Surface engineering of synthetic polymer materials for tissue engineering and regenerative medicine applications
Hassan
Rashidi
,
Jing
Yang
and
Kevin M.
Shakesheff
*
Wolfson Centre for Stem Cells, Tissue Engineering and Modelling, Division of Drug Delivery and Tissue Engineering, School of Pharmacy, University of Nottingham, Nottingham, United Kingdom. E-mail: kevin.shakesheff@nottingham.ac.uk
First published on 7th August 2014
Abstract
When using polymer materials as scaffolds for tissue engineering or regenerative medicine applications the initial, and often lasting, interaction between cells and the material are via surfaces. Surface engineering is an important strategy in materials fabrication to control and tailor cell interactions whilst preserving desirable bulk materials properties. Surface engineering methods have been described that can strongly influence cell adhesion, migration, proliferation, differentiation and functionality. This review aims to categorise the current strategies for modifying surface chemistry and/or topography in terms of the resultant change in cell behaviour.
1. The requirement for surface engineering
A tissue-engineering scaffold serves as a template for tissue regeneration and plays an important role in cell adhesion, migration proliferation, and differentiation. Many synthetic biodegradable polymers have been developed to provide a 3D environment for cell growth and tissue formation. These include poly(α-hydroxy acids),1 poly(propylene fumarate),2 poly(orthoester),3 polycarbonate,4 polyurethanes,5,6 poly-3-hydroxybutyrate7 and polyphosphazenes.8 Some of the synthetic biodegradable polymers such as poly(α-hydroxyacids) including poly(lactic acid), poly(glycolic acid) and their copolymer poly(lactic acid-co-glycolic acid) have gained FDA approval for certain biomedical applications.When these synthetic materials are used in vitro or in vivo they will encounter a protein rich medium that conditions the material surface.9–12 The protein rich medium may be the cell culture medium added with cells for in vitro applications or the local interstitial fluid at the site of implantation for in vivo applications. The surface conditioning is driven by the thermodynamics of protein adsorption. The chemical properties of the material will strongly influence the final composition of the adsorbed protein layers and, in turn, this will influence the response of local cells and tissues. The choice of synthetic material to be used in a scaffold is driven by issues such as the processability of the polymer, the chemistry of bulk biodegradation, mechanical properties and logistical issues of cost, compatibility with sterilisation techniques and shelf life. These properties do not necessarily ensure that the surface properties of the final scaffold are optimal for protein and cell interaction.
Hence, there is often a requirement to change the surface properties of scaffold materials without changing bulk properties. Surface engineering strategies to enhance protein and cell interactions fall into 2 distinct categories; chemical and topographical modifications. These strategies are often inspired by the features of extracellular matrices (ECM). The contribution of nano-scale features of ECM to the modulation of cell-matrix signalling and cell behaviour is a well-known phenomenon. ECM consists of multiple proteins and polysaccharides, which act as a natural tissue scaffold. It has a crucial role in cell survival, proliferation and differentiation by providing spatial, biochemical and mechanical cues.13,14 ECM intrinsic factors are involved in the activation of intracellular signalling pathways through adhesion receptors, such as integrins.15–17 Moreover, it has been well documented that physical cues and topography of the ECM orchestrate cell behaviour through a phenomenon known as cellular guidance.18–20
Techniques and approaches to engineer the chemistry and topography of synthetic scaffolds have been reviewed by others previously.12,21–26 Therefore, in the next section we provide a concise summary of approaches and refer the reader to more detailed reviews and original papers for further information.
2. Summary of surface engineering techniques
2.1 Surface chemistry
Broadly the methodologies for changing surface chemistry can be divided into methods that (i) introduce new functional groups onto the scaffold polymer surfaces or (ii) coat the polymer with a thin layer of another polymer or other chemical species. Table 1 summarises example of these approaches.| Technique | Summary of approach | Surface chemistry modification | References |
|---|---|---|---|
| Introduce new functional groups onto scaffold polymer surface | |||
| Surface hydrolysis | Soaking of polymer scaffold in an acidic or alkaline solution. | Hydrolysed ester groups to form carboxylic acid and hydroxyl groups. | 55 |
| Oxygen plasma etching | Etch the substrate material with oxygen plasma to introduce functional groups. | Presentation of oxygen containing functional groups. | 110 |
| UV/ozone treatment | Oxidise the substrate material with UV/ozone at ambient conditions. | Presentation of oxygen and nitrogen containing functional groups. | 75 |
| Covalent grafting of peptides | Graft peptides onto scaffolding materials using coupling reactions such as reactions involving carbodiimide. If functional groups for coupling reaction are not present on the polymer, then it will need to be modified to introduce functional groups for subsequent grafting. | Presentation of grafted peptides. | 74,111 |
| Coating with thin layer of another polymer or chemical species | |||
| Plasma polymerisation | Polymerisation of monomer vapours under mild temperatures on the substrate scaffolding materials for retaining the functional groups of the monomers. | Presentation of the functional groups of the plasma polymers. | 60,112,113 |
| Physical adsorption | Immersing scaffolding materials in protein or peptide grafted polymer solutions. The adsorbed layer physically attaches to the scaffolding material. | Presentation of the physically adsorbed layer. | 56,114 |
| Surface entrapment | A region of the material close to and including the surface is swollen by a partial solvent. The surface modifying agent is dissolved in the partial solvent. When the solvent is removed the modifying agent is trapped at the surface. | Presentation of biomolecules or polymers containing cell adhesion peptides. | 57,115 |
| Layer by layer assembly | The substrate is first modified with a charged layer; another layer with opposite charge is then applied onto the first layer. Thicker coatings can be achieved by repeating the process. | Presentation of self-assembled bilayers. | 54,116 |
| In situ apatite formation | Apatite is formed on the scaffolding materials by soaking the scaffold in simulated body fluids. | Surface formed apatite. | 109,117 |
The choice of method is driven by the type of polymer used to form the scaffold, the scaffold structure and final surface chemistry required. Direct chemical modification of the surface requires the chemistry of the synthetic polymer to permit the modification (e.g. to undergo hydrolysis). Coating methods are applicable to different scaffolds depending on the surface charge, solvent interaction and surface energy. A final factor to consider is the scaffold structure. Small pore networks within the scaffold can restrict access to coating materials especially in plasma polymerisation.
2.2 Surface topography
The importance of surface topography on cell behaviour has been realised for nearly a century. According to Moore et al.27 topographical cues were used for the first time to culture embryonic frog cells on coverslips covered by spider web.28 Two decades later, Weiss et al. demonstrated the in vitro arrangement of aligned nerve fibres on rod-like fibrin.29 Recently, the utilisation of new nanofabrication technologies has enabled further advancement of the above mentioned pioneering studies in order to investigate the effect of micro- and nanotopography on cell behaviour and function.30–32 More recently, mathematical algorithms were used to fabricate chips of poly(lactic acid) with 2176 different random and nonbiased topographic features to study how parameters of the mathematical algorithm correlate with cellular responses.33 Such an approach can be applied to unravel complex and still incompletely understood interplay between cells and topographic features.Advances in micro- and nano-scale fabrication techniques have enabled incorporation of micro- to nano-scale topographic features onto various substrate surfaces. Recent developments have come from adaptation of techniques routinely used in the production of semiconductors by the electronics industry. Photolithography was the first among these technologies which was utilised to create topographic patterns of 5–100 micrometers for stem cell research.34 Increasing demand to create smaller length scale patterns led to the development of alternative methodologies such as electron beam lithography to create topographic features as small as 3–5 nm.35 Lithographically established patterns can be transferred into supporting substrate through an etch process. In order to fabricate samples directly to substrates for biological experiments, Nickel shims can be prepared from master samples to replicate patterns either by hot embossing or injection moulding.36
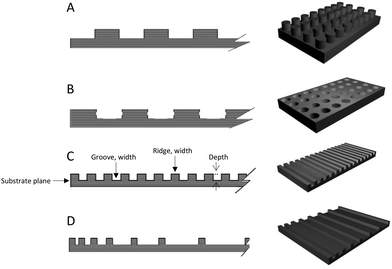
Nanotopographic geometries can be categorised into anisotropic topography like nanogrooved and aligned fibres or isotropic topography such as nanopillar/post and nanopit. In addition, nanofeatures can be created evenly through substrate or distributed unevenly (i.e. varying groove width) to create topographic gradients (Fig. 1 and 2).
 | ||
| Fig. 2 Scanning electron microscopy of nanopits with hexagonal, square, near square order with a random displacement of ±50 nm and random arrangements, respectively. Reprinted by permission from Macmillan Publishers Ltd: Nature Materials.32 | ||
2.3 Combined modification of topography and chemistry
Besides the conventional approach of varying an individual surface property (chemical or physical), combinatorial approaches have also been used to investigate the combined effect of both chemical and topographical properties of substrates.37–42 Furthermore, the effect of orthogonal-gradients of chemical and physical modifications was studied to elucidate the combinatorial effects of both attributes on cell behaviour.43 Although in most cases a sequential strategy have been taken to impose both modifications onto surface, more recently new methods has been developed to impose both modifications in one step such as reactive imprint lithography (RIL).44 In RIL, deprotection reaction of tert-butyl ester groups during imprinting at elevated temperature is exploited to obtain activated and structured surface over large area which can be functionalised with established methods of surface chemistry. RIL has advantages over previously introduced methodology using a strong organic acids for wet-chemical deprotection of the tert-butyl ester groups, introduced by Embrechts and colleagues.45 In addition, lower levels of intramolecular anhydride formed during manufacturing of modified surfaces using RIL compare to thermolysis of free surfaces.443. Functional outcomes of surface engineering
Ultimately the wide range of surface engineering strategies highlighted in section 2 has been developed to influence cell behaviour on contact with the scaffold. In this section we have defined 4 desirable cell behaviours and review the diverse range of surface engineering approaches that have targeted each behaviour type.3.1 Cell polarity, adhesion and migration
One of the most pronounced effects of topography is on cell polarity. The importance of cell polarity and geometry on cell function is well documented.30,46 Cell polarity is critical in organ development and loss of cell polarity has been associated with pathological conditions, such as cancer metastasis.47,48 A wide range of cell types respond to nanotopographical cues by elongating and aligning parallel to nanogrooves. Morphological responses of cells to nanopit and nanopost features are subtle and vary from reduction of spreading to constant or increased filopodia formation. Polarity of cultured cells is significantly influenced by the variety of topographical features. On denser and narrower grooves the polarity of cultured cells increased, whereas in wider grooves cells were not as polarized.49,50The alteration of surface topography also influences adherence and migration of cells. In general, nanopits and nanoposts reduce cell adhesion, while enhanced adhesion can be achieved on nanograting substrates. In contrast to random trajectories of migration on flat surfaces, increased overall migration velocities are observed in the direction of the grating axis on nanogratings, while little information is available regarding cell migration on nanoposts and nanopillars (Table 2). Despite extensive knowledge regarding fundamental mechanisms of cell migration, little is known about molecular mechanism by which directional migration is promoted by local 3D architectures.51 Although recent advances suggest decreased mechanical forces through down-regulation and high turnover of a focal adhesion protein known as zyxin as the underlying mechanism of focal adhesion remodelling in human mesenchymal stem cells (hMSCs) cultured on 350 nm gratings,52 further studies must be aimed to fully elucidate mechanisms by which local 3D topography encourages directional cell migration. In addition, further studies are required to further examine the influence of feature geometry and size on adhesion.
| Cell type | Substrate material | Physical modification | Functional outcome | References |
|---|---|---|---|---|
| PDMS, poly(dimethylsiloxane); Si, silicon; PUA, poly(urethane acrylate). | ||||
| bPASMCs | PDMS | Microposts, 2–10 μm in diameter, 3–50 μm in height | Cells adhesion, spread across and deflected multiple posts | 31 |
| hCECs | Si | Nanogrooves, 70 nm width, 600 nm depth | Elongation and alignment along micrometer- and nanometer-sized grooves and ridges | 118,119 |
| hECs | PDMS | Nanogrooves, 1200 nm width, 600 nm depth | Elongation along ridges, formation of well-defined capillary tubes following induction by Matrigel | 93 |
| NIH 3T3 fibroblast | PUA | Gradient Microgrooves, 1–9.1 μm | Enhanced adherence to denser features; alignment, elongation and bias migration along the direction of ridges | 50 |
Similarly, cell adhesion and spreading vary following the introduction of different chemical groups or combinations of physical and chemical modifications onto substrates (Tables 3 and 4). Using model surfaces of self-assembled monolayers of silanes, attachment of human fibroblasts was found to be highest on amine and carboxylic acid terminated surfaces. Cell spreading was also highest on these two surfaces. In comparison, –CH3, –PEG and –OH terminated surfaces showed much lower cell attachment and spreading. The amount of protein adsorbed to the –CH3, amine and carboxylic acid presenting surfaces were similar despite the significantly lower amount of cell attachment and spreading on –CH3 surfaces.53 In another study, the attachment of rabbit ear chondrocytes on poly(L-lactide) acid (PLLA) scaffold was significantly improved after aminolysis in 1,6-hexanediamine solution, and was furthered improved by coating with a three bilayer of chondroitin sulphate/collagen I.54 The aminolysis requires the immersion of PLLA scaffolds in 1,6-hexanediamine at elevated temperature, which can damage the bulk property of the scaffold. Adhesion of smooth muscle cells was also found to be increased on hydrolysed PGA mesh compared to un-treated mesh.55 However, the degradation of PGA in sodium hydroxide solution during the hydrolysis process was significant, with a fibre diameter loss of 50% in approximately 6 minutes. Physical adsorption of fibronectin also increased cell adhesion of osteoblasts on PLGA porous scaffolds. A concentration of 200 nM of fibronectin with an incubation time of 2 hours was found to be effective to promote cell adhesion.56 This method is a simple one-step procedure; however, the stability of the adsorbed protein layer is a concern. The coverage and conformation of the adsorbed protein layer may also depend on the chemistry, surface energy and surface charge of the substrate. The attachment of MC3T3-E1 osteoblasts was found to be increased on the gelatin entrapped surface.57 Gelatin particles were used as sacrificial materials to generate porous PLLA scaffolds. During the dissolution and leaching out stage of the gelatin particles within the PLLA scaffolds, some of the gelatin molecules are trapped within the PLLA material, as verified by ATR-FTIR and X-ray Photoelectron Spectroscopy. The entrapment requires the selection of a right solvent that can dissolve the molecules that promote cell adhesion and swells the substrate material. This requirement can hinder the application of this method to different adhesion-promoting molecules and scaffolding materials. Surface chemistry has also been reported to affect the polarity of epithelial cells. Laminin-111 coated PLGA nanofibre scaffolds have been found to promote mature tight junctions.58
| Cell type | Substrate material | Chemical modification | Functional outcome | References |
|---|---|---|---|---|
| AHDCS, adult human-derived corneal stromal cells; bVSMCs, bovine vascular smooth muscle cells; HBC, hydroxybutyl chitosan; hCECs, human corneal epithelial cells; hECs, human endothelial cells; PAA, poly(acrylamide); PMMA, poly(methyl methacrylate); ppAAm, plasma polymerised allylamine; TCPS, tissue culture poly(styrene). | ||||
| bVSMCs | PGA | Surface hydrolysis, hydrolyse ester groups and form carboxylic acid and hydroxyl groups | Increased cell adhesion and seeding density | 55 |
| Rat osteosarcoma cell line | TCPS | Plasma copolymerisation of acrylic acid and 1,7 octadiene | Improved cell adhesion to plasma copolymer surface | 112 |
| Human keratinocytes | TCPS | Plasma copolymerisation of acrylic acid/1,7 octadiene and allyl amine/1,7 octadiene | Improved adhesion of keratinocytes on acrylic acid/1,7 octadiene with low concentration of carboxylic acid groups in similar level to collagen-I | 60 |
| Human osteoprogenitor | PDLLA & PLGA | Physical adsorption of RGD–PLL or fibronectin to PDLLA substrate | Enhanced adhesion and spreading following both modifications, successful osteogenic differentiation into mature osteogenic phenotype | 56 |
| Bovine aortic endothelial cells | PDLLA | Adsorption of PLL–GRGDS | Increased in spreading, inhibition of spreading at high concentration of PLL–GRGDS | 114 |
| Human fibroblast | Glass or silicon | Self-assembled monolayers, Silanisation of glass or silicon surfaces with silanes terminated with CH3, Br, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 or PEG. CH2 or PEG. |
Strong adhesion, spreading, fibronectin formation and growth and enhanced activity of integrins on –COOH and –NH2 terminated surface, weak interaction with –CH3, –PEG and –OH | 53 |
| MC3T3-E1 osteoblasts | PLA | Surface entrapment of gelatin | Increased hydrophilicity following gelatine entrapment, Significant enhancement of cell adhesion and proliferation | 57 |
| 3T3 fibroblast | Glass | Plasma polymerisation of allyl amine and hexane | Increased cell density in the core of millimetre-scale size scaffolds | 113 |
| NIH 3T3 fibroblasts, human megakaryocytic M07e | Glass, gold, titanium oxide, various polymers such as PTFE and PS | Covalent bond to inorganic oxides and amine containing organic surfaces. | Water contact angle change to similar values after coating various substrates with dopamine, Significant attachment of fibroblasts after coating various substrate with dopamine and PEG–SH | 120 |
| SaOS-2 osteosarcoma cell line | Gelatin/bioglass composite | In situ apatite formation | Enhanced attachment and secretion of ECM | 117 |
| Cell type | Substrate material | Chemical modification | Physical modification | Functional outcome | References |
|---|---|---|---|---|---|
| PLL: poly-L-lysine; PHBV: Poly(3-hydroxybutyrate-co-3-hydroxyvalerate); PET: poly(ethyleneterephthalate); rHPN: rat hippocampal pyramid neurons. | |||||
| Rat hippocampal neurons | Glass | Adsorption of PLL | Microgrooves, 20–40 & 50–100 μm width, 5 μm depth | Effective guidance of neuritis outgrowth and number | 37 |
| MC3T3-E1 S14 Osteoblasts | PHBV | Adsorption of fibronectin or immobilisation of alkaline phosphatase | Microgrooves, 1–10 μm width, 10–30 μm depth & micropits, 4 μm width, 5 μm depth | Improved cell adhesion and alignment | 38 |
| Human keratinocyte | PET | Plasma deposition of acrylic acid (cell adhesive) and poly(ethylene oxide (cell repulsive) | Conical nanoposts, 117 ± 5 nm height | Improved cell adhesion | 39 |
| Human umbilical vein endothelial cells | PET | Plasma deposition of acetaldehyde | Aligned fibres, 100 μm diameter | promotion of cell attachment and spreading, formation of focal adhesion | 41 |
| rHPN | Glass | Adsorption of PLL | Microgrooved pattern, 2 & 15 μm width, 1 μm depth | promotion of specifically polarized morphology by guidance cue pattern | 42 |
Plasma polymers with carboxylic acid and nitrogen containing functionalities are found to increase the adhesion of keratinocytes.59,60 These plasma polymerised polymers are formed on the top of substrates under mild temperatures which do not destroy the functionalities of the monomers. The thickness of the plasma polymer layers can be controlled within the nanometer range by varying parameters such as deposition time. The diffusion of plasma polymers into pores is dependent on the pore size,61 which limits the application of this technique to scaffolds with large pores and relatively small construct sizes.
Covalently grafting of proteins and peptides offers advantages over physical adsorption in terms of improving the stability of the modification. Chondrocytes from cartilage tissue of rabbit ears showed higher adhesion after 24 hours culture on PLLA with covalently tethered collagen compared to bare PLLA.62 To graft collagen to PLLA, hydroperoxide groups were first introduced onto the PLLA surface by treating the material with UV and hydrogen peroxide. Carboxyl groups were then introduced onto the PLLA surface by grafting methacrylic acids to form PLLA-g-PMAA which was later activated by 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide for subsequent conjugation to collagen. This method requires the treatment of scaffolding materials in harsh chemicals and processing conditions which can damage the bulk properties and requires a multiple-step procedure including extensive washing to remove chemical residues. A similar procedure was applied to covalently graft collagen to PCL.63
Surface chemistry has also been employed to pattern surfaces with two distinct chemistries for the co-culture of hepatocytes and NIH 3T3-J2 cells. In this study, borosilicate wafers were first coated with a layer of photoresist, patterns were then applied to the substrate using UV through a mask, and the photoresist on these patterned areas was removed for subsequent covalent tether of collagen. The remaining photoresist was then removed in acetone by sonication. Hepatocytes selectively adhered to the collagen coated areas and NIH 3T3-J2 cells attached to the rest of borosilicate surfaces.64 A simpler method of patterning surfaces has been achieved by inkjet printing of collagen solutions onto (2-[methoxy(polyethyleneoxy)propyl]trichlorosilane treated glass slides. The collagen-free parts of the surfaces were then coated with poly(L-lysine) (PLL). Hepatocytes were first seeded, and adhered only to the collagen coated surfaces. Fibroblasts were then allowed to adhere to the PLL coated regions.65 More recently, a novel micropatterning technique introduced using combination of aerosol deposition (airbrushing) of ECM proteins through microstencil and plasma polymerisation in order to create complex patterns of hydrophilic regions on poly(dimethylsiloxane) (PDMS) surface.66
It is important to note that some surface modifications can change the bulk properties, e.g. biodegradation, of the biodegradable polymers. For example, surface modification using wet-chemical processes can lead to a faster degradation rate and reduction of mechanical performance.67 Ozone oxidation, UV- and γ-radiation also lead to degradation of biodegradable polymers.68–70 In contrast, plasma-assisted surface modification offers a method to incorporate functional groups on biodegradable polymers without altering the bulk properties.67
3.2 Proliferation
Nanotopography has been shown to affect other cellular functions including self-renewal and proliferation (Table 5). Several studies have been conducted to evaluate the effect of nanotopography on proliferation of various cell types including mouse embryonic stem cells (mESC),71 human embryonic stem cells (hESC)72 and endothelial cells.73 Enhancement of self-renewal and proliferation was observed in mouse embryonic stem cells cultured on a nanofibrillar scaffold in comparison with a tissue culture plastic surface in the presence of leukemia inhibitory factor. Enhanced expression of Nanog and activation of small GTPase RAC and phosphoinositide 3-kinase were suggested as molecular mechanisms for higher self-renewal of mESC cultured on nanofibrillar scaffold in comparison to flat surface.71 In another study, a significant enhancement of proliferation was observed in surfaces with optimal groove width and wettability following combinatorial modifications of surfaces.43| Cell type | Material | Chemical modification | Physical modification | Functional outcome | References |
|---|---|---|---|---|---|
| bFGF, basic fibroblast growth factor; hiPSCs, human induced pluripotency stem cells; hMECs, human microvascular endothelial cells; HUVEC, human umbilical vein endothelial cells; mESCs, mouse embryonic stem cells; PAS, peptide-acrylate surfaces; PCL, poly(caprolactone); PES, poly(ethersulfone); PET, poly(ethyleneterephthalate); PMMA, poly(methyl methacrylate; ppAAm, plasma polymerised allylamine; PS, spin-cast thin polystyrene; rNSC, rat neural stem cells; TCPS, tissue culture poly(styrene). | |||||
| mESCs | Polyamide | — | Randomly oriented nanofibres, 280 nm average diameter | Promotion of proliferation and self-renewal of mESCs through Rac, PI3 K/AKT signalling | 71 |
| hMSCs | Glass | Self-assembled monolayers, Silanisation of the surface. | — | The –CH3 surfaces maintained the hMSC phenotype. The –NH2 and –SH-modified surfaces promoted and maintained osteogenesis both in the presence and absence of biological stimuli. The –OH and –COOH-modified surfaces promoted and maintained chondrogenesis under both basal and chondrogenic stimulated conditions. | 105 |
| 3T3 dermal fibroblast | PMMA | Plasma deposition (gradient of ppAAm) | Gradient of microgrooves, 5–95 μm width, 3 μm depth | Significant increase in cell proliferation in area with optimal groove width and wettability | 43 |
| rNSC | PES | — | Nanofibres, 273 ± 45 to 1452 ± 312 nm diameter | Lower proliferation compared to cells cultured on laminin-coated 2D surface in the presence of bFGF, lower degree of cell aggregation and higher degree of proliferation and cell spreading as the fibre diameter decreased | 86 |
| hESCs | PAS | Covalent conjugation | — | Supporting self-renewal in chemically-defined, xeno-free medium comparable to that on Matrigel™, retain of normal karyotype | 74 |
| hESCs | PS | Oxygen plasma etching | — | Maintenance of self-renewal and stable karyotype comparable to that on Matrigel™, multi-germ layer in vitro differentiation | 76 |
| hESCs & hiPSCs | PS | UV/ozone treatment | — | Maintenance of self-renewal at optimised UV dose comparable to that on Matrigel™ | 75 |
| hMECs | PET | — | Ripples & Walls, 300 nm & 1.5 μm | Induced proliferation as a result of nuclear accumulation of β-catenin | 73 |
| HUVECs | PCL | Aminolysis and covalent grafting of collagen | — | Significantly improved cell adhesion and proliferation | 63 |
| hESCs | Polystyrene | — | Nanopillars, 50–400 nm height | Maintenance of Oct4 expression in absence of bFGF, downregulation of Oct4 in presence of bFGF in honeycomb configuration | 72 |
In a study by Schernthaner and colleagues, nuclear accumulation of β-catenin and activation of specific β-catenin target genes was suggested as the mechanism responsible for higher rates of proliferation in endothelial cells (ECs) cultured on a polymer culture substrate with laser-generated nanopatterns.73
It has been shown that chemically modified polyacrylates and polystyrene surfaces can support stem cell self-renewal. Self-renewal of embryonic stem cells in defined medium was found to be similar on carboxylic acid containing acrylate surfaces tethered with peptides from vitronectin and bone sialoprotein and on Matrigel.74 Both oxygen plasma and UV/ozone treatment were found to be able to modify a polystyrene surface to support self-renewal of human embryonic stem cells.75,76
High throughput methods have been developed to screen large numbers of different materials and their surface chemistries.77,78 Some polyacrylates after coating with serum have been identified to support self-renewal of human embryonic stem cells.78 Certain integrins binding proteins, such as vitronectin, have been found to play an important role in controlling stem cell fate.
3.3. Differentiation
Nanotopography can be utilized to promote the differentiation of cells into various lineages (summarised in Table 6) as demonstrated in case of ESCs79 and hMSCs.32,80| Cell type | Material | Physical modification | Functional outcome | References |
|---|---|---|---|---|
| HBC, hydroxybutyl chitosan. | ||||
| hMSCs & osteoprogenitors | PMMA | Nanopits, 120 nm diameter, 100 nm depth | Stimulation of hMSC osteogenic differentiation on disordered nanoscale features in absence of osteogenic supplements | 32 |
| hMSCs | HBC & HBC/collagen | Aligned nanofibres, 200–900 nm average diameter | Enhanced alignment, upregulation of myogenic gene markers | 85 |
| hMSCs | PDMS | Nano- and microgratings, 350 nm, 1 or 10 μm width, 350 nm depth | Significant up-regulation of neuronal markers compared to micropatterned and unpatterned, further enhancement of differentiation in presence of biochemical cues such as retinoic acid | 80 |
| mESC | PCL | Aligned nanofibres, 250 nm average diameter | Neural differentiation of mESCs seeded directly onto PCL nanofibres, minimal astrocytic differentiation. | 121 |
| hMSCs | PUA | Nanoposts, 150, 400 & 600 nm diameter, Nanogratings, 150, 400 & 600 width | Higher ALP activity and higher expression of osteogenic markers in cells cultured on patterned surface compared to unpatterned PUA in presence of osteogenic medium | 83 |
| hESCs | PDMS | Nanopillars, 35–400 nm diameter | Enhanced neuronal yield by increasing pillar height from 25–400 nm, ∼80% neuronal differentiation on higher pillar height in first 96 h in absence of biochemical factors | 87 |
| Human primary osteoblast | PCL | Micropits, 300 nm depth, 20, 30 & 40 μm diameter | Osteogenic differentiation with most pronounce effect in 30 μm pits | 122 |
| hMSCs | PDMS | Nanogratings, 250 nm depth, 250 nm width | Up-regulation of neurogenic and myogenic differentiation markers in hMSCs cultured on nanograting compared to microgratings and unpatterned | 92 |
| hESCs | PCL | Nanopits, 100 nm depth, 120 nm diameter | Enhanced mesodermal differentiation in comparison with planar surface | 79 |
Nanotopographical features have been used to direct hMSCs differentiation particularly into an osteogenic lineage.32,81–83 Induction of osteogenic differentiation has been achieved by long-term culture of hMSCs on varying degree of disordered nanopits made from poly(methylmethylacrylate) (PMMA) in the absence of inducing signals, suggesting physical nanostructures might be sufficient to induce differentiation;32 However, enhanced osteogenic differentiation of hMSCs was achieved following induction of cultured hMSCs on surfaces with nanofeatures in combination with osteogenic medium suggesting optimal conditions can be achieved by using combinations of both physical cues and soluble factors.83 Work by Zouani et al. suggests that not only the width but also the depth of nanopatterns is important to elicit differentiation.84 These studies demonstrated that surface topography can be used to directly bias cell fate decisions.
Nanotopographic induction of differentiation towards other fates has also been investigated such as myogenic85 and neurogenic differentiation.80,86,87 Higher differentiation tendency toward neural lineages have been demonstrated in embryonic or adult stem cells cultured on engineered surfaces in various studies. One such example is the differentiation of hMSCs into neuronal-like cells following culture on nanograting of 350 nm width.80 Similar to You and colleagues’ observation,83 further enhancement of differentiation was achieved by the synergistic effect of both nanotopography and biochemical cues such as retinoic acid.80 In addition, enhanced neuronal differentiation have been reported in neuronal progenitors when co-cultured with astrocytes on micropatterns larger than 10 μm88,89 and electrospun polyamide nanofibers90 due to synergistic effects of soluble factors released by astrocytes. Although the molecular mechanism driving differentiation by nanotopography is largely unknown, recent studies suggested involvement of integrin-activated focal adhesion kinase.91,92
Nanotopographic features have also been investigated to encourage formation of multicellular structures with enhanced functionality. The importance of nanoscale cues in directing organization and function has been shown in human endothelial progenitor cells (hEPCs) cultured on both planar and nanograting substrates where hEPCs cultured on nanograting substrates formed multicellular band structures while cells cultured on planar substrate formed confluent monolayers.93 In a more recent study, it was demonstrated that endothelial cells (ECs) were aligned on both nanofibrillar and micropatterned channels, down-regulated adhesion proteins and chemokines and reduced adhesiveness to monocytes and platelets.94 Using a combination of electron beam and photolithography, a 3 layer tubular scaffold with a coaxial arrangement were fabricated which can be used as a vascular tissue engineering scaffold.95
Since the contractile property of cardiac tissue is directly related to cellular elongation and orientation, formation of anisotropic myocardium has also been investigated through various topographic features. In a pioneering study, faster propagation of action potential was observed in neonatal rat ventricular myocytes cultured on abraded microchannels on a poly(vinyl)chloride substrate.96 In another study, the effect of microtopography on intracellular calcium dynamics was investigated on cardiomyocytes cultured on a PDMS substrate with trapezoidal grooves with a depth of 50 μm and 120 μm spacing between adjacent triangular ridges.97 Increased diastolic and systolic intracellular calcium following electrical stimulation at higher and all stimulation frequencies, respectively, demonstrated that microstructure can directly influence cardiomyocytes intracellular calcium dynamics. To further investigate the influence of topography on functionality of cardiomyocytes, contractile forces generated by cardiomyocytes cultured on flat and a 10 μm-wide microcantilevers was measured in another study.98 Cells cultured on microcantilevers showed anisotropic actin organisation and had 65–85% higher contractile forces compared with flat surfaces. In addition, it was shown that expression of junctional markers such as N-cadherin and connexion-43 upregulated in presence of certain arrangements of micropillars, further suggesting enhancement of cardiomyocytes functionality by surface topography.99
Neural tissue engineering is another area, where topographical guidance has been utilised to further enhance functionality. Several approaches have been taken to fabricate conduits containing microchannels using various materials including PLGA and PCL.100,101 Moore et al. fabricated PLGA conduits with distinct channels running parallel along the length of the scaffold using injection moulding with rapid solvent evaporation.102 Krych et al. demonstrated that greater regeneration can be achieved following implantation of Schwann cell-seeded PLGA conduits with 450 μm over 600 μm diameter microchannels.103 Rutkowski and colleagues also demonstrated that improved functionality can be achieved following the introduction of grooves within lumens with a width of 10 μm, depth of 4.3 μm and spacing of 10 μm in rats with 1 cm sciatic nerve transections.104
It has been demonstrated that differentiation potential can be altered following chemical modification of surfaces (Table 7). The differentiation of bone marrow derived mesenchymal stem cells was studied on a range of silane modified surfaces. –CH3 surfaces maintained the MSC phenotype. –NH2 and –SH modified surfaces promoted and maintained osteogenesis both in the presence and absence of biological stimuli.105 Human mesenchymal stem cells were cultured on polystyrene surfaces modified with photoreactive azidophenyl derivatives of three different chargeable polymers: poly(acrylic acid), polyallylamine and poly(ethylene glycol). The polyallylamine surface supported cell adhesion and proliferation and also promoted chondrogenic differentiation.106 The synthesis of sulphated glycosaminoglycan from bovine chondrocytes have been found to be highest on cationic PLGA microcarriers coated with PLGA-g-poly(L-lysine) graft copolymers compared with on hydrophobic and negatively charged PLGA, respectively.107 In addition, functional groups have been tethered to induce differentiation of human mesenchymal stem cells. Primary amine, t-butyl, phosphate, tetrafluorobutyl and methacrylic acid functional groups containing methacrylates were incorporated into poly(ethylene glycol)dimethacrylate at sufficiently low concentration to study the effect of these small molecules on the differentiation of mesenchymal stem cells. Protein and gene expression altered by these molecules were analysed for stem cells cultured in the gels.108
| Cell type | Material | Chemical modification | Functional outcome | References |
|---|---|---|---|---|
| PAAc, poly(acrylic acid); PAAm, poly(allylamine); PEG, poly(ethylene glycol). | ||||
| Myoblasts | Alginate | Covalent coupling | Adherence, proliferation, fusion and expression of heavy-chain myosin (a differentiation marker) following GRGDY modification of alginate surface | 123 |
| Human osteoprogenitor | PDLLA & PLGA | Physical adsorption of RGD-poly(L-lysine) or fibronectin to PDLLA substrate | Enhanced adhesion and spreading following both modifications, successful osteogenic differentiation into mature osteogenic phenotype | 56 |
| hECs | PLLA | Layer by layer assembly | Increased adhesion, proliferation and secretion of von Willebrand factor | 116 |
| hMSCs | Glass | Surface entrapment | –NH2 and –SH-modified surfaces promoted and maintained osteogenesis, chondrogenic differentiation on –NH2-modified surface in presence of chondrogenic medium but not on –SH-modified surface, control and –CH3-modified surface maintained MSC phenotype but lack differentiation stimuli | 105 |
| Rabbit ear chondrocytes | PLLA | Layer by layer assembly of chondroitin sulphate and collagen type-I onto PLLA | Improved cell attachment, proliferation, cytoviability and GAG secretion following introduction of chondroitin sulphate and collagen type I onto PLLA | 54 |
| hMSCs | PAAc, PAAm & PEG | Physical coating | Negatively charged surface supported adhesion and proliferation while positively charged PAAm supported cell adhesion, proliferation and differentiation, enhanced chondrogenic differentiation on PEG and PAAm-modified surface | 106 |
Table 8 summarises reports in which topography and chemistry were both controlled with the aim of controlling cell differentiation. For example Lie et al. demonstrated that not only adhesion of MC3T3-E1 osteoblasts was enhanced on nano-fibrous gelatin scaffolds with in situ formed apatite, enhanced proliferation and differentiation were also observed following incorporation of apatite compared with nano-fibrous gelatine alone.109
| Cell type | Material | Chemical modification | Physical modification | Functional outcome | References |
|---|---|---|---|---|---|
| MC3T3-E1 osteoblasts | Gelatine | in situ apatite formation | Nanofibre | Enhanced cell adhesion and proliferation, higher mechanical strength and enhanced osteoblastic differentiation following incorporation of apatite | 109 |
| PC12 | PAA | Adsorption of BSA | Microwells (10 μm in diameter) connected by 1 μm microchannels | Selective attachment, growth and differentiation, control over number of neuritis outgrowth | 40 |
| hMSCs | PET | Covalent immobilisation of –RGD peptide | Nanopits, 10–100 nm depth | Promotion of adhesion without noticeable differentiation on 10 nm, induced differentiation into osteoblast-like cells on 100 nm features | 84 |
4. Prospective
This review has attempted to consider the role of surface engineering from the perspective of the functional outcomes in terms of changes in cell response. It is apparent that cell polarity, adhesion, proliferation and differentiation can be influenced by a wide range of surface properties. The breadth of available surface engineering techniques should be beneficial for clinical translation because these cell responses can be achieved on virtually any bulk material and for many tissue types.In summarising the current literature it is apparent that there is a shortage of studies on the combined effects of chemistry and topography. In the body, most cells interact with surfaces arranged within a 3D architecture. The relationship between multiple surface-to-cell interactions and 3D space are essential in determining tissue patterning, repair or regeneration. The combination of topographical and chemical changes opens up a huge design space for new surfaces. This, in turn, requires high throughput and perhaps combinatorial mechanisms of creating surfaces and analysing their interactions with cells.
More mechanistic studies are required to shed light on the response of cells to surface properties. In most studies we measure single (or a small selection) of desirable functional outcomes. These outcomes can be achieved via multiple cellular pathways and understanding, and possibly controlling, these pathways through combined chemistry and topography will more closely mimic the role of the ECM.
Surface engineering methods should be attractive for clinical translation of synthetic scaffolds. The technologies reviewed here are generally inexpensive to use and from a regulatory perspective are scalable and easy to quantity control. Separating bulk and surface properties should allow enhancements in cell and tissue interaction without the need to redesign bulk properties.
Acknowledgements
The authors are grateful to Dr Nikolaj Gadegaard for providing figures and Hadi Marashinia for assistance with the illustrations. KMS acknowledges funding from BBSRC Biotechnology and Biological Sciences Research Council (BB/G010579/1). KMS has received funding from the European Research Council under the European Community's Seventh Framework Programme (FP7/2007-2013)/ERC grant agreement 227845.References
- R. K. Kulkarni, et al., Polylactic acid for surgical implants, Arch. Surg., 1966, 93(5), 839–843 CrossRef CAS.
- J. P. Fisher, et al., Soft and hard tissue response to photocrosslinked poly(propylene fumarate) scaffolds in a rabbit model, J. Biomed. Mater. Res., 2002, 59(3), 547–556 CrossRef CAS PubMed.
- J. Heller, et al., Poly(ortho esters): synthesis, characterization, properties and uses, Adv. Drug Delivery Rev., 2002, 54(7), 1015–1039 CrossRef CAS.
- S. J. Lee, et al., Response of MG63 osteoblast-like cells onto polycarbonate membrane surfaces with different micropore sizes, Biomaterials, 2004, 25(19), 4699–4707 CrossRef CAS PubMed.
- J. P. Santerre, et al., Understanding the biodegradation of polyurethanes: From classical implants to tissue engineering materials, Biomaterials, 2005, 26(35), 7457–7470 CrossRef CAS PubMed.
- J. J. Guan, et al., Preparation and characterization of highly porous, biodegradable polyurethane scaffolds for soft tissue applications, Biomaterials, 2005, 26(18), 3961–3971 CrossRef CAS PubMed.
- G. Q. Chen and Q. Wu, The application of polyhydroxyalkanoates as tissue engineering materials, Biomaterials, 2005, 26(33), 6565–6578 CrossRef CAS PubMed.
- M. T. Conconi, et al., In vitro evaluation of poly bis(ethyl alanato)phosphazene as a scaffold for bone tissue engineering, Tissue Eng., 2006, 12(4), 811–819 CrossRef CAS PubMed.
- L. Vroman, et al., Interaction of high molecular-weight kininogen, factor-XII, and fibrinogen in plasma at interfaces, Blood, 1980, 55(1), 156–159 CAS.
- S. H. Lee and E. Ruckenstein, adsorption of proteins onto polymeric surfaces of different hydrophilicities – a case-study with bovine serum-albumin, J. Colloid Interface Sci., 1988, 125(2), 365–379 CrossRef CAS.
- S. M. Slack and T. A. Horbett, Changes in fibrinogen adsorbed to segmented polyurethanes and hydroxyethylmethacrylate-ethylmethacrylate copolymers, J. Biomed. Mater. Res., 1992, 26(12), 1633–1649 CrossRef CAS PubMed.
- A. de Mel, B. G. Cousins and A. M. Seifalian, Surface modification of biomaterials: a quest for blood compatibility, Int. J. Biomater., 2012, 2012, 707863 Search PubMed.
- R. O. Hynes, The Extracellular Matrix: Not Just Pretty Fibrils, Science, 2009, 326(5957), 1216–1219 CrossRef CAS PubMed.
- M. M. Stevens and J. H. George, Exploring and engineering the cell surface interface, Science, 2005, 310(5751), 1135–1138 CrossRef CAS PubMed.
- R. O. Hynes, Integrins: Bidirectional, allosteric signaling machines, Cell, 2002, 110(6), 673–687 CrossRef CAS.
- A. L. Berrier and K. M. Yamada, Cell-matrix adhesion, J. Cell Physiol., 2007, 213(3), 565–573 CrossRef CAS PubMed.
- K. R. Legate, S. A. Wickstrom and R. Fassler, Genetic and cell biological analysis of integrin outside-in signaling, Genes Dev., 2009, 23(4), 397–418 CrossRef CAS PubMed.
- P. Clark, et al., cell guidance by ultrafine topography invitro, J. Cell Sci., 1991, 99, 73–77 Search PubMed.
- R. B. Dickinson, S. Guido and R. T. Tranquillo, Biased cell-migration of fibroblasts exhibiting contact guidance in oriented collagen gels, Ann. Biomed. Eng., 1994, 22(4), 342–356 CrossRef CAS.
- R. J. Pelham and Y. L. Wang, Cell locomotion and focal adhesions are regulated by substrate flexibility, Proc. Natl. Acad. Sci. U. S. A., 1997, 94(25), 13661–13665 CrossRef CAS.
- C. M. Chan, T. M. Ko and H. Hiraoka, Polymer surface modification by plasmas and photons, Surf. Sci. Rep., 1996, 24(1–2), 3–54 Search PubMed.
- Z. W. Ma, Z. W. Mao and C. Y. Gao, Surface modification and property analysis of biomedical polymers used for tissue engineering, Colloids Surf., B, 2007, 60(2), 137–157 CrossRef CAS PubMed.
- R. Vasita, K. Shanmugam and D. S. Katti, Improved biomaterials for tissue engineering applications: Surface modification of polymers, Curr. Top. Med. Chem., 2008, 8(4), 341–353 CrossRef CAS.
- N. M. Alves, et al., Controlling Cell Behavior Through the Design of Polymer Surfaces, Small, 2010, 6(20), 2208–2220 CrossRef CAS PubMed.
- S. R. Meyers and M. W. Grinstaff, Biocompatible and Bioactive Surface Modifications for Prolonged In Vivo Efficacy, Chem. Rev., 2012, 112(3), 1615–1632 CrossRef CAS PubMed.
- L. C. Kam, K. Shen and M. L. Dustin, Micro- and Nanoscale Engineering of Cell Signaling, in Annual Review of Biomedical Engineering, ed. M. L. Yarmush, 2013, vol. 15, pp. 305–326 Search PubMed.
- S. W. Moore and M. P. Sheetz, Biophysics of Substrate Interaction: Influence on Neural Motility, Differentiation, and Repair, Dev. Neurobiol., 2011, 71(11), 1090–1101 CrossRef CAS PubMed.
- R. G. Harrison, The reaction of embryonic cells to solid structures, J. Exp. Zool., 1914, 17(4), 521–544 CrossRef PubMed.
- P. Weiss, In vitro experiments on the factors determining the course of the outgrowing nerve fiber, J. Experimental Zoology, 1934, 68(3), 393–448 CrossRef PubMed.
- C. S. Chen, et al., Geometric control of cell life and death, Science, 1997, 276(5317), 1425–1428 CrossRef CAS.
- J. L. Tan, et al., Cells lying on a bed of microneedles: An approach to isolate mechanical force, Proc. Natl. Acad. Sci. U. S. A., 2003, 100(4), 1484–1489 CrossRef CAS PubMed.
- M. J. Dalby, et al., The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder, Nat. Mater., 2007, 6(12), 997–1003 CrossRef CAS PubMed.
- H. V. Unadkat, et al., An algorithm-based topographical biomaterials library to instruct cell fate, Proc. Natl. Acad. Sci. U. S. A., 2011, 108(40), 16565–16570 CrossRef CAS PubMed.
- A. Curtis and C. Wilkinson, Topographical control of cells, Biomaterials, 1997, 18(24), 1573–1583 CrossRef CAS.
- C. Vieu, et al., Electron beam lithography: resolution limits and applications, Appl. Surf. Sci., 2000, 164, 111–117 CrossRef CAS.
- R. McMurray, M. J. Dalby and N. Gadegaard, Nanopatterned Surface for Biomedical Applications, Biomedical Engineering, Trends in Material Science, in Biomedical Engineering, Trends in Materials Science, ed. A. Laskovski, InTech, 2011 Search PubMed.
- J. Y. Zhang, et al., Combined topographical and chemical micropatterns for templating neuronal networks, Biomaterials, 2006, 27(33), 5734–5739 CrossRef CAS PubMed.
- H. Kenar, et al., Chemical and topographical modification of PHBV surface to promote osteoblast alignment and confinement, J. Biomed. Mater. Res., Part A, 2008, 85A(4), 1001–1010 CrossRef CAS PubMed.
- E. Sardella, et al., Nano-structured cell-adhesive and cell-repulsive plasma-deposited coatings: Chemical and topographical effects on keratinocyte adhesion, Plasma Processes Polym., 2008, 5(6), 540–551 CrossRef CAS PubMed.
- G. Dos Reis, et al., Direct Microfabrication of Topographical and Chemical Cues for the Guided Growth of Neural Cell Networks on Polyamidoamine Hydrogels, Macromol. Biosci., 2010, 10(8), 842–852 CrossRef CAS PubMed.
- A. Hadjizadeh, Acetaldehyde plasma polymer-coated PET fibers for endothelial cell patterning: Chemical, topographical, and biological analysis, J. Biomed. Mater. Res., Part B, 2010, 94(1), 11–21 Search PubMed.
- A. C. Greene, et al., Combined chemical and topographical guidance cues for directing cytoarchitectural polarization in primary neurons, Biomaterials, 2011, 32(34), 8860–8869 CrossRef CAS PubMed.
- J. Yang, et al., A High-Throughput Assay of Cell-Surface Interactions using Topographical and Chemical Gradients, Adv. Mater., 2009, 21(3), 300–304 CrossRef CAS PubMed.
- J. Duvigneau, et al., Reactive Imprint Lithography: Combined Topographical Patterning and Chemical Surface Functionalization of Polystyrene-block-poly(tert-butyl acrylate) Films, Adv. Funct. Mater., 2010, 20(3), 460–468 CrossRef CAS PubMed.
- A. Embrechts, et al., Inverted microcontact printing on polystyrene-block-poly(tert-butyl acrylate) films: A versatile approach to fabricate structured biointerfaces across the length scales, Langmuir, 2008, 24(16), 8841–8849 CrossRef CAS PubMed.
- R. McBeath, et al., Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment, Dev. Cell, 2004, 6(4), 483–495 CrossRef CAS.
- D. M. Bryant and K. E. Mostov, From cells to organs: building polarized tissue, Nat. Rev. Mol. Cell Biol., 2008, 9(11), 887–901 CrossRef CAS PubMed.
- D. M. Bryant, et al., A molecular network for de novo generation of the apical surface and lumen, Nat. Cell Biol., 2010, 12(11), 1035–1045 CrossRef CAS PubMed.
- M. Arnold, et al., Induction of cell polarization and migration by a gradient of nanoscale variations in adhesive ligand spacing, Nano Lett., 2008, 8(7), 2063–2069 CrossRef CAS PubMed.
- D. H. Kim, et al., Mechanosensitivity of fibroblast cell shape and movement to anisotropic substratum topography gradients, Biomaterials, 2009, 30(29), 5433–5444 CrossRef CAS PubMed.
- R. J. Petrie, A. D. Doyle and K. M. Yamada, Random versus directionally persistent cell migration, Nat. Rev. Mol. Cell Biol., 2009, 10(8), 538–549 CrossRef CAS PubMed.
- K. Kulangara, et al., Nanotopography as modulator of human mesenchymal stem cell function, Biomaterials, 2012, 33(20), 4998–5003 CrossRef CAS PubMed.
- N. Faucheux, et al., Self-assembled monolayers with different terminating groups as model substrates for cell adhesion studies, Biomaterials, 2004, 25(14), 2721–2730 CrossRef CAS PubMed.
- Y. H. Gong, et al., Layer-by-layer assembly of chondroitin sulfate and collagen on aminolyzed pOly(L-lactic acid) porous scaffolds to enhance their chondrogenesis, Acta Biomater., 2007, 3(5), 677–685 CrossRef CAS PubMed.
- J. M. Gao, L. Niklason and R. Langer, Surface hydrolysis of poly(glycolic acid) meshes increases the seeding density of vascular smooth muscle cells, J. Biomed. Mater. Res., 1998, 42(3), 417–424 CrossRef CAS.
- X. B. Yang, et al., Human osteoprogenitor growth and differentiation on synthetic biodegradable structures after surface modification, Bone, 2001, 29(6), 523–531 CrossRef CAS.
- X. H. Liu, Y. J. Won and P. X. Ma, Surface modification of interconnected porous scaffolds, J. Biomed. Mater. Res., Part A, 2005, 74A(1), 84–91 CrossRef CAS PubMed.
- S. I. Cantara, et al., Selective functionalization of nanofiber scaffolds to regulate salivary gland epithelial cell proliferation and polarity, Biomaterials, 2012, 33(33), 8372–8382 CrossRef CAS PubMed.
- A. J. Beck, et al., Development of a plasma-polymerized surface suitable for the transplantation of keratinocyte-melanocyte cocultures for patients with vitiligo, Tissue Eng., 2003, 9(6), 1123–1131 CrossRef CAS PubMed.
- R. M. France, et al., Attachment of human keratinocytes to plasma co-polymers of acrylic acid octa-1,7-diene and allyl amine octa-1,7-diene, J. Mater. Chem., 1998, 8(1), 37–42 RSC.
- M. Zelzer, et al., Influence of the Plasma Sheath on Plasma Polymer Deposition in Advance of a Mask and down Pores, J. Phys. Chem. B, 2009, 113(25), 8487–8494 CrossRef CAS PubMed.
- Z. W. Ma, et al., Immobilization of natural macromolecules on poly-L-lactic acid membrane surface in order to improve its cytocompatibility, J. Biomed. Mater. Res., 2002, 63(6), 838–847 CrossRef CAS PubMed.
- S. Yuan, et al., Surface modification of polycaprolactone substrates using collagen-conjugated poly(methacrylic acid) brushes for the regulation of cell proliferation and endothelialisation, J. Mater. Chem., 2012, 22(26), 13039–13049 RSC.
- S. N. Bhatia, M. L. Yarmush and M. Toner, Controlling cell interactions by micropatterning in co-cultures: Hepatocytes and 3T3 fibroblasts, J. Biomed. Mater. Res., 1997, 34(2), 189–199 CrossRef CAS.
- A. Zarowna-Dabrowska, et al., Generation of primary hepatocyte microarrays by piezoelectric printing, Colloids Surf., B, 2012, 89, 126–132 CrossRef CAS PubMed.
- I. Paik, et al., Rapid micropatterning of cell lines and human pluripotent stem cells on elastomeric membranes, Biotechnol. Bioeng., 2012, 109(10), 2630–2641 CrossRef CAS PubMed.
- T. Desmet, et al., Nonthermal Plasma Technology as a Versatile Strategy for Polymeric Biomaterials Surface Modification: A Review, Biomacromolecules, 2009, 10(9), 2351–2378 CrossRef CAS PubMed.
- L. Montanari, et al., Gamma irradiation effects on poly(DL-lactictide-co-glycolide) microspheres, J. Controlled Release, 1998, 56(1–3), 219–229 CrossRef CAS.
- S. C. J. Loo, C. P. Ooi and Y. C. F. Boey, Radiation effects on poly(lactide-co-glycolide) (PLGA) and poly(L-lactide) (PLLA), Polym. Degrad. Stab., 2004, 83(2), 259–265 CrossRef CAS.
- M. H. Ho, et al., Efficient modification on PLLA by ozone treatment for biomedical applications, Macromol. Biosci., 2007, 7(4), 467–474 CrossRef CAS PubMed.
- A. Nur-E-Kamal, et al., Three-dimensional nanofibrillar surfaces promote self-renewal in mouse embryonic stem cells, Stem Cells, 2006, 24(2), 426–433 CrossRef PubMed.
- Y. P. Kong, et al., Expression of Oct4 in human embryonic stem cells is dependent on nanotopographical configuration, Acta Biomater., 2013, 9(5), 6369–6380 CrossRef CAS PubMed.
- M. Schernthaner, et al., Nanopatterned polymer substrates promote endothelial proliferation by initiation of beta-catenin transcriptional signaling, Acta Biomater., 2012, 8(8), 2953–2962 CrossRef CAS PubMed.
- Z. Melkoumian, et al., Synthetic peptide-acrylate surfaces for long-term self-renewal and cardiomyocyte differentiation of human embryonic stem cells, Nat. Biotechnol., 2010, 28(6), 606–610 CrossRef CAS PubMed.
- K. Saha, et al., Surface-engineered substrates for improved human pluripotent stem cell culture under fully defined conditions, Proc. Natl. Acad. Sci. U. S. A., 2011, 108(46), 18714–18719 CrossRef CAS PubMed.
- M. M. Mahlstedt, et al., Maintenance of Pluripotency in Human Embryonic Stem Cells Cultured on a Synthetic Substrate in Conditioned Medium, Biotechnol. Bioeng., 2010, 105(1), 130–140 CrossRef CAS PubMed.
- M. D. Disney and P. H. Seeberger, The use of carbohydrate microarrays to study carbohydrate-cell interactions and to detect pathogens, Chem. Biol., 2004, 11(12), 1701–1707 CrossRef CAS PubMed.
- Y. Mei, et al., Combinatorial development of biomaterials for clonal growth of human pluripotent stem cells, Nat. Mater., 2010, 9(9), 768–778 CrossRef CAS PubMed.
- E. Kingham, et al., Nanotopographical Cues Augment Mesenchymal Differentiation of Human Embryonic Stem Cells, Small, 2013, 9(12), 2140–2151 CrossRef CAS PubMed.
- E. K. F. Yim, S. W. Pang and K. W. Leong, Synthetic nanostructures inducing differentiation of human mesenchymal stem cells into neuronal lineage, Exp. Cell Res., 2007, 313(9), 1820–1829 CrossRef CAS PubMed.
- S. Oh, et al., Stem cell fate dictated solely by altered nanotube dimension, Proc. Natl. Acad. Sci. U. S. A., 2009, 106(7), 2130–2135 CrossRef CAS PubMed.
- T. Sjostrom, et al., Fabrication of pillar-like titania nanostructures on titanium and their interactions with human skeletal stem cells, Acta Biomater., 2009, 5(5), 1433–1441 CrossRef CAS PubMed.
- M. H. You, et al., Synergistically Enhanced Osteogenic Differentiation of Human Mesenchymal Stem Cells by Culture on Nanostructured Surfaces with Induction Media, Biomacromolecules, 2010, 11(7), 1856–1862 CrossRef CAS PubMed.
- O. F. Zouani, et al., Altered nanofeature size dictates stem cell differentiation, J. Cell Sci., 2012, 125(5), 1217–1224 CrossRef CAS PubMed.
- J. M. Dang and K. W. Leong, Myogenic induction of aligned mesenchymal stem cell sheets by culture on thermally responsive electrospun nanofibers, Adv. Mater., 2007, 19(19), 2775–2779 CrossRef CAS PubMed.
- G. T. Christopherson, H. Song and H. Q. Mao, The influence of fiber diameter of electrospun substrates on neural stem cell differentiation and proliferation, Biomaterials, 2009, 30(4), 556–564 CrossRef CAS PubMed.
- E. Migliorini, et al., Acceleration of Neuronal Precursors Differentiation Induced by Substrate Nanotopography, Biotechnol. Bioeng., 2011, 108(11), 2736–2746 CrossRef CAS PubMed.
- J. B. Recknor, D. S. Sakaguchi and S. K. Mallapragada, Directed growth and selective differentiation of neural progenitor cells on micropatterned polymer substrates, Biomaterials, 2006, 27(22), 4098–4108 CrossRef CAS PubMed.
- J. S. Oh, et al., Soluble factors from neocortical astrocytes enhance neuronal differentiation of neural progenitor cells from adult rat hippocampus on micropatterned polymer substrates, J. Biomed. Mater. Res., Part A, 2009, 91A(2), 575–585 CrossRef CAS PubMed.
- R. Delgado-Rivera, et al., Increased FGF-2 secretion and ability to support neurite outgrowth by astrocytes cultured on polyamide nanofibrillar matrices, Matrix Biol., 2009, 28(3), 137–147 CrossRef CAS PubMed.
- S. W. Kuo, et al., Regulation of the fate of human mesenchymal stem cells by mechanical and stereo-topographical cues provided by silicon nanowires, Biomaterials, 2012, 33(20), 5013–5022 CrossRef CAS PubMed.
- B. K. K. Teo, et al., Nanotopography Modulates Mechanotransduction of Stem Cells and Induces Differentiation through Focal Adhesion Kinase, ACS Nano, 2013, 7(6), 4785–4798 CrossRef CAS PubMed.
- C. J. Bettinger, et al., Enhancement of in vitro capillary tube formation by substrate nanotopography, Adv. Mater., 2008, 20(1), 99–103 CrossRef CAS PubMed.
- N. F. Huang, et al., Spatial patterning of endothelium modulates cell morphology, adhesiveness and transcriptional signature, Biomaterials, 2013, 34(12), 2928–2937 CrossRef CAS PubMed.
- K. Seunarine, et al., Biodegradable polymer tubes with litho graphically controlled 3D micro- and nanotopography, Microelectron. Eng., 2008, 85(5–6), 1350–1354 CrossRef CAS PubMed.
- N. Bursac, et al., Cardiomyocyte cultures with controlled macroscopic anisotropy – A model for functional electrophysiological studies of cardiac muscle, Circ. Res., 2002, 91(12), E45–E54 CrossRef CAS.
- L. H. Yin, H. Bien and E. Entcheva, Scaffold topography alters intracellular calcium dynamics in cultured cardiomyocyte networks, Am. J. Physiol. Heart Circ. Physiol., 2004, 287(3), H1276–H1285 CrossRef CAS PubMed.
- J. Kim, et al., Quantitative evaluation of cardiomyocyte contractility in a 3D microenvironment, J. Biomech., 2008, 41(11), 2396–2401 CrossRef PubMed.
- A. A. Patel, T. A. Desai and S. Kumar, Microtopographical assembly of cardiomyocytes, Integr. Biol., 2011, 3(10), 1011–1019 RSC.
- D. Y. Wong, et al., Macro-architectures in spinal cord scaffold implants influence regeneration, J. Neurotrauma, 2008, 25(8), 1027–1037 CrossRef PubMed.
- L. M. He, et al., Manufacture of PLGA Multiple-Channel Conduits with Precise Hierarchical Pore Architectures and In Vitro/Vivo Evaluation for Spinal Cord Injury, Tissue Eng., Part C, 2009, 15(2), 243–255 CrossRef CAS PubMed.
- M. J. Moore, et al., Multiple-channel scaffolds to promote spinal cord axon regeneration, Biomaterials, 2006, 27(3), 419–429 CrossRef CAS PubMed.
- A. J. Krych, et al., Relationship between scaffold channel diameter and number of regenerating axons in the transected rat spinal cord, Acta Biomater., 2009, 5(7), 2551–2559 CrossRef CAS PubMed.
- G. E. Rutkowski, et al., Synergistic effects of micropatterned biodegradable conduits and Schwann cells on sciatic nerve regeneration, J. Neural Eng., 2004, 1(3), 151–157 CrossRef PubMed.
- J. M. Curran, R. Chen and J. A. Hunt, The guidance of human mesenchymal stem cell differentiation in vitro by controlled modifications to the cell substrate, Biomaterials, 2006, 27(27), 4783–4793 CrossRef CAS PubMed.
- L. Guo, et al., Chondrogenic differentiation of human mesenchymal stem cells on photoreactive polymer-modified surfaces, Biomaterials, 2008, 29(1), 23–32 CrossRef CAS PubMed.
- K. W. Chun, et al., Biodegradable PLGA microcarriers for injectable delivery of chondrocytes: Effect of surface modification on cell attachment and function, Biotechnol. Prog., 2004, 20(6), 1797–1801 CrossRef CAS PubMed.
- D. S. W. Benoit, et al., Small functional groups for controlled differentiation of hydrogel-encapsulated human mesenchymal stem cells, Nat. Mater., 2008, 7(10), 816–823 CrossRef CAS PubMed.
- X. Liu, et al., Biomimetic nanofibrous gelatin/apatite composite scaffolds for bone tissue engineering, Biomaterials, 2009, 30(12), 2252–2258 CrossRef CAS PubMed.
- M. M. Mahlstedt, et al., Maintenance of Pluripotency in Human Embryonic Stem Cells Cultured on a Synthetic Substrate in Conditioned Medium, Biotechnol. Bioeng., 2010, 105(1), 130–140 CrossRef CAS PubMed.
- S. P. Massia and J. A. Hubbell, Human endothelial-cell interactions with surface-coupled adhesion peptides on a nonadhesive glass substrate and 2 polymeric biomaterials, J. Biomed. Mater. Res., 1991, 25(2), 223–242 CrossRef CAS PubMed.
- R. Daw, et al., Plasma copolymer surfaces of acrylic acid 1,7 octadiene: Surface characterisation and the attachment of ROS 17/2.8 osteoblast-like cells, Biomaterials, 1998, 19(19), 1717–1725 CrossRef CAS.
- J. J. A. Barry, et al., Using a core-sheath distribution of surface chemistry through 3D tissue engineering scaffolds to control cell ingress, Adv. Mater., 2006, 18(11), 1406–1410 CrossRef CAS PubMed.
- R. A. Quirk, et al., Poly(L-lysine)-GRGDS as a biomimetic surface modifier for poly(lactic acid), Biomaterials, 2001, 22(8), 865–872 CrossRef CAS.
- R. A. Quirk, et al., Surface engineering of poly(lactic acid) by entrapment of modifying species, Macromolecules, 2000, 33(2), 258–260 CrossRef CAS.
- Y. B. Zhu, et al., Layer-by-layer assembly to modify poly(L-lactic acid) surface toward improving its cytocompatibility to human endothelial cells, Biomacromolecules, 2003, 4(2), 446–452 CrossRef CAS PubMed.
- M. Mozafari, et al., Biomimetic formation of apatite on the surface of porous gelatin/bioactive glass nanocomposite scaffolds, Appl. Surf. Sci., 2010, 257(5), 1740–1749 CrossRef CAS PubMed.
- A. I. Teixeira, et al., Epithelial contact guidance on well-defined micro- and nanostructured substrates, J. Cell Sci., 2003, 116(10), 1881–1892 CrossRef CAS PubMed.
- N. W. Karuri, et al., Biological length scale topography enhances cell-substratum adhesion of human corneal epithelial cells, J. Cell Sci., 2004, 117(15), 3153–3164 CrossRef CAS PubMed.
- H. Lee, et al., Mussel-inspired surface chemistry for multifunctional coatings, Science, 2007, 318(5849), 426–430 CrossRef CAS PubMed.
- J. W. Xie, et al., The differentiation of embryonic stem cells seeded on electrospun nanofibers into neural lineages, Biomaterials, 2009, 30(3), 354–362 CrossRef CAS PubMed.
- A. Wilkinson, et al., Biomimetic microtopography to enhance osteogenesis in vitro, Acta Biomater., 2011, 7(7), 2919–2925 CrossRef CAS PubMed.
- J. A. Rowley, G. Madlambayan and D. J. Mooney, Alginate hydrogels as synthetic extracellular matrix materials, Biomaterials, 1999, 20(1), 45–53 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |