Palladium-mediated bioorthogonal conjugation of dual-functionalised nanoparticles and their cellular delivery†
Frank
Thielbeer‡
,
Sunay V.
Chankeshwara‡
,
Emma M. V.
Johansson
,
Neil
Norouzi
and
Mark
Bradley
*
School of Chemistry, University of Edinburgh, West Mains Road, EH9 3JJ, Edinburgh, UK. E-mail: Mark.Bradley@ed.ac.uk; Fax: +44 (0)131 777 0344; Tel: +44 (0)131 650 4820
First published on 11th October 2012
Abstract
Nanoparticles have gained considerable significance in the life sciences due to their ability to be internalised by living cells and the relative ease with which they can be functionalised with cargos ranging from molecular sensors to biomacromolecules. However, the scope of available bioconjugation methods is limited and new bioorthogonal methods are much sought after. Herein, we present dual functionalised (HO)2B/H2N-polymeric nanoparticles which can be conjugated via amide bond formation and/or Pd-mediated Suzuki–Miyaura cross-coupling in a chemoselective and bioorthogonal manner. These dual-functionalised particles were found to be efficiently taken up by mammalian cells without toxicity and were successfully employed in the cellular delivery and intracellular release of a “turn-on” molecular probe thereby demonstrating the potential use of the new particles for chemical biology applications.
Introduction
Intracellular delivery of functional macromolecules using nano and micro-sized polymeric particles is widely used in the life sciences due to their efficiency in being able to deliver cargos into cells in a benign manner.1 Sensors conjugated to particles have, for example, been utilised to reveal important insights concerning the physiological state of a cell, such as intracellular pH,2 ion concentrations,3 and the functional states of proteins.4 In addition, particles have been employed to deliver a wide range of bioactive compounds into cells including drugs,5,6 proteins7 and nucleic acids;8,9 moreover; fluorescently labelled particles have been used to track and sort cells.10,11Non-degradable polystyrene nanoparticles have been used in vitro cellular uptake11a and in vivo tracking11b experiments because of their non-immunogenic properties and low cytotoxicity. They have been used to track the stem cells in the generation of chimera, perhaps the most robust proof possible of non-toxicity.11c In addition, the particle's size and surface modification can easily be varied.1,11a
The ability to functionalise non-degradable nanoparticles such as polymeric, magnetic or quantum dots is extremely relevant due to their proposed biomedical applications. Such nanoparticles can be used as a multi-tasking platform, for instance, the combination of fluorescent markers and MRI contrast agents or photothermal therapy and hyperthermia agents would allow simultaneous diagnosis and therapeutic application of internalised nanoparticles.12
Typical bioconjugation techniques, such as thiol–maleimide addition or amide coupling reactions are applied in the construction of particle bioconjugates.13,14 However, a variety of competing nucleophiles and electrophiles present in complex biomolecules hamper their use. Huisgen cycloaddition chemistry can be used to ligate alkyne-functionalised cargos onto azide-functionalised particles in a bioorthogonal fashion, but typically requires copper ion catalysis.15,16 The efficient, selective and benign bioconjugation of cargo molecules onto nanoparticles therefore remains an important challenge, and one that becomes even more complex when particles are required to carry two or more cargos.17 This can be achieved using particles with multiple bioorthogonal, functional groups on their surface. Stucky and co-workers have prepared dual-functionalised amine and azide particles18 while Li et al. synthesised a block copolymer, containing amine, acylhydrazide and azide groups, which was used to coat and functionalise gold nanoparticles.19
Recently, we have demonstrated the synthesis and reaction monitoring of aminomethyl-functionalised nanoparticles which can be further derivatised with a variety of reactive functional groups.20 However, to boost the particle properties, we aimed to introduce an additional functionality allowing chemoselective conjugation onto the particle's surface.
Inspired by the challenges and demands of synthesising novel nanoparticles while developing new conjugation techniques, herein we describe the development of dual (HO)2B/H2N-functionalised polymeric nanoparticles. Further, we investigated their derivatisation via orthogonal Pd-mediated Suzuki–Miyaura cross-coupling and amide bond formation, and demonstrate the benefits of these dual-functionalised nanoparticles by efficient cellular uptake, intracellular cargo liberation and organelle targeting.
Results and discussion
Particle synthesis
Lately, carbon–carbon bond formation via Pd-catalysed reactions, has been used to modify proteins,21 label cell membranes,22 and to carry out novel intracellular chemistry23 in a manner that is fully orthogonal to amide bond formation. To the best of our knowledge Pd-mediated nanoparticle functionalisation has not been reported and we therefore targeted this approach to allow bioorthogonal nanoparticle conjugation.The (HO)2B/H2N-particles (5–8) were prepared by emulsifier-free emulsion polymerisation of styrene 1, cross-linker divinylbenzene 2 (2%), 4-vinylbenzylamine hydrochloride 3 (VBAH, 3%), and varying amounts of 4-vinylbenzylboronic acid 4 (VBA, 0–9%) (Scheme 1a). The particles 5–8 all had a hydrodynamic diameter between 200 and 260 nm as determined by dynamic light scattering (Scheme 1b, Table 1, Fig. S1†) regardless of the level of VBA incorporation. However, they showed varying polydispersity indices (PDI) (Table 1).
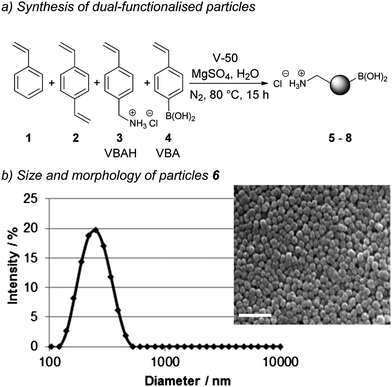 | ||
| Scheme 1 Synthesis and analysis of dual-functionalised (HO)2B/H2N-particles via emulsifier-free emulsion polymerisation: (a) particle synthesis; and (b) size analysis of particles 6 by dynamic light scattering and scanning electron microscopy (240 nm, PDI 0.071). Scale bar equals 1 μm. | ||
| Particles | Size [nm] (PDI) | H2N-groups [μmol g−1] | (HO)2B-group [μmol g−1] |
|---|---|---|---|
| 5 (0% VBA) | 229 (0.060) | (82 ± 14) | (2 ± 1) |
| 6 (3% VBA) | 240 (0.071) | (129 ± 15) | (62 ± 1) |
| 7 (6% VBA) | 260 (0.190) | (163 ± 5) | (116 ± 2) |
| 8 (9% VBA) | 211 (0.313) | (76 ± 3) | (204 ± 1) |
Particles 6, polymerised with 3% VBA, had a PDI of 0.071, similar to particles 5 synthesised without VBA (0.060), both being classified as monodisperse (PDI < 0.1). Further increase in VBA levels lead to polydisperse particles with PDIs of 0.190 (6% VBA, 7) and 0.313 (9% VBA, 8) which were confirmed by scanning electron microscopy (Scheme 1b, Fig. S1†). Presumably, 7 and 8 were polydisperse resulting from increased acid–base interactions between VBAH 3 and VBA 4 disturbing the VBAH-mediated micelle formation during the polymerisation.24
The dual-functionalisation of the nanoparticles was quantitatively verified by ninhydrin analysis for amine loading and ICP-OES analysis25 for boronic acid loading levels (Table 1). The addition of 3% VBA in the synthesis gave particles 6 with a boronic acid loading of 62 μmol g−1. An increase in VBA levels (6% or 9%) resulted in an approximately 2-fold (116 μmol g−1, 7) and 3-fold (204 μmol g−1, 8) increase in boron content. Due to the good polydispersity, in combination with the good boronic acid and aminomethyl loadings, particles 6 were selected for Pd-mediated conjugation and biological studies.
Palladium-mediated particle conjugation
In order to study Pd-mediated Suzuki–Miyaura cross-coupling onto the particles 5-iodofluorescein109 was coupled onto 6 (Scheme 2a) with various Pd salts screened in physiological buffers. This allowed monitoring of the conjugation reaction by increases in particle fluorescence in addition to a decrease in the boron content (Table S5†) with the best reaction conditions identified to be in PBS at 37 °C with the water soluble complex of 2-amino-4,6-dihydroxypyrimidine–Pd(OAc)2 as catalyst.26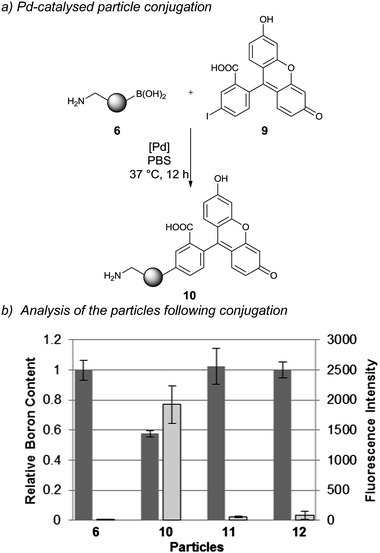 | ||
| Scheme 2 Pd-catalysed particle conjugation: (a) (HO)2B/H2N-particle conjugation with 5-iodofluorescein using as a catalyst the sodium salt complex of Pd(OAc)2 and 2-amino-4,6-dihydroxypyrimidine; and (b) analysis of particle conjugation by boron consumption (via ICP-OES analysis) and fluorescence intensity of particles (via flow cytometry) (n = 3). Dark grey = boron content and light grey = fluorescence intensity of particles. | ||
Following the coupling, two independent techniques, ICP-OES and flow cytometry, were applied to verify the degree of fluorescein conjugation. ICP-OES analysis of 10 revealed a 40% reduction in the boron content (Scheme 2b, dark grey columns), while flow cytometry analysis of 10 revealed a 500-fold increase in particle fluorescence compared to the precursor particles 6 (Scheme 2b, light grey columns). Two control reactions were run, one in which 5-iodofluorescein was replaced with fluorescein and a second in which the catalyst was removed from the reaction mixture. As expected, both control reactions resulted in unchanged boron content and particle fluorescence.
To confirm the reproducibility of palladium-mediated particle conjugation, the optimised conditions were performed on a model system in triplicate and the efficiency of conjugation was determined by measuring the boron consumption using ICP-OES. The conjugation reactions were highly reproducible (38 ± 4% conjugation efficiency). Quantitative yields were not achieved presumably due to the distribution of the boronic acid groups between the particle's “surface” and “interior” with coupling to the latter increasingly more difficult due to the steric demands,27 in agreement with recent reports that demonstrated that modifications of azide-functionalised nanoparticles with alkyne modified fluorescein by Cu-catalysed click chemistry was dependent on the polymerisation process, surfactants used and accessible azide groups.28 Advantages of the Pd-mediated Suzuki–Miyaura cross-coupling over the Cu-catalysed click reaction are the benign nature of the Pd catalyst,29 while oxidation of biological substrates has also been observed with Cu-catalysed click chemistry due to the hydrogen peroxide and superoxide radicals produced by copper ion mediated catalytic oxidation of sodium ascorbate by molecular oxygen.16
Palladium catalysed reactions have been used to modify proteins,21 label cell membranes,22 and to carry out novel intracellular chemistry23 without any observed apparent toxicity or substrate sensitivity towards palladium. The above results thus confirmed the ability of Suzuki–Miyaura cross-coupling reactions to conjugate cargo molecules onto the (HO)2B/H2N-particles in a biocompatible manner.
Cellular uptake and viability
Following the successful Pd-mediated conjugation of fluorophore 9 onto the particles, the cellular uptake of the fluorescein conjugated particles 10 into HEK293T and HeLa cells was analysed by flow cytometry. This demonstrated successful internalisation of the particles with increasing particle concentrations lead to increasing intracellular fluorescence (Fig. 1, external fluorescence quenched with trypan blue.30 For histograms and microscopy images see Fig. S5 and S6†). A 14-fold increase in cellular fluorescence was observed with the highest particle concentration (75 μg mL−1) with no apparent cellular toxicity (Fig. S15†).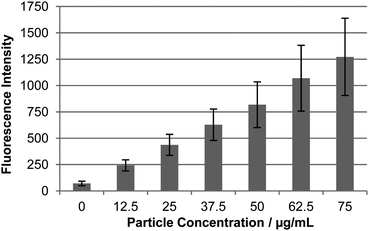 | ||
| Fig. 1 Cellular uptake of Pd-mediated fluorescein conjugated particles 10 in HEK293T cells. Relative uptake by flow cytometry (external fluorescence was quenched by the addition of 2% trypan blue30). For microscopic images and histograms see Fig. S5 and S6†). | ||
Boronic acid groups are known for their high affinity towards diols which has been used to develop glucose sensors.31 As described above, the Pd-coupling is not quantitative leaving boronic acid groups on the nanoparticle surface. To investigate if the boronic acid groups could induce cellular toxicity, cell viability assays were also carried out with non-functionalised (HO)2B/H2N-nanoparticles 6 and demonstrated no cellular toxicity (Fig. S13†). These results demonstrated that the (HO)2B/H2N-nanoparticles, are readily functionalised by Suzuki–Miyaura cross-coupling, that the modified particles are efficiently taken up by the cells without any toxicity from the residual boronic acid particles and that Pd-levels on the particles are negligible.32
Bioorthogonal dual-functionalisation of the particles
The (HO)2B/H2N-nanoparticles 6 were subsequently dual functionalised to carry a Cy5 dye and cargo moiety which, upon cellular uptake, will be released from the particles (Scheme 3a). The Cy5 dye on the particles would enable cellular uptake quantification, while the cargo moiety (a peptide) was designed to contain a “turn-on” fluorescence signal that would only become active following intracellular release of the cargo. This was based on a cleavable disulphide linker connecting a fluorescence quencher (methyl red) and fluorescein to the cargo. Upon cellular uptake, intracellular cleavage of the disulphide bond would release the cargo from the particles allowing “turn-on” of fluorescence (Scheme 3a) (as a control the disulphide bond was replaced with a hydrocarbon linkage). The cargo moiety was chosen to be a short anionic peptide sequence, DDGD, which would avoid leakage of the released fluorescein out of the cell.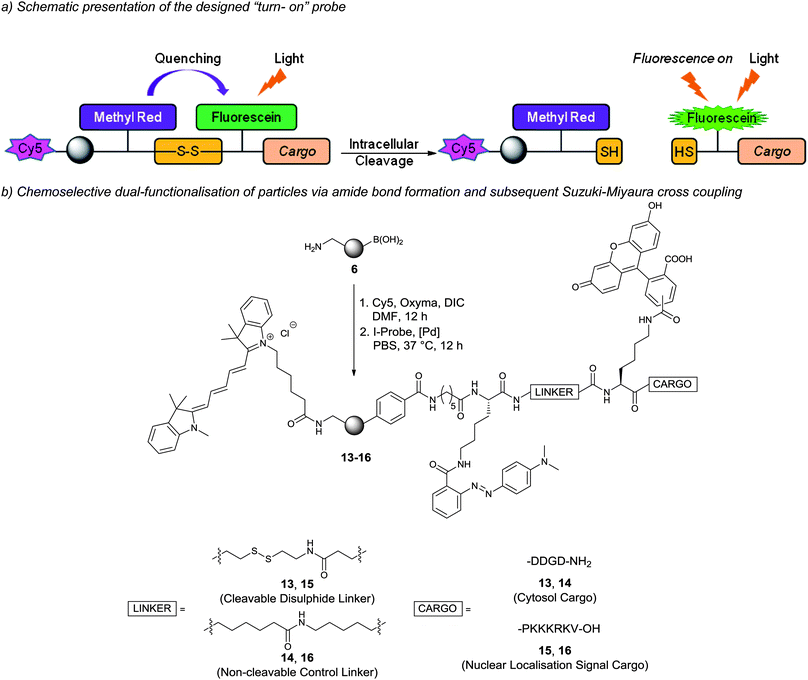 | ||
| Scheme 3 Dual-functionalised particles and intracellular fluorescein release: (a) probe design and the mechanism of intracellular cargo release and fluorescence “turn-on”; (b) dual-functionalisation of particles by conjugation of Cy5 via amide formation and subsequent Suzuki–Miyaura cross-coupling of iodo-aryl probes onto particles. 13 = cytosol targeted probe and 15 = nucleus targeted probe (14 and 16 are negative control probes with non-cleavable linkages). | ||
The particle construct 13 was prepared by coupling Cy5 via carbodiimide chemistry while the (HO)2B-groups on the particle surface allowed bioorthogonal conjugation of an unprotected peptide cargo containing an aryl-iodide via Suzuki–Miyaura cross-coupling (Scheme 3b, Fig. S2†). ICP-OES measurement of the particles revealed a decrease in the boron content for particles 13 (23%). The ability of 13 to release fluorescein was investigated in vitro by treatment with dithiothreitol (DTT) followed by analysis of the fluorescence of the particles supernatant. Fluorescence was only detected after DTT (10 mM) treatment of the disulphide-linked probe. As expected, control particles 14 did not release fluorescein (Fig. S3†).
Particles 13 and 14 were incubated with HEK293T and HeLa cells and cellular uptake monitored by flow cytometry detecting Cy5. The Cy5 fluorescence was equal for cells incubated with particles 13 and 14 confirming similar intracellular uptake (Fig. S8†). However, after 48 h incubation a intracellular fluorescence signal ”turn-on” was detected, demonstrating the intracellular cleavage of the disulphide bond and subsequent release of the fluorescein peptide reporter from particles 13 (Fig. 2). Confocal fluorescence microscopy verified linker-dependent cargo release from the particles and cytosolic localisation (Fig. 3). In contrast, the control particles 14 did not release the fluorescent reporter (Fig. 2 and 3).
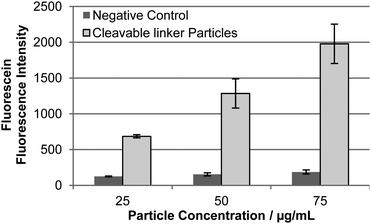 | ||
| Fig. 2 Quantification of intracellular fluorescein release from particles in HEK293T cells as determined by flow cytometry after 24 h incubation. Light grey = disulphide-linked particles 13; and dark grey = control particles 14. (n = 3). | ||
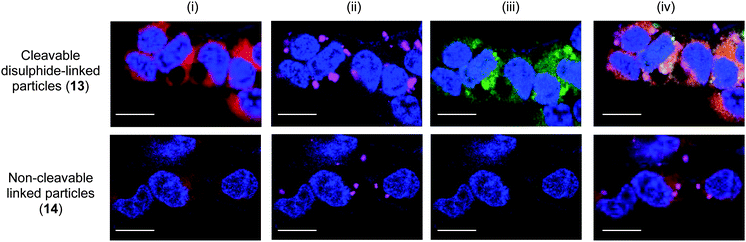 | ||
| Fig. 3 Confocal fluorescence microscopy images of HEK293T cells following incubation with cytosol targeted probe 13 and non-cleavable control probe 14 (50 μg mL−1). (i) Blue = nucleus stain Hoechst 33342 (λex = 404 nm), red = cytoplasm stain celltracker red CMTPX (λex = 594 nm); (ii) blue = nucleus stain, magenta = Cy5-labeled particles (λex = 633 nm); (iii) blue = nucleus stain, green = released fluorescein (λex = 488 nm); (iv) overlay. Scale bar: 50 μm. | ||
Finally, a particle construct was prepared with the intention of delivering the cargo into the cell nucleus. Thus a cargo containing the peptide PKKKRKV, a known nuclear localisation signal (NLS), and the previously described fluorescein–methyl red quencher system was conjugated onto the Cy5-labelled particles affording particles 15 (cleavable disulphide linker) and the non-cleavable control 16 (Scheme 3b). The synthesis of the particles followed the previously established route via coupling of Cy5 onto (HO)2B/H2N-particles following the probe conjugation via Suzuki–Miyaura cross-coupling of an iodo-aryl tagged unprotected peptide (Scheme 3b).
As expected, uptake of particles 15 resulted in fluorescent “turn-on” in HEK293T and HeLa cells with fluorescein accumulated into the cell nucleus (Fig. 4). In contrast, the control particles 16 did not release the NLS-construct and fluorescein was not observed in the nucleus (Fig. 4). These studies demonstrate the advantages and applications of dual-functionalisation of particles. The attachment of an independent Cy5-label on the particles allowed quantitative measurement of cellular uptake of the particles while the cargo-sensor construct was readily conjugated to the particle and demonstrated intracellular release of the cargo by “turn-on” of fluorescence.
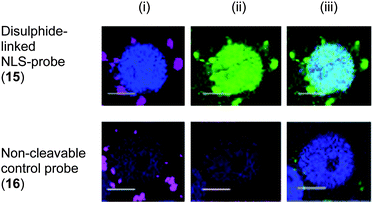 | ||
| Fig. 4 Release of NLS-fluorescein construct and translocation into the nucleus of HEK293T cells from cleavable disulphide-linked particles 15 and from non-cleavable control particles 16 (50 μg mL−1): (i) blue nucleus stain Hoechst 33342 (λex = 404 nm), magenta: Cy5-labeled particles (λex = 633 nm); (ii) blue: nucleus stain, red: cytoplasm stain celltracker red CMTPX (λex = 594 nm), green: released NLS-fluorescein (λex = 488 nm); and (iii) overlay. Scale bar: 25 μm. | ||
Conclusions
In conclusion, the preparation of dual-functionalised nanoparticles containing bioorthogonal boronic acid and aminomethyl groups was successfully demonstrated. The boronic acid groups on the particles allowed the conjugation of complex peptide-reporter cargos by Pd-mediated Suzuki–Miyaura cross-coupling. This conjugation method is reported for the first time and extends the repertoire of benign bioconjugation methods in a bioorthogonal fashion. The modified particles with attached cargos were successfully internalised into cells without cellular toxicity and the applications of these particles demonstrated by intracellular delivery and release of cargos with organelle targeting. Future applications of the dual-functionalised particles as well as the conjugation technique may address the awaiting challenges and opportunities in the field of nanoparticles in chemical biology.Experimental section
Particle synthesis
Styrene 1 (122 μL, 1 mmol, freshly washed with 4 × 25% NaOH and 4 × brine), divinylbenzene 2 (3.1 μL, 22 μmol, 2 mol% with regards to styrene, freshly washed with 4 × 25% NaOH and 4 × brine), VBAH 3 (5.9 mg, 35 μmol, 3 mol%), 4-vinylbenzylboronic acid 4 (0%; 3%: 5.2 mg, 35 μmol; 6% 10.4 mg, 70 μmol; 9% 15.5 mg, 105 μmol), 2,2-azobis (2-methylpropionamidine) dihydrochloride (V-50) (1.6 mg, 6 μmol) and MgSO4 (0.4 mg, 3 μmol) were added to N2 purged water (2 mL). The reaction mixture was stirred at room temperature under atmosphere of N2 for 15 min and subsequently stirred at 80 °C for 15 h. Upon cooling to room temperature, the particles were collected by centrifugation (15 min at 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g, Eppendorf 5430R) and washed with DMF (3 × 2 mL), MeOH (3 × 2 mL) and water (3 × 2 mL). The particles were stored in water at a solid content of 20 mg mL−1.
000g, Eppendorf 5430R) and washed with DMF (3 × 2 mL), MeOH (3 × 2 mL) and water (3 × 2 mL). The particles were stored in water at a solid content of 20 mg mL−1.
General procedure for Pd-mediated particle conjugation
Conjugation of iodated cargo was carried out by Suzuki–Miyaura cross-coupling. A solution of cargo (40 μmol, 50 equiv.) in DMSO (4 μL) was prepared and added to a suspension of (HO)2B/H2N-particles (0.8 μmol, 1 equiv.) in PBS (1000 μL, pH 7.4) and sonicated for 1 min. Afterwards, catalyst stock solution (2 μmol, 0.2 μL, 5%) were added and the reaction mixture shaken at 1400 rpm at 37 °C for 12 h. The particle mixture was washed in water (3 × 1 mL) and stored in PBS at a concentration of 10 mg mL−1.Cellular uptake
HEK293T and HeLa were plated in DMEM supplemented with 10% (v/v) heat inactivated fetal bovine serum, L-glutamine (4 mM) and antibiotics (100 units per mL penicillin and 100 units per L streptomycin) growth media in a 24-well plate at a density of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per well and grown for 24 h at 37 °C and 5% CO2. Thereafter, particles in media (12.5–75 μg mL−1) were added and incubated with the cells for 24 h. Cells were washed with PBS (2 × 350 μL) and incubated for a further 24 h. After incubation, cells were washed with PBS (2 × 350 μL), and imaged under a Leica DM IRB fluorescence microscope or fluorescence pictures were taken on a Leica SP5 confocal microscope. Zeiss 510 Meta software was used for digital acquisition. Images were processed using AutoQuant X software. Quantitative flow cytometric analysis was carried out following the above mentioned particle incubation procedure. Following washing, cells were harvested with trypsin/EDTA and analysed by flow cytometry (BD FACSAria using FlowJo Version 7.6.3 software for data analysis). Flow cytometry was carried out in triplicate and by addition of 2% trypan blue to quench any extracellular fluorescence.30
000 cells per well and grown for 24 h at 37 °C and 5% CO2. Thereafter, particles in media (12.5–75 μg mL−1) were added and incubated with the cells for 24 h. Cells were washed with PBS (2 × 350 μL) and incubated for a further 24 h. After incubation, cells were washed with PBS (2 × 350 μL), and imaged under a Leica DM IRB fluorescence microscope or fluorescence pictures were taken on a Leica SP5 confocal microscope. Zeiss 510 Meta software was used for digital acquisition. Images were processed using AutoQuant X software. Quantitative flow cytometric analysis was carried out following the above mentioned particle incubation procedure. Following washing, cells were harvested with trypsin/EDTA and analysed by flow cytometry (BD FACSAria using FlowJo Version 7.6.3 software for data analysis). Flow cytometry was carried out in triplicate and by addition of 2% trypan blue to quench any extracellular fluorescence.30
Acknowledgements
The authors would like to thank the MRC UK (F.T.), the European Commission for Marie Curie International Incoming Fellowship to S.V.C. (Project No. PIIF-GA-2009-254238) and Dr Annamaria Lilienkampf for helpful discussions.Notes and references
- (a) L. M. Alexander, S. Pernagallo, A. Livigni, R. M. Sánchez-Martín, J. M. Brickman and M. Bradley, Mol. BioSyst., 2010, 6, 399–409 RSC; (b) S. C. Balmert and S. R. Little, Adv. Mater., 2012, 24, 3757–3778 CrossRef CAS , and references cited therein. A. Unciti-Broceta, J. J. Díaz-Mochón, R. M. Sánchez-Martín and M. Bradley, Acc. Chem. Res., 2012, 45, 1140–1152 Search PubMed.
- M. Bradley, L. Alexander, K. Duncan, M. Chennaoui, A. C. Jones and R. M. Sánchez-Martín, Bioorg. Med. Chem. Lett., 2008, 18, 313–317 CrossRef CAS.
- R. M. Sánchez-Martín, M. Cuttle, S. Mittoo and M. Bradley, Angew. Chem., Int. Ed., 2006, 45, 5472–5474 CrossRef.
- K. Kim, M. Lee, H. Park, J.-H. Kim, S. Kim, H. Chung, K. Choi, I.-S. Kim, B. L. Seong and I. C. Kwon, J. Am. Chem. Soc., 2006, 128, 3490–3491 CrossRef CAS.
- J.-Z. Du, X.-J. Du, C.-Q. Mao and J. Wang, J. Am. Chem. Soc., 2011, 133, 17560–17563 CrossRef CAS.
- T. C. Yih and M. Al-Fandi, J. Cell. Biochem., 2006, 97, 1184–1190 CrossRef CAS.
- R. M. Sanchez-Martin, L. Alexander, M. Muzerelle, J. M. Cardenas-Maestre, A. Tsakiridis, J. M. Brickman and M. Bradley, ChemBioChem, 2009, 10, 1453–1456 CrossRef CAS.
- L. M. Alexander, R. M. Sánchez-Martín and M. Bradley, Bioconjugate Chem., 2009, 20, 422–426 CrossRef CAS.
- J. G. Borger, J. M. Cardenas-Maestre, R. Zamoyska and R. M. Sanchez-Martin, Bioconjugate Chem., 2011, 22, 1904–1908 CrossRef CAS.
- F. Thielbeer, S. V. Chankeshwara and M. Bradley, Biomacromolecules, 2011, 12, 4386–4391 CrossRef CAS.
- For the application of non-degradable nanoparticles see: (a) R. M. Sanchez-Martin, M. Muzerelle, N. Chitkul, S. E. How, S. Mittoo and M. Bradley, ChemBioChem, 2005, 6, 1341–1345 CrossRef CAS; (b) K. Dhaliwal, L. Alexander, G. Escher, A. Unciti-Broceta, M. Jansen, N. Mcdonald, J. M. Cardenas-Maestre, R. Sanchez-Martin, J. Simpson, C. Haslett and M. Bradley, Faraday Discuss., 2011, 149, 107–114 RSC; (c) A. Tsakiridis, L. M. Alexander, N. Gennet, R. M. Sanchez-Martin, A. Livigni, M. Li, M. Bradley and J. M. Brickman, Biomaterials, 2009, 30, 5853–5861 CrossRef CAS.
- D. B. Tada, L. L. R. Vono, E. L. Duarte, R. Itri, P. K. Kiyohara, M. S. Baptista and L. M. Rossi, Langmuir, 2007, 23, 8194–8199 CrossRef CAS.
- W. R. Algar, D. E. Prasuhn, M. H. Stewart, T. L. Jennings, J. B. Blanco-Canosa, P. E. Dawson and I. L. Medintz, Bioconjugate Chem., 2011, 22, 825–858 CrossRef CAS.
- J. M. Cardenas-Maestre, S. Panadero-Fajardo, A. M. Perez-Lopez and R. M. Sanchez-Martin, J. Mater. Chem., 2011, 21, 12735–12743 RSC.
- R. K. Iha, K. L. Wooley, A. M. Nyström, D. J. Burke, M. J. Kade and C. J. Hawker, Chem. Rev., 2009, 109, 5620–5686 CrossRef CAS.
- V. Hong, S. I. Presolski, C. Ma and M. G. Finn, Angew. Chem., Int. Ed., 2009, 48, 9879–9883 CrossRef CAS.
- S. S. Kelkar and T. M. Reineke, Bioconjugate Chem., 2011, 22, 1879–1903 CrossRef CAS.
- Z. An, W. Tang, M. Wu, Z. Jiao and G. D. Stucky, Chem. Commun., 2008, 6501–6503 RSC.
- X. Li, J. Guo, J. Asong, M. A. Wolfert and G.-J. Boons, J. Am. Chem. Soc., 2011, 133, 11147–11153 CrossRef CAS.
- F. Thielbeer, K. Donaldson and M. Bradley, Bioconjugate Chem., 2011, 22, 144–150 CrossRef CAS.
- C. D. Spicer and B. G. Davis, Chem. Commun., 2011, 47, 1698–1700 RSC.
- C. D. Spicer, T. Triemer and B. G. Davis, J. Am. Chem. Soc., 2012, 134, 800–803 CrossRef CAS.
- R. M. Yusop, A. Unciti-Broceta, E. M. V. Johansson, R. M. Sánchez-Martín and M. Bradley, Nat. Chem., 2011, 3, 241–245 CrossRef.
- C. S. Chern, Prog. Polym. Sci., 2006, 31, 443–486 CrossRef CAS.
- N. Vogel, C. P. Hauser, K. Schuller, K. Landfester and C. K. Weiss, Macromol. Chem. Phys., 2010, 211, 1355–1368 CrossRef CAS.
- J. M. Chalker, C. S. C. Wood and B. G. Davis, J. Am. Chem. Soc., 2009, 131, 16346–16347 CrossRef CAS.
- A. Hennig, H. Borcherding, C. Jaeger, S. Hatami, C. Würth, A. Hoffmann, K. Hoffmann, T. Thiele, U. Schedler and U. Resch-Genger, J. Am. Chem. Soc., 2012, 134, 8268–8276 CrossRef CAS.
- K. Ouadahi, E. Allard, B. Oberleitner and C. Larpent, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 314–328 CrossRef CAS.
- D. A. Fleming, C. J. Thode and M. E. Williams, Chem. Mater., 2006, 18, 2327–2334 CrossRef CAS.
- V. L. Mosiman, B. K. Patterson, L. Canterero and C. L. Goolsby, Cytometry, 1997, 30, 151–156 CrossRef CAS.
- H. Shibata, Y. J. Heo, T. Okitsu, Y. Matsunaga, T. Kawanishi and S. Takeuchi, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 17894–17898 CrossRef CAS.
- The residual Pd content of 10 was investigated by ICP-OES analysis and revealed 44 ng of palladium in 75 μg of particle suspension.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthesis of VBAH, 5-iodofluorescein, anionic and NLS peptide-reporters, particle analysis, preparation of particles 13–16 (amide bond coupling and Pd-mediated coupling), ICP-OES analysis (for palladium and boron quantification), DTT-induced fluorescein release, histograms and microscopy images of cells following fluorescein-conjugated particle uptake, quantification of Cy5 fluorescence after incubation with dual-functionalised particles, MTT assays and HeLa cell data. See DOI: 10.1039/c2sc20706k |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2013 |
