Magnetic composite biomaterials for tissue engineering
Sara
Gil
and
João F.
Mano
*
3B's Research Group – Biomaterials, Biodegradables and Biomimetics, ICVS/3B's – PT Government Associate Laboratory, AvePark, Zona Industrial da Gandra, S. Cláudio do Barco, 4806-909 Taipas, Guimarães, Portugal. E-mail: sara.gil@dep.uminho.pt; jmano@dep.uminho.pt; Fax: +351253510909; Fax: +351253510909; Tel: +351-253510912 Tel: +351-253510904
First published on 28th March 2014
Abstract
Magnetic nanoparticles (MNPs) have been increasingly used in tissue engineering and regenerative medicine. These particles have been mainly employed as elements directly incorporated into cells or interacting with cell membranes; however, MNPs are now being combined with biomaterials to create other functionalities of the structural framework used to support cells, namely for controlling cellular responses and for enhancing drug delivery and release. This mini-review summarizes and highlights the latest developments and applications of polymeric/ceramic biomimetic scaffolds and hydrogels that contain MNPs for such purposes, also addressing future perspectives for the use of these magnetic composite biomaterials in biomedicine.
Introduction
Magnetic materials have been widely used in biomedicine. The preparation of stronger and smaller permanent magnets allowed the creation of more delicate biomedical applications like in the fields of ophthalmology (magnetically assisted cataract surgery), dentistry (temporary fixing prosthesis), cardiology and gastroenterology (guiding catheters through the body), and neurology (navigating within the brain).1 In particular, much effort has been devoted to the synthesis of magnetic nanosized materials, due to their small size and unusual superparamagnetic properties.2–5 Magnetite (Fe3O4) and maghemite (γ-Fe2O3) are the most common iron oxides used in biomedicine due to their low toxicity, relative ease of functionalization and high magnetization at room temperature.5–8 Such materials are easily fabricated into the shape of nanoparticles.9 Magnetic nanoparticles (MNPs) exhibit a superparamagnetic behavior at sizes below 20 nm, demonstrating high potential for in vivo applications because they do not retain any remanent magnetization upon removal of the magnetic field, which prevents aggregation and enables them to redisperse rapidly after withdrawing the magnetic field.3,5,8,10MNPs and magnetic liposomes have been increasingly exploited in the field of biomedicine. They have controllable size (from a few nanometers up to tens of nanometers) which is compatible with those of viruses (20–450 nm), proteins (5–50 nm) and genes (2 nm wide by 10–100 nm long). Besides their small size, nanoparticles and liposomes can be functionalized with other materials enabling their interaction and specific binding to other biological entities, and enhancing their colloidal stability and biocompatibility. Also, through the action of a magnetic field it is possible to trace and control the localization of these nanomaterials within the human body, through minimally invasive methods.3–5
Tissue engineering (TE) is an interdisciplinary field, exploiting biological and engineering principles that, when combined with suitable biochemical factors, allows for the development of functional substitutes for lost or damaged tissue.11 An emerging TE strategy, named magnetic-force based tissue engineering, employs cells that have been magnetically labeled with MNPs or magnetic cationic liposomes (MCLs) in the biofabrication of more complex tissue constructs.8,12 For example, the cellular culture and co-culture techniques applying this principle can be used in magnetic cell patterning, magnetic cell seeding and magnetic cell levitation.12 Such work mainly focused on the direct contact between MNPs or MCLs and cells. In another perspective, magnetic elements can be combined with biomaterials that are usually used as structural frameworks for supporting cells to attach, proliferate and differentiate. Such a strategy could allow for the production of hybrid structures with enhanced functionalities, including devices that are able to provide mechanical stimuli to cells or to deliver on demand growth factors (GFs) or other bioactive molecules. This mini-review provides an overview of the latest developments of polymeric/ceramic scaffolds and hydrogels that contain magnetic particles for such purposes. The results reported so far indicate increased interest of researchers in these topics, foreseeing that magnetic particles can be used as stimuli to influence cellular activity, as well as cell proliferation and differentiation, and will bring new prospects and major improvements to the fields of drug delivery and tissue regeneration.
Cellular behavior
Stem cell behavior is highly influenced by the physical properties of the scaffold and the chemical/biochemical landscape over its surface. However, other external factors may affect cellular behavior, such as mechanical stimulation. Many studies have shown that cell and tissue growth increase in response to mechanical stresses generated by the mobility of the surface matrix or by fluid flow.13–15 This has been the basis for the development of bioreactors.16Mechanotransduction is a well known pathway by which cells convert physical stimuli into biochemical activity. For many TE and regenerative medicine applications, mechanical cues provide important stimuli to the cells that promote the production of functional tissue matrix. For example, the differentiation of mesenchymal stem cells into bone, cartilage, muscle and connective tissue is particularly conditioned by mechanical cues.17,18 However, applying the correct stress profiles to cells growing in a 3D scaffold within a bioreactor or within a patient's body has proven difficult.
To provide mechanical stimuli similar to those experienced in vivo by cells, the in vivo environment must be mimicked inside the bioreactor. Currently, the available bioreactors do not allow the application of spatially varying stresses in three dimensions in order to form complex tissue structures. Direct magnetic actuation can provide the application of controlled forces in order to precisely regulate cellular functions. In this context, MNPs can be attached to specific ion channels present on the cellular membrane, acting as stress generators.19 Cells can thus be mechanically conditioned by magnetic remote actuation. Cartmell and co-workers demonstrated that mechanical stimulation of primary human osteoblast cells by adhered magnetic particles promoted the regeneration of the bone matrix when under the influence of a magnetic field.20
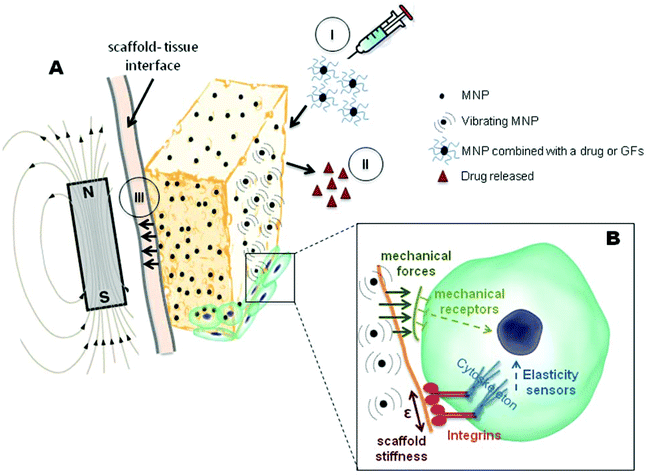
However, a possibility that has seldom been considered is the application of MNPs in tissue regeneration by incorporating them into scaffolds. These superparamagnetic scaffolds can be “activated” through the application of an external magnetic field. The field acts on the nanoparticles along the gradient vector, producing compressive or tensile forces that are sensed by the cells in the scaffold (Fig. 1). The forces necessary to activate the mechanosensitive channels via cell membrane deformation are really small (on the order of picoNewtons).21 Therefore we believe that superparamagnetic scaffolds can provide the necessary cues for stimulating stem cell differentiation.
X. B. Zeng and co-workers investigated the behavior of rat osteoblast and mice preosteoblast cells on a series of MNP-hydroxyapatite (HA) magnetic scaffolds with different MNP contents (from 0 to 2 wt%).22 The results demonstrated the positive influence of MNP-HA scaffolds on cell adhesion, proliferation and differentiation when compared to non-magnetic HA scaffolds, suggesting enhanced cell behavior due to the incorporation of MNPs.14,22 Furthermore, these results were significantly intensified when the MNP-HA scaffolds were under the influence of an external static magnetic field, suggesting a likely synergistic effect between the magnetic scaffolds and the exterior magnetic field.22–24 Likewise, a positive correlation between MNP content and cell proliferation was observed.
With sizes of 20 nm or less, MNPs become superparamagnetic and behave like common materials in the absence of an external magnetic field. However, at the nanoscale level, each MNP in the scaffold acts like a single magnetic domain, providing micromotions at the interface between cells and the scaffold that might affect the ion channels on the cell membrane, and trigger the mechanotransduction pathway.25 Nevertheless, as explained earlier, once MNPs are exposed to a magnetic field, they are rapidly magnetized providing enhanced therapeutic effects.26
Although the majority of research groups attribute the direct effect of MNPs on cell activity only to magnetism, we cannot rule out the possibility raised by Y. Sapir and co-workers, which states that in addition to the scaffold magnetic properties, the integration of MNPs into the scaffolds also changes the surface roughness of the scaffold pore walls and scaffold stiffness.27 In fact, scaffold elasticity properties are known to influence cell behavior.28–30 Adhesive forces are formed when a cell binds to a certain substrate. These forces are generated by the cellular cytoskeleton, allowing the cell to spread. Substrate stiffness is a parameter that allows controlling cell behavior and the extent of cell spreading (Fig. 1).31,32
Sapir et al. observed an enhanced effect on metabolic cell activity in the MNP-scaffold when exposed to a magnetic field. They did not report an increase in proliferation, but rather an induction of other cellular processes such as cell organization.27 However, to clarify whether this is solely due to the magnetic component of the scaffolds, it would be interesting to perform a more systematic study, where a scaffold impregnated with non-magnetic particles, but with the same elastic/storage modulus as an MNP impregnated one, would act as a control.
It can be concluded that magnetic scaffolds clearly have an influence on cellular aspects such as adhesion, proliferation and differentiation. The MNP-impregnated scaffolds demonstrate an enhanced effect on cell behavior, promoting the remote activation of the mechanotransduction pathway which in turn triggers the biochemical one. Magnetic scaffolds present an excellent alternative and improvement in bioreactor and scaffold design, as they can provide mechanical cues that can be enhanced upon the application of a remotely generated external magnetic field. From the research done so far, it is evident that few papers have clearly studied the mechanism by which cell behavior is influenced. Some authors attribute such differences to scaffold magnetic properties, which are synergistically enhanced when under the action of a magnetic field, but others also state that changes in surface topography and scaffold stiffness are parameters that also need to be considered. In the future it would be important to increase the systematization of the studies related to the fundamental understanding of the effect of the presence of MNPs in biomaterials (with and without the application of an external magnetic field), isolating for example stiffness/topography components.
Tissue engineering applications
Drug delivery
For the complete biological and histomorphological maturation of tissues, synthetic systems, able to control the delivery of bioactive systems, are particularly promising as devices for enhancing tissue regeneration. Therefore scaffolds capable of mimicking the molecular regulatory characteristics, combined with an adequate three-dimensional (3D) architecture, are necessary for guiding functional angiogenesis, for controlling stem cell proliferation and differentiation, and for tissue repairing.33,34Through the introduction of MNPs into the scaffold, unique properties are imparted to the resulting material. In particular, nanocomposites sensitive to the magnetic field exhibit the specific property of being responsive to remote actuation, thus allowing high control of the release of therapeutic agents by the influence of an external magnetic field.
Controlled drug delivery is mainly aimed at the sustained delivery of therapeutic substances over a prolonged period of time. Nevertheless, pulsatile drug delivery is also very attractive.35 Through an adequate scaffold design and time/intensity control of the external field one could, in principle, achieve zero-order or more complex (e.g. pulsatile) delivery profiles, capable of mimicking the physiological needs of bioactive agents, and thus leading to optimum drug delivery.36
The use of magnetic scaffolds responsive to an “on demand” magnetic field allows overcoming of the limitations faced by conventional scaffolds. These are often pre-loaded with GFs or other therapeutic molecules, resulting in devices with limited control of the release profile.37,38 Also, systems with a constant release rate, very popular in the pharmaceutical field, may not be adequate in TE strategies. The body's need for a drug during a regenerative process is not always constant;38 thereby it is believed that magnetic scaffolds could provide a controlled delivery that could be compatible with the endogenous production and availability of GFs, hormones and other bioactive molecules.
Such principles were already validated in the field of drug release systems, especially using composite hydrogels. For example, R. Langer and co-workers designed a magnetic subcutaneous implant based on ethylene-vinyl acetate copolymer (EVAc) hydrogels able to liberate insulin at higher rates, upon demand.39 When the device was implanted in diabetic mice the glucose level was maintained at a nearly constant value. However, when a magnetic field was applied the blood glucose level clearly decreased. The movements induced by the field on the magnet inside the implanted hydrogel exerted pressure on the implant's matrix, causing the squeezing of the drug out of the pores. Therefore the control of the delivery of insulin could be achieved by different parameters such as the frequency, strength and duration of the external magnetic field.
V. M. Paoli and co-workers studied the effect of an oscillating magnetic field on the morphology and release profile of dextran-Rhodamine (Dex-R) from magnetic collagen hydrogels containing nanoparticles and microparticles. Regarding drug release profiles, it was observed that the release rate followed an exponential profile for both formulations. However the amount of drug released is almost doubled upon application of the magnetic field.40
The change in the release profile in the system described is limited to the control of the magnification of the release when the field is turned on. However, we consider that the combination of different magnetic stimuli with the design and composition of hydrogel structures could bring new perspectives to the drug delivery field and help to obtain release profiles other than first order release. For example, S. Y. Chen and co-workers fabricated a magnetic hydrogel by mixing poly(vinyl alcohol) with Fe3O4 MNPs through freeze–thawing cycles, and studied the drug release profile when under a pulsed magnetic field.41 When the field is “switched on” MNPs aggregate together instantly, producing a bulk magnetic moment and causing a rapid reduction of the hydrogel's porosity. In this state, the rate of drug release is at a lower level and the hydrogel possesses a “closed” configuration. However, when the magnetic field is “switched off”, the hydrogel returns to its original geometrical conformation (swelling rate increases) resulting in a burst-like release profile that turns back to the normal diffusion mode shortly after the burst. On the other hand, other groups reported different results, stating that the application of an external magnetic field causes a rapid burst in the drug release.36 Again, considering the design of the scaffolds, they could also be advantageous for the stabilization of drugs or GFs if those were attached to MNPs that are impregnated in the scaffold. In this way they would always be available for cells encapsulated within the scaffold as opposed to the dispersed molecules.42,43
The studies performed so far have thus shown that hydrogels containing magnetic elements may exhibit distinct, and even opposite, drug release behaviors upon the action of an external magnetic field. In the future, we could even envisage more sophisticated devices. For example, the combination of magnetic nanoparticles with responsive polymers44 could open new prospects for externally mediated treatments in vivo not only including drug release, but also hyperthermia and combinations thereof.45
In tissue engineering there is a constant need for a spatially controlled delivery of cells and/or specific GFs to foment rapid and well organized cell scaffold colonization. However, there is a limitation on the amount of biological material that can be incorporated into a scaffold before implantation. In this context, magnetic responsive scaffolds can be also envisaged as reloading systems for long term biochemical stimuli. Such scaffolds could function as fixed stations capable of attracting and fixing, for example nano/micro-magnetic particles containing the required therapeutic molecules via magnetic driving. These particles could be administrated on demand and be directed to the implanted scaffold, delivering the cargo in that place.
Although magnetic responsive composites have been developed for the delivery of therapeutics to treat different diseases, they are not yet optimized to be used specifically for regenerative purposes. We believe that there is immense potential for both fundamental and applied research in this field that should clearly need the cooperation of multidisciplinary teams.
Tissue regeneration
Strategies used nowadays for tissue regeneration do not often promote a successful growth of tissue nor the complete integration of scaffolds into the tissue. Successful regeneration largely depends on interface interactions between cells and scaffolds. Therefore, scaffolds should not act as static elements. They should be “activated” during cell colonization, re-structuring their architecture according to the different mechanical and anatomical characteristics and to the different maturation phases of the tissue. As previously discussed, one of the most important stimuli that promote cellular differentiation into bone, cartilage, muscle and connective tissue is the mechanical one, and magnetic stimulation emerges as a possible means of achieving this stimulation in vivo.The application of static or alternating magnetic fields in clinical studies has already been reported by some groups, and has been proven beneficial in regeneration, integration, and in-growth of tissues into ceramics.46,47 Also, as commented before, the incorporation of magnetic nanoparticles into scaffolds used in tissue engineering has already been validated as having a beneficial role in cellular behavior in in vitro studies. Therefore, we consider that the incorporation of MNPs into scaffolds acting synergistically with the magnetic field in vivo would improve cellular proliferation and differentiation, and promote an enhancement in tissue integration into the scaffold, a crucial step towards the clinical applications of the composites. Besides, another factor that should be taken into consideration is the fixation of scaffolds in the defect that could, in principle, be improved with the help of an external magnetic field (Fig. 1). An efficient mechanical fixation would prevent macro and microscopic movements at the interface between the scaffold and body tissue, thus enhancing integration of the newly formed tissue.
M. Marcacci and co-workers demonstrated for the first time how collagen magnetic scaffolds, fixed in vivo with external magnets, could induce controlled regeneration in a good 3D pattern.48 Under the effect of the static magnetic field the scaffolds become “activated” and oriented according to the field, thus allowing an oriented ECM deposition, which mimics the site specific collagen/apatite orientation.49
In vivo studies of tissue formation and enhancement mediated by magnetic or superparamagnetic responsive composites have been rarely reported, although pioneering studies have demonstrated valuable improvements in tissue regeneration.24,50,51 In addition to magnetic biomaterial properties, the synergistic effect of an external magnetic field results in the site specific oriented tissue architecture that shortens tissue remodeling by accelerating the balance between mature tissue formation and scaffold degradation. Moreover, this methodology allows one to reduce the strength of the magnetic field applied to the tissues, since weak magnetic force stimulation has a significant effect on the scaffold, and consequently on tissue formation and homogeneity, and on the stability of the scaffold when implanted in the injured site.
Therefore, TE using magnetic composites holds great promise, since it is beneficial in optimizing the control of timing, delivery of GFs, magnetic strength and scaffold fixation in tissue formation allowing control of the processes governing interface regeneration and homeostasis.
Conclusions and future perspectives
Nowadays, magnetic iron oxides, especially in the form of MNPs, are being used in TE applications. In particular, MNPs are being incorporated into scaffolds, providing functional 3D engineered systems which can respond to external magnetic field stimuli.Such magnetic responsive composites can provide other functionalities to implantable devices, either by directly influencing the interaction of the scaffold with the cells (affecting adhesion, proliferation and differentiation) or by their use as smart drug delivery and tissue regeneration systems. Figure 1, resumes some effects that can be explored by such devices in the context of TE, considered in this mini-review. Regarding cell interactions it was possible to conclude that MNPs stimulate cell adhesion, proliferation, and even differentiation, this effect being amplified in the presence of an external magnetic field. Magnetic composites can thus provide local mechanical stimuli to cells, enhancing the regeneration potential of implantable devices.
The magnetic properties of polymeric matrices, especially hydrogels, also allow fine tuning and accurate controlling of drug's release profile in the spatiotemporal context. More work will be necessary in order to improve the drug release profile (including multiple drug release specifically designed to stimulate the regenerative process) using magnetic scaffolds in the presence of a magnetic field.
Furthermore, it is believed that magnetic composites could be useful tools for controlling the delivery and availability of GFs for tissue regeneration, being more cost effective when conjugated with magnetic nanoparticles than when freely available. Moreover, remote actuation could allow for scaffold reloading with these molecules, thus benefitting tissue regeneration. Also, magnetic scaffolds can be fixed in the body by the action of an external magnetic field, overcoming issues related to scaffold micro- and macro-movements in injured sites.
Besides the applications discussed in this review, magnetic composites could offer other possibilities that could be explored in the field of TE. For example, Utkan Demirci and co-workers developed a technique that enables 3D microgel assembly by mimicking the repeating cellular functional units that compose tissues.52 The technology presented herein offers an alternative to known approaches, which face cell seeding limitations and microenvironment control.
Also we envision for the future other kinds of applications, such as magnetic responsive surfaces that could control better the cellular behavior, implantable constructs with the shape controlled by magnetic fields, magnetic beads for stem cell expansion,53 and magnetic hydrogel composites as remotely activated microfluidic devices.54
Acknowledgements
The present work was supported by the Portuguese Foundation for Science and Technology (FCT) through the Suprarelax project (PTDC/FIS/115048/2009).References
- G. T. Gillies, R. C. Ritter, W. C. Broaddus, M. S. Grady, M. A. Howard and R. G. McNeill, Rev. Sci. Instrum., 1994, 65, 533–562 CrossRef CAS.
- Y. W. Jun, J. W. Seo and J. Cheon, Acc. Chem. Res., 2008, 41, 179–189 CrossRef CAS PubMed.
- S. F. Medeiros, A. M. Santos, H. Fessi and A. Elaissari, Int. J. Pharm., 2011, 403, 139–161 CrossRef CAS PubMed.
- Q. A. Pankhurst, J. Conolly, S. K. Jones and J. Dobson, J. Phys. D: Appl. Phys., 2003, 36, 167–181 CrossRef.
- J. M. De Teresa, C. Marquina, D. Serrate, R. F. Pacheco, L. Morellon, P. A. Algarabel and M. R. Ibarra, Int. J. Nanotechnol., 2005, 2, 3–22 CrossRef CAS.
- N. Tran and T. J. Webster, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2009, 1, 336–351 CrossRef CAS PubMed.
- C. Cunha, S. Panseri, D. Lannazo, A. Piperno, A. Pistone, M. Fazio, A. Russo, M. Marcacci and S. Galvagno, Nanotechnology, 2012, 23, 465102 CrossRef CAS PubMed.
- J. L. Corchero and A. Villaverde, Trends Biotechnol., 2009, 27, 468–476 CrossRef CAS PubMed.
- C. Yang, J. Wu and Y. Hou, Chem. Commun., 2011, 47, 5130–5141 RSC.
- S. Gil, E. Castro and J. F. Mano, Matter. Lett., 2013, 100, 266–270 CrossRef CAS.
- R. Langer and J. P. Vacanti, Science, 1993, 260, 920–926 CAS.
- E. Castro and J. F. Mano, J. Biomed. Nanotechnol., 2013, 9, 1129–1136 CrossRef CAS PubMed.
- S. Nomura and T. Takano-Yamamoto, Matrix Biol., 2000, 19, 91–96 CrossRef CAS PubMed.
- K. Lai, W. Jiang, J. Z. Tang, Y. Wu, B. He, G. Wang and Z. Gou, R. Soc. Chem. Adv., 2012, 2, 13007–13017 CAS.
- G. Bao and S. Suresh, Nat. Mater., 2003, 2, 715–725 CrossRef CAS PubMed.
- L. E. Freed and G. Vunjak-Novakovic, in Principles of Tissue Engineering, ed. R. P. Lanza, R. Langer and J. Vacanti, Elsevier, San Diego, 2nd edn, 2000, ch. 13, pp. 143–156 Search PubMed.
- M. Wright, P. Jobanputra, C. Bavington, D. M. Salter and G. Nuki, Clin. Sci., 1996, 90, 61–71 CrossRef CAS PubMed.
- Q. A. Pankhurst, N. T. K. Thanh, S. K. Jones and J. Dobson, J. Phys. D: Appl. Phys., 2009, 42 Search PubMed.
- J. Dobson, S. H. Cartmell, A. Keramane and A. J. E. Haj, IEEE Trans. Nanobioscience, 2006, 5, 173–177 CrossRef PubMed.
- S. H. Cartmell, J. Dobson, S. B. Verschueren and A. J. E. Haj, IEEE Trans. Nanobioscience, 2002, 1, 92–97 CrossRef PubMed.
- L. M. Walker, A. Holm, L. Cooling, L. Maxwell, A. Oberg, T. Sundgvist and A. J. E. Haj, FEBS Lett., 1999, 459, 39–42 CrossRef CAS PubMed.
- X. B. Zeng, H. Hu, L. Q. Xie, F. Lan, W. Jiang, Y. Wu and Z. W. Gu, Int. J. Nanomedicine, 2012, 7, 3365–3378 CrossRef CAS PubMed.
- J. T. Kannarkat, J. Battogtokh, J. Philip, O. C. Wilson and P. M. Mehl, J. Appl. Phys., 2010, 107, 307–373 CrossRef.
- J. Meng, B. Xiao, Y. Zhang, J. Liu, H. Xue, J. Lei, H. Kong, Y. Huang, Z. Jin, N. Gu and H. Xu, Sci. Rep., 2013, 3, 2655–2662 Search PubMed.
- S. Hughes, A. J. El Haj and J. Dobson, Med. Eng. Phys., 2005, 27, 754–762 CrossRef PubMed.
- A. K. Gupta and M. Gupta, Biomaterials, 2005, 26, 3995–4021 CrossRef CAS PubMed.
- Y. Sapir, S. Cohen, G. Friedman and B. Plovak, Biomaterials, 2012, 33, 4100–4109 CrossRef CAS PubMed.
- B. Bhana, R. K. Iver, W. L. Chen, R. Zhao, K. L. Sider, M. Likhitpanichkul, C. A. Simmons and M. Radisic, Biotechnol. Bioeng., 2010, 105, 1148–1160 CAS.
- A. L. Sieminski, R. P. Hebbel and K. J. Gooch, Exp. Cell Res., 2004, 297, 574–584 CrossRef CAS PubMed.
- A. M. Ross, Z. Jiang, M. Bastmeyer and J. Lahann, Small, 2012, 8, 336–355 CrossRef CAS PubMed.
- T. Yeung, P. C. Georges, L. A. Flanagan, B. Marq, M. Ortiz, M. Funaki, N. Zahir, W. Ming, V. Weaver and P. A. Janmey, Cell Motil. Cytoskeleton, 2005, 60, 24–34 CrossRef PubMed.
- N. M. Alves, I. Pashkuleva, R. L. Reis and J. F. Mano, Small, 2010, 6, 2208–2220 CrossRef CAS PubMed.
- W. M. Saltzman and W. L. Olbricht, Nat. Rev. Drug Discovery, 2002, 1, 177–186 CrossRef CAS PubMed.
- M. Biondi, F. Ungaro, F. Quaglia and P. A. Netti, Adv. Drug Delivery Rev., 2008, 60, 229–242 CrossRef CAS PubMed.
- T. Bussemer, I. Otto and R. Bodmeier, Crit. Rev. Ther. Drug Carrier Syst., 2001, 18, 433–458 CrossRef CAS PubMed.
- N. S. Satarkar and J. Z. Hilt, J. Controlled Release, 2008, 130, 246–251 CrossRef CAS PubMed.
- V. E. Santos, M. E. Gomes, J. F. Mano and R. L. Reis, Tissue Eng. B Rev., 2013, 19, 308–326 CrossRef PubMed.
- V. E. Santos, M. E. Gomes, J. F. Mano and R. L. Reis, Tissue Eng. B Rev., 2013, 19, 327–352 CrossRef PubMed.
- J. Kost, J. Wolfrum and R. Langer, J. Biomed. Res., 1987, 21, 1367–1373 CrossRef CAS PubMed.
- V. M. De Paoli, S. H. Lacerda, L. Spinu, B. Ingberg, Z. Rosenweig and N. Rosenweig, Langmuir, 2006, 22, 5894–5899 CrossRef CAS PubMed.
- T. Y. Liu, S. H. Hu, T. Y. Liu, D. M. Liu and S. Y. Chen, Langmuir, 2006, 22, 5974–5978 CrossRef CAS PubMed.
- O. Ziv-Polat, H. Skaat, A. Shahar and S. Margel, Int. J. Nanomedicine, 2012, 7, 1259–1254 CrossRef CAS PubMed.
- H. Skaat, O. Ziv-Polat, A. Shahar, D. Last, Y. Mardor and S. Margel, Adv. Healthc. Mater., 2012, 1, 168–171 CrossRef CAS PubMed.
- J. F. Mano, Adv. Eng. Mater., 2008, 10, 515–527 CrossRef CAS.
- S. B. Campbell, M. Patenaude and T. Hoare, Biomacromolecules, 2013, 14, 644–653 CrossRef CAS PubMed.
- K. L. Grace, W. J. Revell and M. Brookes, Orthopedics, 1998, 21, 297–302 CAS.
- P. A. Glazer, M. R. Heilmann, J. C. Lotz and D. S. Bradford, Spine, 1997, 22, 2351–2356 CrossRef CAS PubMed.
- S. Panseri, A. Russo, M. Sartori, G. Giavaresi, M. Sandri, M. Fini, M. C. Maltarello, T. Shelvakova, A. Ortolani, A. Visani, V. Dediu, A. Tampieri and M. Marcacci, Bone, 2013, 56, 432–439 CrossRef CAS PubMed.
- S. Bakbak, R. Kayacan and O. Akkuş, J. Biomech., 2011, 44, 11 CrossRef.
- S. Panseri, A. Russo, G. Giavaresi, M. Sartori, F. Veronesi, M. Fini, D. M. Salter, A. Ortolani, A. Strazzari, A. Visani, C. Diogini, N. Bock, M. Sandri, A. Tampieri and M. Marcacci, J. Biomed. Mater. Res., Part A, 2012, 100, 2278–2286 CAS.
- S. Panseri, C. Cunha, T. D'Alessandro, M. Sandri, A. Russo, G. Giavaresi, M. Marcacci, C. T. Hung and A. Tampieri, PLoS One, 2012, 7, 38710–38718 Search PubMed.
- F. Xu, C. M. Wu, V. Rengarajan, T. D. Finley, H. O. Keles, Y. Sung, B. Li, U. A. Gurkan and U. Demirci, Adv. Mater., 2011, 23, 4254–4260 CrossRef CAS PubMed.
- W. Song, M. B. Oliveira, P. Sher, S. Gil and J. F. Mano, Biomed. Mater., 2013, 8, 045008 CrossRef PubMed.
- N. S. Satarkar, W. Zhang, R. E. Eitel and J. Z. Hilt, Lab Chip, 2009, 9, 1773–1779 RSC.
| This journal is © The Royal Society of Chemistry 2014 |