mol. Med., 2005, 83, 377–385 CrossRef.
- L. W. Zhang, W. Bäumer and N. A. Monteiro-Riviere, Nanomedicine, 2011, 6, 777–791 CrossRef CAS.
- Y. Zhang, M. K. So and J. Rao, Nano Lett., 2006, 6, 1988–1992 CrossRef CAS.
- J. K. Jaiswal, H. Mattoussi, J. M. Mauro and S. M. Simon, Nat. Biotechnol., 2002, 21, 47–51 CrossRef.
- H. Duan and S. Nie, J. Am. Chem. Soc., 2007, 129, 3333–3338 CrossRef CAS.
- S. Wang, W. Lu, O. Tovmachenko, U. S. Rai, H. Yu and P. C. Ray, Chem. Phys. Lett., 2008, 463, 145–149 CrossRef CAS.
- A. M. Alkilany, P. K. Nagaria, C. R. Hexel, T. J. Shaw, C. J. Murphy and M. D. Wyatt, Small, 2009, 5, 701–708 CrossRef CAS.
- H. J. Parab, H. M. Chen, T.-C. Lai, J. H. Huang, P. H. Chen, R.-S. Liu, M. Hsiao, C.-H. Chen, D.-P. Tsai and Y.-K. Hwu, J. Phys. Chem. C, 2009, 113, 7574–7578 CAS.
- T. S. Hauck, A. A. Ghazani and W. C. W. Chan, Small, 2007, 4, 153–159 CrossRef.
- Y. Qiu, Y. Liu, L. Wang, L. Xu, R. Bai, Y. Ji, X. Wu, Y. Zhao, Y. Li and C. Chen, Biomaterials, 2010, 31, 7606–7619 CrossRef CAS.
- Y. J. Kim, S. I. Yang and J. C. Ryu, Mol. Cell. Toxicol., 2010, 6, 119–125 CrossRef CAS.
- R. Foldbjerg, D. A. Dang and H. Autrup, Arch. Toxicol., 2011, 85, 743–750 CrossRef CAS.
- S. Arora, J. Jain, J. Rajwade and K. Paknikar, Toxicol. Lett., 2008, 179, 93–100 CrossRef CAS.
- C. Carlson, S. Hussain, A. Schrand, L. K. Braydich-Stolle, K. Hess, R. Jones and J. Schlager, J. Phys. Chem. B, 2008, 112, 13608–13619 CrossRef CAS.
- S. Kim, J. E. Choi, J. Choi, K. H. Chung, K. Park, J. Yi and D. Y. Ryu, Toxicol. In Vitro, 2009, 23, 1076–1084 CrossRef CAS.
- R. Foldbjerg, P. Olesen, M. Hougaard, D. A. Dang, H. J. Hoffmann and H. Autrup, Toxicol. Lett., 2009, 190, 156–162 CrossRef CAS.
- S. Liau, D. Read, W. Pugh, J. Furr and A. Russell, Lett. Appl. Microbiol., 1997, 25, 279–283 CAS.
- E. Navarro, F. Piccapietra, B. Wagner, F. Marconi, R. Kaegi, N. Odzak, L. Sigg and R. Behra, Environ. Sci. Technol., 2008, 42, 8959–8964 CrossRef CAS.
- C. Beer, R. Foldbjerg, Y. Hayashi and H. Autrup, Toxicol. Lett., 2012, 208, 286–292 CrossRef CAS.
- E. Oberdörster, Environ. Health Perspect., 2004, 112, 1058–1062 CrossRef.
- Y. Niwa and N. Iwai, Environ. Health Prev. Med., 2006, 11, 292–297 CrossRef CAS.
- C. M. Sayes, A. M. Gobin, K. D. Ausman, J. Mendez, J. L. West and V. L. Colvin, Biomaterials, 2005, 26, 7587–7595 CrossRef CAS.
- C. M. Sayes, J. D. Fortner, W. Guo, D. Lyon, A. M. Boyd, K. D. Ausman, Y. J. Tao, B. Sitharaman, L. J. Wilson and J. B. Hughes, Nano Lett., 2004, 4, 1881–1887 CrossRef CAS.
- A. Don Porto Carero, P. Hoet, L. Verschaeve, G. Schoeters and B. Nemery, Environ. Mol. Mutagen., 2001, 37, 155–163 CrossRef CAS.
- L. Braydich-Stolle, S. Hussain, J. J. Schlager and M. C. Hofmann, Toxicol. Sci., 2005, 88, 412–419 CrossRef CAS.
- Y. Zhang, S. F. Ali, E. Dervishi, Y. Xu, Z. Li, D. Casciano and A. S. Biris, ACS Nano, 2010, 4, 3181–3186 CrossRef CAS.
- S. Hirano, Y. Fujitani, A. Furuyama and S. Kanno, Toxicol. Appl. Pharmacol., 2010, 249, 8–15 CrossRef CAS.
- B. Zhong, W. Whong and T. Ong, Mutat. Res., Genet. Toxicol. Environ. Mutagen., 1997, 393, 181–187 CrossRef CAS.
- C. Ge, F. Lao, W. Li, Y. Li, C. Chen, Y. Qiu, X. Mao, B. Li, Z. Chai and Y. Zhao, Anal. Chem., 2008, 80, 9426–9634 CrossRef CAS.
- N. W. S. Kam, T. C. Jessop, P. A. Wender and H. Dai, J. Am. Chem. Soc., 2004, 126, 6850–6851 CrossRef CAS.
- L. W. Zhang, L. Zeng, A. R. Barron and N. A. Monteiro-Riviere, Internet J. Toxicol., 2007, 26, 103–113 CrossRef CAS.
- A. A. Shvedova, A. Pietroiusti, B. Fadeel and V. E. Kagan, Toxicol. Appl. Pharmacol., 2012, 261, 121–133 CrossRef CAS.
- C. C. Ge, Y. Li, J. J. Yin, Y. Liu, L. M. Wang, Y. L. Zhao and C. Y. Chen, NPG Asia Mater., 2012, 4, e32 CrossRef.
- L. Meng, R. Chen, L. M. Wang, P. Wang, C. Z. Li, R. Bai, Y. L. Zhao, H. Autrup and C. Y. Chen, Small, DOI:10.1002/smll.201201388, ASAP.
- I. Fenoglio, M. Tomatis, D. Lison, J. Muller, A. Fonseca, J. B. Nagy and B. Fubini, Free Radical Biol. Med., 2006, 40, 1227–1233 CrossRef CAS.
- L. Meng, A. Jiang, R. Chen, C. Li, L. Wang, Y. Qu, P. Wang, Y. Zhao and C. Chen, Toxicology, 2012 DOI:10.1016/j.tox.2012.11.011 , ASAP.
- V. Raffa, G. Ciofani, O. Vittorio, C. Riggio and A. Cuschieri, Nanomedicine, 2010, 5, 89–97 CrossRef CAS.
- L. Lacerda, H. Ali-Boucetta, M. A. Herrero, G. Pastorin, A. Bianco, M. Prato and K. Kostarelos, Nanomedicine, 2008, 3, 149–161 CrossRef CAS.
- X. Sun, Z. Liu, K. Welsher, J. T. Robinson, A. Goodwin, S. Zaric and H. Dai, Nano Res., 2008, 1, 203–212 CrossRef CAS.
- K. Yang, S. Zhang, G. Zhang, X. Sun, S. T. Lee and Z. Liu, Nano Lett., 2010, 10, 3318–3323 CrossRef CAS.
- W. Hu, C. Peng, W. Luo, M. Lv, X. Li, D. Li, Q. Huang and C. Fan, ACS Nano, 2010, 4, 4317–4323 CrossRef CAS.
- K. Wang, J. Ruan, H. Song, J. Zhang, Y. Wo, S. Guo and D. Cui, Nanoscale Res. Lett., 2011, 6, 8 Search PubMed.
- S. R. Ryoo, Y. K. Kim, M. H. Kim and D. H. Min, ACS Nano, 2010, 4, 6587–6598 CrossRef CAS.
- Y. Chang, S. T. Yang, J. H. Liu, E. Dong, Y. Wang, A. Cao, Y. Liu and H. Wang, Toxicol. Lett., 2011, 200, 201–210 CrossRef CAS.
- P. Rivera Gil, G. Oberdörster, A. Elder, V. Puntes and W. J. Parak, ACS Nano, 2010, 4, 5527–5531 CrossRef CAS.
- A. Sasidharan, L. Panchakarla, P. Chandran, D. Menon, S. Nair, C. Rao and M. Koyakutty, Nanoscale, 2011, 3, 2461–2464 RSC.
- Y. Li, Y. Liu, Y. Fu, T. Wei, L. Le Guyader, G. Gao, R. S. Liu, Y. Z. Chang and C. Chen, Biomaterials, 2012, 33, 402–411 CrossRef CAS.
- H. Zhou, K. Zhao, W. Li, N. Yang, Y. Liu, C. Chen and T. Wei, Biomaterials, 2012, 33, 6933–6942 CrossRef CAS.
- E. Chang, N. Thekkek, W. W. Yu, V. L. Colvin and R. Drezek, Small, 2006, 2, 1412–1417 CrossRef CAS.
- A. Hoshino, K. Fujioka, T. Oku, M. Suga, Y. F. Sasaki, T. Ohta, M. Yasuhara, K. Suzuki and K. Yamamoto, Nano Lett., 2004, 4, 2163–2169 CrossRef CAS.
- A. M. Derfus, W. C. W. Chan and S. N. Bhatia, Nano Lett., 2004, 4, 11–18 CrossRef CAS.
- M. A. Hines and P. Guyot-Sionnest, J. Phys. Chem., 1996, 100, 468–471 CrossRef CAS.
- X. Peng, M. C. Schlamp, A. V. Kadavanich and A. Alivisatos, J. Am. Chem. Soc., 1997, 119, 7019–7029 CrossRef CAS.
- N. A. Monteiro-Riviere and A. O. Inman, Carbon, 2006, 44, 1070–1078 CrossRef CAS.
- M. Zhu, G. Nie, H. Meng, T. Xia, A. Nel and Y. Zhao, Acc. Chem. Res., 2013, 46, 622–631 CrossRef CAS.
- S. Mitragotri and J. Lahann, Nat. Mater., 2009, 8, 15–23 CrossRef CAS.
- H. S. Choi, W. Liu, P. Misra, E. Tanaka, J. P. Zimmer, B. I. Ipe, M. G. Bawendi and J. V. Frangioni, Nat. Biotechnol., 2007, 25, 1165–1170 CrossRef CAS.
- F. Lux, A. Mignot, P. Mowat, C. Louis, S. Dufort, C. Bernhard, F. Denat, F. Boschetti, C. Brunet, R. Antoine, P. Dugourd, S. Laurent, L. Vander Elst, R. Muller, L. Sancey, V. Josserand, J. L. Coll, V. Stupar, E. Barbier, C. Remy, A. Broisat, C. Ghezzi, G. Le Duc, S. Roux, P. Perriat and O. Tillement, Angew. Chem., Int. Ed., 2011, 50, 12299–12303 CrossRef CAS.
- C. Zhou, M. Long, Y. P. Qin, X. K. Sun and J. Zheng, Angew. Chem., Int. Ed., 2011, 50, 3168–3172 CrossRef CAS.
- B. D. Chithrani, A. A. Ghazani and W. C. W. Chan, Nano Lett., 2006, 6, 662–668 CrossRef CAS.
- W. Jiang, B. Y. S. Kim, J. T. Rutka and W. C. W. Chan, Nat. Nanotechnol., 2008, 3, 145–150 CrossRef CAS.
- M. V. D. Z. Park, A. M. Neigh, J. P. Vermeulen, L. J. J. de la Fonteyne, H. W. Verharen, J. J. Briede, H. van Loveren and W. H. de Jong, Biomaterials, 2011, 32, 9810–9817 CrossRef CAS.
- Y. Yuan, C. S. Liu, J. C. Qjan, J. Wang and Y. Zhang, Biomaterials, 2010, 31, 730–740 CrossRef CAS.
- X. W. Ma, Y. Y. Wu, S. B. Jin, Y. Tian, X. N. Zhang, Y. L. Zhao, L. Yu and X. J. Liang, ACS Nano, 2011, 5, 8629–8639 CrossRef CAS.
- S. Kim, W. K. Oh, Y. S. Jeong, J. Y. Hong, B. R. Cho, J. S. Hahn and J. Jang, Biomaterials, 2011, 32, 2342–2350 CrossRef CAS.
- J. A. Champion, A. Walker and S. Mitragotri, Pharm. Res., 2008, 25, 1815–1821 CrossRef CAS.
- Y. Pan, S. Neuss, A. Leifert, M. Fischler, F. Wen, U. Simon, G. Schmid, W. Brandau and W. Jahnen-Dechent, Small, 2007, 3, 1941–1949 CrossRef CAS.
- M. Tsoli, H. Kuhn, W. Brandau, H. Esche and G. Schmid, Small, 2005, 1, 841–844 CrossRef CAS.
- N. J. Hao, L. L. Li, Q. Zhang, X. L. Huang, X. W. Meng, Y. Q. Zhang, D. Chen, F. Q. Tang and L. F. Li, Microporous Mesoporous Mater., 2012, 162, 14–23 CrossRef CAS.
- X. L. Huang, L. L. Li, T. L. Liu, N. J. Hao, H. Y. Liu, D. Chen and F. Q. Tang, ACS Nano, 2011, 5, 5390–5399 CrossRef CAS.
- X. L. Huang, X. Teng, D. Chen, F. Q. Tang and J. Q. He, Biomaterials, 2010, 31, 438–448 CrossRef CAS.
- S. E. A. Gratton, P. A. Ropp, P. D. Pohlhaus, J. C. Luft, V. J. Madden, M. E. Napier and J. M. DeSimone, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 11613–11618 CrossRef CAS.
- S. Muro, C. Garnacho, J. A. Champion, J. Leferovich, C. Gajewski, E. H. Schuchman, S. Mitragotri and V. R. Muzykantov, Mol. Ther., 2008, 16, 1450–1458 CrossRef CAS.
- L. Florez, C. Herrmann, J. M. Cramer, C. P. Hauser, K. Koynov, K. Landfester, D. Crespy and V. Mailander, Small, 2012, 8, 2222–2230 CrossRef CAS.
- J. A. Champion and S. Mitragotri, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 4930–4934 CrossRef CAS.
- P. Decuzzi, R. Pasqualini, W. Arap and M. Ferrari, Pharm. Res., 2009, 26, 235–243 CrossRef CAS.
- P. Decuzzi and M. Ferrari, Biomaterials, 2006, 27, 5307–5314 CrossRef CAS.
- P. Decuzzi, B. Godin, T. Tanaka, S. Y. Lee, C. Chiappini, X. Liu and M. Ferrari, J. Controlled Release, 2010, 141, 320–327 CrossRef CAS.
- R. S. Liu, Controlled Nanofabrication: Advances and Applications, Pan Stanford Publishing, Singapore, 2013 Search PubMed.
- E. C. Cho, J. W. Xie, P. A. Wurm and Y. N. Xia, Nano Lett., 2009, 9, 1080–1084 CrossRef CAS.
- C. Wilhelm, C. Billotey, J. Roger, J. N. Pons, J. C. Bacri and F. Gazeau, Biomaterials, 2003, 24, 1001–1011 CrossRef CAS.
- A. L. Martin, L. M. Bernas, B. K. Rutt, P. J. Foster and E. R. Gillies, Bioconjugate Chem., 2008, 19, 2375–2384 CrossRef CAS.
- R. R. Arvizo, O. R. Miranda, M. A. Thompson, C. M. Pabelick, R. Bhattacharya, J. D. Robertson, V. M. Rotello, Y. S. Prakash and P. Mukherjee, Nano Lett., 2010, 10, 2543–2548 CrossRef CAS.
- J. P. Ryman-Rasmussen, J. E. Riviere and N. A. Monteiro-Riviere, Nano Lett., 2007, 7, 1344–1348 CrossRef CAS.
- O. Harush-Frenkel, N. Debotton, S. Benita and Y. Altschuler, Biochem. Biophys. Res. Commun., 2007, 353, 26–32 CrossRef CAS.
- T. Xia, M. Kovochich, M. Liong, H. Meng, S. Kabehie, S. George, J. I. Zink and A. E. Nel, ACS Nano, 2009, 3, 3273–3286 CrossRef CAS.
- A. E. Nel, L. Madler, D. Velegol, T. Xia, E. M. V. Hoek, P. Somasundaran, F. Klaessig, V. Castranova and M. Thompson, Nat. Mater., 2009, 8, 543–557 CrossRef CAS.
- C. C. Ge, J. F. Du, L. N. Zhao, L. M. Wang, Y. Liu, D. H. Li, Y. L. Yang, R. H. Zhou, Y. L. Zhao, Z. F. Chai and C. Y. Chen, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 16968–16973 CrossRef CAS.
- S. J. Tan, N. R. Jana, S. J. Gao, P. K. Patra and J. Y. Ying, Chem. Mater., 2010, 22, 2239–2247 CrossRef CAS.
- B. Naim, D. Zbaida, S. Dagan, R. Kapon and Z. Reich, EMBO J., 2009, 28, 2697–2705 CrossRef CAS.
- E. R. Zubarev, J. Xu, A. Sayyad and J. D. Gibson, J. Am. Chem. Soc., 2006, 128, 15098–15099 CrossRef CAS.
- H. W. Duan, M. Kuang, D. Y. Wang, D. G. Kurth and H. Mohwald, Angew. Chem., Int. Ed., 2005, 44, 1717–1720 CrossRef CAS.
- H. Wang, Y. Zhao, Y. Wu, Y. L. Hu, K. H. Nan, G. J. Nie and H. Chen, Biomaterials, 2011, 32, 8281–8290 CrossRef CAS.
- Q. Xu, Y. Liu, S. Su, W. Li, C. Chen and Y. Wu, Biomaterials, 2012, 33, 1627–1639 CrossRef CAS.
- R. Gref, Y. Minamitake, M. T. Peracchia, V. Trubetskoy, V. Torchilin and R. Langer, Science, 1994, 263, 1600–1603 CAS.
- I. Lynch and K. A. Dawson, Nano Today, 2008, 3, 40–47 CrossRef CAS.
- C. D. Walkey and W. C. W. Chan, Chem. Soc. Rev., 2012, 41, 2780–2799 RSC.
- M. A. Dobrovolskaia, A. K. Patri, J. W. Zheng, J. D. Clogston, N. Ayub, P. Aggarwal, B. W. Neun, J. B. Hall and S. E. McNeil, Nanomedicine, 2009, 5, 106–117 CrossRef CAS.
- M. P. Monopoli, D. Walczyk, A. Campbell, G. Elia, I. Lynch, F. B. Bombelli and K. A. Dawson, J. Am. Chem. Soc., 2011, 133, 2525–2534 CrossRef CAS.
- I. Lynch, A. Salvati and K. A. Dawson, Nat. Nanotechnol., 2009, 4, 546–547 CrossRef CAS.
- Z. J. Zhu, T. Posati, D. F. Moyano, R. Tang, B. Yan, R. W. Vachet and V. M. Rotello, Small, 2012, 8, 2659–2663 CrossRef CAS.
- C. D. Walkey and W. C. W. Chan, Chem. Soc. Rev., 2012, 41, 2780–2799 RSC.
- P. V. AshaRani, G. L. K. Mun, M. P. Hande and S. Valiyaveettil, ACS Nano, 2009, 3, 279–290 CrossRef CAS.
- Y. Zhu, W. X. Li, Q. N. Li, Y. G. Li, Y. F. Li, X. Y. Zhang and Q. Huang, Carbon, 2009, 47, 1351–1358 CrossRef CAS.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2013 |
