Simple density-based particle separation in a microfluidic chip†
Daisuke
Sugiyama‡
a,
Yuki
Teshima
b,
Kenichi
Yamanaka
c,
Maria Portia
Briones-Nagata
a,
Masatoshi
Maeki
b,
Kenichi
Yamashita
ab,
Masashi
Takahashi§
d and
Masaya
Miyazaki
*ab
aMeasurement Solution Research Center, National Institute of Advanced Industrial Science and Technology (AIST), 807-1 Shuku, Tosu, Saga 841-0052, Japan. E-mail: m.miyazaki@aist.go.jp; Fax: +81-942-81-3627; Tel: +81-942-81-4059
bDepartment of Molecular and Material Sciences, Interdisciplinary Graduate School of Engineering Science, Kyushu University, 6-1 Kasuga-koen, Kasuga, Fukuoka 816-8580, Japan
cDepartment of Applied Biological Sciences, Faculty of Agriculture, Saga University, 1 Honjo, Saga, Saga 840-8502, Japan
dKyushu Okinawa Agricultural Research Center, National Agriculture and Food Research Organization (NARO), 2421 Suya, Koshi, Kumamoto 861-1192, Japan
First published on 17th October 2013
Abstract
We investigate the behaviour of a simple microfluidic device designed to separate particles based on density. The device consists of a separation-channel with three inlet and two outlet channels. The particle samples were loaded in the middle of the main channel. The results of separation experiments using model particles provide clear evidence that this approach can be used to achieve good separation of particles of close density populations. Particle separation was found more favourable with low flow rates. Forces exerted on particles were modelled by Stokes' law and found to be consistent with experimental results. One advantage of this microfluidic system is low exposure of the sample to hydrodynamic shear stresses compared with conventional methods. This device may be adopted to precisely handle single cells and easily interface with other tools and separation techniques.
Introduction
Biological, chemical, and medical processes involving complex fluids with particles (e.g., blood) often involve preparative separation of particles, cells and even molecules for use in subsequent procedures. Many useful techniques have been developed for cell sorting. Particle separation methods can be divided into three groups: (1) those that use gravity or centrifugal force, in which the particles move relative to the suspending agent; (2) separations based upon optical, acoustic radiation, dielectrophoretic and magnetic forces, and (3) those that use chromatographic principles, where particles move at the same velocity as the fluid. Cell and soft particle separation and sorting are essential steps in biology research. This area has attracted great attention from both academia and industry.1–3 Density-based particle separation techniques find applications in separation of specific cells, bacteria, or environmental particles.The field of microfluidics concerns the miniaturization of conventional devices or methods to operate on restricted volumes of liquids on the microlitre scale. Microfluidic systems allow handling of lower reagent and sample volumes with advantages such as low cost, operational simplicity, high throughput, potential for integration and automation, and higher precision.
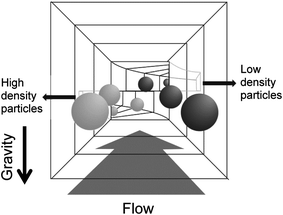
The separation and sorting of cells are essential techniques in microfluidic applications.2 In recent years, a number of researchers have reported particle separation using microfluidic devices. In general, most microfluidic cell and particle separation methods can be categorized as filtering based on geometry,4 hydrodynamics,5 and cell mobility.6 Dielectrophoresis-activated sorting,7 magnetic-activated cell sorting,8 as well as fluorescence-activated cell sorting9 have also been proposed for cell or particle separation. Recently, some techniques used a stably formed pinched flow,10 similar to pinched flow11 or Dean vortices in curved channels,12 ultrasound,13 electric fields,14 magnetic fields,15 or centrifugal force to separate the micro-sized particles and cells. These methods rely on size-based separation. However, there are only a few reports on density-based separation in the microfluidic format.16 In this study, we describe a technique for density-based separation of submillimeter-sized particles using model particles of approximately the same sizes and mass. The experiment was performed in a simple microfluidic device under minimal exposure to hydrodynamic shear stress conditions, based on Stokes' equation (Fig. 1).
Results and discussion
The microfluidic device used in this study is shown in Fig. 2. The system consists of central inlet and horizontally separated outlets at the end of a straight separating channel. The particles were injected into the separating channel through the inlet. The sucrose solution, acting as the mobile phase, was injected through the two side inlets. Through laminar flow in the separation-channel, the particles moved upward or downward, depending on their densities (see the ESI, Video†). The flow passed through the top and bottom outlets. The high-density particles, which were denser than the mobile phase, were collected from the lower outlet, while the low-density particles rose up in the separation-channel and were collected from the upper outlet (Fig. 1). The moving distances of these particles were estimated from Stokes' equation (eqn (1)).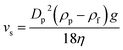 | (1) |
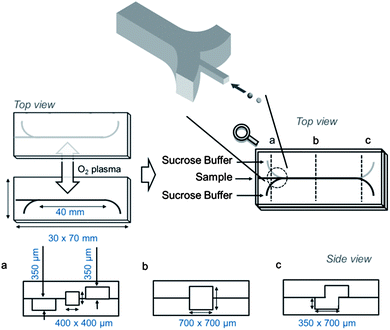 | ||
| Fig. 2 Schematic diagrams showing the design of the microfluidic particle separation device used in this study. | ||
Initially, we investigated the proper injection rate of the particles. The particle solutions of different concentrations were each loaded into the channel from the inlet at a constant flow rate of 10 μl min−1. When the particle concentration was below 1 × 104 particles ml−1, injection was smoothly achieved. At higher particle concentrations (>1 × 106 particles ml−1), clogging within the loading channel occurred. Therefore, precise control of the particle concentration is necessary to prevent clogging and achieve good separation.
Next, we evaluated the separation performance of the microfluidic device at a density resolution of 0.01 g ml−1. We expected the high-density (white) particles to move to the lower part of the microchannel, while the low-density (black) particles were expected to move to the upper part of the microchannel. Using Stokes' equation, the required minimum residence time to transport the particles along 400 μm was estimated at 29 s. We examined separation at a flow rate of 30 μl min−1. This flow rate was employed based on various considerations. The channel length and flow rate are influencing factors that determine the separation of particles under the specified environment. The flow rate should be tuned in order to achieve efficient separation. By employing different flow rates, we found out that the most efficient separation under a 40 mm channel length was at 30 μl min−1, given a transport distance of 400 μm and a calculated minimum residence time of 29 s based on Stokes' equation. Separation of particles was achieved by utilizing the difference in the sedimentation rates i.e., different lateral displacement along the transport distance as controlled by the residence time. At constant volume of the separation channel, the residence time will be shorter with a faster flow rate. However, the system characteristics described above achieved efficient particle separation. As shown in Fig. 4, the white particles moved to the bottom part, while the black particles moved to the upper part of the microchannel. The degree of separation was calculated from the amount of particles recovered at both outlet channels. At this flow rate, 90% degree of separation was achieved.
The relationship between residence time and separation rate was examined at different mobile phase flow rates (Table 1). The residence time was shorter with a faster flow rate. We varied the flow rates from lower values (20 and 30 μl min−1) to higher values (60, 90, and 150 μl min−1) to investigate their effects on the driving force for particle separation. The lower flow rates (20 and 30 μl min−1) yielded higher separation rates. Conversely, the degree of separation decreased when higher flow rates (60, 90, and 150 μl min−1) were applied. These results showed good correlation with calculations using Stokes' equation, which demonstrated that efficient separation requires residence times longer than 29 s (flow rate lower than 40 μl min−1). At higher flow rates, this residence time is not achieved and the degree of separation decreases. These results demonstrate the ability of our device to separate particles based on differences in density in accordance with Stokes' law.
| Flow rate [μl min−1] | Residence time [s] | Degree of separation [%] |
|---|---|---|
| 20 | 60 | 90 |
| 30 | 39 | 90 |
| 60 | 20 | 85 |
| 90 | 13 | 65 |
| 150 | 8 | 55 |
A number of researchers have demonstrated continuous particle separation methods in microfluidic devices. However, these methods require the application of an external force that may cause damage to cells or soft particles. Moreover, particles were separated purely based on size, as in the case of hydrodynamic particle separation. Huh et al. reported hydrodynamic separation amplification, by a method that combined field-driven separation with hydrodynamic-assisted separation to accomplish rapid size profiling and mass-dependent separation of particles.11 However, this particle separation method is limited to separation of particle populations with relatively large size differences and may fail to achieve separation in cases where two particles are of similar size and shape but different densities.
The novel particle separation device developed in our study was tested using model particles of similar sizes and mass. We demonstrated the separation of submillimeter-sized particles based on density differences, where different size particles exhibited different moving velocities. Theoretically, this microfluidic device may separate much smaller particles using longer residence times. However, in long devices featuring winding channels, Dean vortices at corners may affect separation.
We therefore consider our straight channel device to be appropriate, although for separation of smaller particles requiring longer residence time, this device may be impractical. A 100% separation using this technique is unattainable in cases of smaller particles requiring longer residence time, with such failure resulting from the natural convection within a straight channel. Further studies are required to overcome these problems and to optimize separation using our device.
For practical applications, particle separation techniques require separation based not only on differences in particle density but also on size and shape. In this experiment, we showed the separation of uniform-sized spherical particles. The technique did not use centrifugation or other outer forces. The sorting chip is expected to be capable of separating cells of similar size and shape having different densities, such as matured and immature oocytes or fertilized ovum, by exploiting the advantage of a microfluidic system through sorting with low hydrodynamic shear stresses.
Conclusions
We developed a novel particle separation technique based on density. This method used a simple microfluidic device that exposed the sample to minimal hydrodynamic shear stresses compared with conventional methods. The measured separation compared well with that expected from Stokes' equation. This separation technique provides a robust method for density-based separation of similar-sized particles and may be expected to allow separation of different cell populations.Experimental procedure
Reagents
Chemicals used in this study were of the highest purity available from Wako Pure Chemical Industries (Osaka, Japan) and used without further purification. The model particles, NIST traceable particle size standards, DUKE STANDARDS™ Microsphere (white particles) and CHROMOSPHERE-T™ (black particles) were purchased from Thermo Fisher Scientific Inc. (Waltham, MA, USA). The particle diameters were 140 and 150 μm, respectively, and the particle densities were adjusted to 1.055 g ml−1 (white particles) and 1.045 g ml−1 (black particles) by centrifugation. The density of a sucrose solution used as the mobile phase was adjusted to 1.05 g ml−1 by increasing the concentration of sucrose. Polyvinyl alcohol (PVA; final concentration: 0.1%) was added to the mobile phase to prevent agglomeration of the particles. The densities of the sucrose solutions were estimated by measuring the weight using a measuring flask.Fabrication of a microfluidic chip
The microfluidic devices were fabricated using a standard PDMS moulding technique as previously described.17,18 The upper and lower plates with channels were designed and assembled as shown in Fig. 2. In brief, the master polymethyl methacrylate (PMMA) plate was fabricated using micromachining techniques. The PDMS prepolymer was poured directly onto a PMMA master plate and cured at an elevated temperature (70 °C for approximately 3 h). The resulting PDMS replica was treated with oxygen plasma prior to assembly of the microfluidic chip. The inlet and outlet made of Teflon tubes were immobilized using PDMS.Separation of model particles
The experimental setup for particle separation is shown in Fig. 3. An inverted microscope was used to observe the separated particles. The solutions were loaded into the microfluidic chip by syringe pumping. The separated particles were recovered from the outlets of the upper and bottom parts of the channel, respectively. The degree of separation (%) was determined by counting the number of white and black particles observed at each outlet and dividing each particle by the original total number, and expressed as a percentage.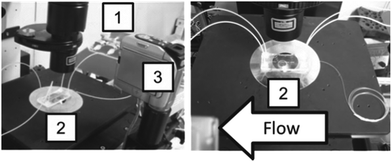 | ||
| Fig. 3 Experimental setup used in this study. Left: overall setup; right: magnified view around the microfluidic device. (1) Syringe pump; (2) microfluidic chip; and (3) CCD camera. | ||
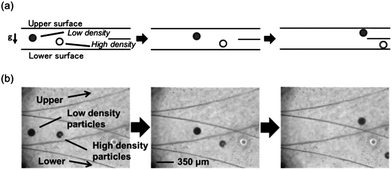 | ||
| Fig. 4 Separation of particles in a microfluidic device; (a) lateral schematic diagram of the particle alignment and separation and (b) photographs of separated model particles under the microscope. | ||
Notes and references
- T. M. Squires and S. R. Quake, Rev. Mod. Phys., 2005, 77, 977 CrossRef CAS.
- P. S. Dittrich and A. Manz, Nat. Rev. Drug Discovery, 2006, 5, 210 CrossRef CAS PubMed.
- Z. Wu, A. Q. Liu and K. Hjort, J. Micromech. Microeng., 2007, 17, 1992 CrossRef CAS.
- P. Sethu, A. Sin and M. Toner, Lab Chip, 2006, 6, 83 RSC.
- M. Yamada and M. Seki, Lab Chip, 2005, 5, 1233 RSC.
- S. S. Shevkoplyas, T. Yoshida, L. L. Munn and M. W. Bitensky, Anal. Chem., 2005, 77, 933 CrossRef CAS PubMed.
- J. Voldman, M. L. Gray, M. Toner and M. A. Schmidt, Anal. Chem., 2002, 74, 3984 CrossRef CAS.
- X. Hu, P. H. Bessette, J. Qian, C. D. Meinhart, P. S. Daugherty and H. T. Soh, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 15757 CrossRef CAS PubMed.
- W. A. Bonner, H. R. Hulett, R. G. Sweet and L. A. Herzenberg, Rev. Sci. Instrum., 1972, 43, 404 CrossRef CAS.
- T. Kawamata, M. Yamada, M. Yasuda and M. Seki, Electrophoresis, 2008, 29, 1423 CrossRef CAS PubMed.
- D. Huh, J. H. Bahng, Y. Ling, H. H. Wei, O. D. Kripfgans, J. B. Fowlkes, J. B. Grotberg and S. Takayama, Anal. Chem., 2007, 79, 1369 CrossRef CAS PubMed.
- D. D. Carlo, D. Irimia, R. G. Tompkins and M. Toner, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 18892 CrossRef PubMed.
- F. Petersson, L. Aberg, A. M. Sward-Nilsson and T. Laurell, Anal. Chem., 2007, 79, 5117 CrossRef CAS PubMed.
- I. Doh and Y. H. Cho, Sens. Actuators, A, 2005, 121, 59 CrossRef CAS PubMed.
- N. Pamme and C. Wilhelm, Lab Chip, 2006, 6, 974 RSC.
- T. Morijiri, S. Sunahiro, M. Senaha, M. Yamada and M. Seki, Microfluid. Nanofluid., 2011, 11, 105 CrossRef.
- J. D. Xu, L. Locascio, M. Gaitan and C. S. Lee, Anal. Chem., 2000, 72, 1930 CrossRef CAS.
- G. S. Fiorini and D. T. Chiu, BioTechniques, 2005, 38, 429 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Supplementary video. Microfluidic separation of particles based on density difference. Through the laminar flow in the separation channel, the high-density particles being denser than the mobile phase (sucrose) are harvested from the lower outlet while the low-density particles have the tendency to travel into the upper branch of the bifurcating channel. Sucrose is transported at a 20 μl min−1 flow rate. See DOI: 10.1039/c3ay40971f |
| ‡ Present address: Q-may Laboratory Co., 1116 Miyake, Taketa, Oita 878-0007, Japan. |
| § Present address: Division of Agrobiology, Graduate School of Agriculture, Hokkaido University, Sapporo, Hokkaido 060-0808, Japan. |
| This journal is © The Royal Society of Chemistry 2014 |