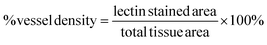Evaluation of MMP substrate concentration and specificity for neovascularization of hydrogel scaffolds
S.
Sokic
a,
M. C.
Christenson
a,
J. C.
Larson
a,
A. A.
Appel
ab,
E. M.
Brey
ab and
G.
Papavasiliou
*a
aDepartment of Biomedical Engineering, Illinois Institute of Technology, 3255 South Dearborn Street, Wishnick Hall Room 314, Chicago, IL 60616-3793, USA. E-mail: papavasiliou@iit.edu; Fax: +1 (312) 567-5770; Tel: +1 (312) 567-5959
bResearch Service, Hines Veterans Administration Hospital, Hines, IL, USA
First published on 7th July 2014
Abstract
Controlled vascular response in scaffolds following implantation remains a significant clinical challenge. A critical biomaterial design criterion is the synchronization of the rates of scaffold degradation and vascularized tissue formation. Matrix metalloproteinases (MMPs) are key enzymes that regulate neovascularization and extracellular matrix remodelling. Synthetic protease-sensitive hydrogels offer controllable environments for investigating the role of matrix degradation on neovascularization. In this study, PEG hydrogels containing MMP-sensitive peptides with increased catalytic activity for MMPs expressed during neovascularization were investigated. Scaffolds were functionalized with MMP-2-, MMP-14- or general collagenase-sensitive peptides and with varying peptide concentration using crosslinkers containing one (SSite) or multiple (TSite) repeats of each protease-sensitive sequence. Increasing peptide concentration enhanced the degradation kinetics of scaffolds functionalized with MMP-specific sequences while 80% of the collagenase-sensitive scaffolds remained upon exposure to MMP-2 and MMP-14. In vitro neovascularization was consistent with in vivo tissue invasion with significantly increased invasion occurring within SSite MMP-specific as compared to collagenase-sensitive hydrogels and with further invasion in TSite as compared to SSite hydrogels regardless of peptide specificity. All scaffolds supported in vivo neovascularization; however, this was not dependent on peptide specificity. These findings demonstrate that peptide concentration and specificity regulate in vivo scaffold degradation, neovascularization and matrix remodelling.
Introduction
The clinical success of tissue engineered scaffolds used for the replacement and reconstruction of damaged and/or diseased tissues and organs relies on the ability to induce rapid, stable and functional neovascularization (new blood vessel formation) within the implant. Neovascularization is a highly coordinated process that is dependent on the interactions of multiple cell types with spatially and temporally regulated physical, mechanical and biochemical cues provided by the extracellular matrix (ECM). These interactions include proteolytic degradation of the basement membrane, cell migration and proliferation within the ECM in response to angiogenic stimuli, ECM remodelling, lumen formation, maturation and return to quiescence.1 Synthetic biomaterial scaffolds designed to integrate key signals of the native ECM microenvironment in a controllable and systematic manner offer great potential for stimulating vascularized tissue regeneration.A critical biomaterial design criterion is the synchronization of the rates of scaffold degradation and vascularized tissue formation.2 Ideally, the scaffold should undergo degradation at a rate sufficient to maintain the structural integrity of the newly formed tissue as well as to induce formation of rapid and stable vasculature. A key class of enzymes that regulate neovascularization and ECM degradation and remodeling are matrix metalloproteinases (MMPs). MMPs reportedly produced by endothelial cells include MMP-2, MMP-9, and MMP-14. Specifically, MMP-2 is responsible for the degradation of basement membrane proteins. MMP-14 has a well-documented role in guiding endothelial cell function including migration, formation of guidance channels and lumens, and vessel stabilization.3
Synthetic hydrogel scaffolds of poly(ethylene glycol) (PEG) crosslinked with peptide sequences susceptible to degradation by cell secreted enzymes including MMPs have shown promise for support of vascularized tissue formation.4–8 Initial development of these protease sensitive hydrogels utilized the collagenase-cleavable peptide sequences GGL↓GPAGGK9–11 as well as GPQG↓IAGQ6,12,13 derived from the alpha chain of type I collagen. These peptide substrates are generally cleavable by multiple MMP enzymes and previous studies have indicated that their incorporation into scaffolds result in relatively slow degradation times thereby limiting the rate of in vivo tissue remodelling.14
In recent years, a number of methods have been developed to identify peptide sequences with increased specificity and sensitivity to distinct MMPs. These include phage display libraries, positional scanning peptide libraries and mixture-based oriented peptide libraries.15 Using a combinatorial method of mixture-based oriented libraries, cleavage site motifs with increased catalytic activity for six different enzymes in the MMP family have been previously identified.15 Previous work by Patterson and Hubbell incorporated over fifteen of these MMP-sensitive peptide sequences into synthetic PEG scaffolds and screened their susceptibility to degradation by MMP-1 and MMP-2 enzymes.14 In general, it was found that incorporation of peptide sequences with increased MMP catalytic activity and sensitivity in hydrogels resulted in significantly faster scaffold degradation times when exposed to MMP-1 and MMP-2 enzymes, increased fibroblast cell proliferation and spreading within these synthetic matrices in vitro and in enhanced cell invasion from aortic ring segments.14 In a more recent study, the use of MMP-sensitive peptide sequences with increased specificity to MMP-9 and MMP-14 enzymes were incorporated into PEG scaffolds as a means of enabling selective control of invasion of specific cell types in vitro.16 Invasion of vascular smooth muscle cells and fibroblasts was completely prevented in scaffolds crosslinked with an MMP-9-sensitive peptide sequence, while the incorporation of an MMP-14 specific sequence significantly enhanced the invasion of smooth muscle cells but impeded fibroblast invasion.16 While previous studies have shown that the inclusion of more specific MMP-cleavable peptide sequences in scaffolds leads to enhancements in protease-mediated degradation and selective invasion of specific cell types in vitro, it is not entirely clear whether peptide specificity facilitates tissue remodeling and vascularization in vivo as multiple cells and cell–matrix interactions are involved in these processes. To our knowledge, the influence of MMP peptide specificity and protease-mediated scaffold degradation rate on neovascularization and tissue formation in vivo has yet to be elucidated.
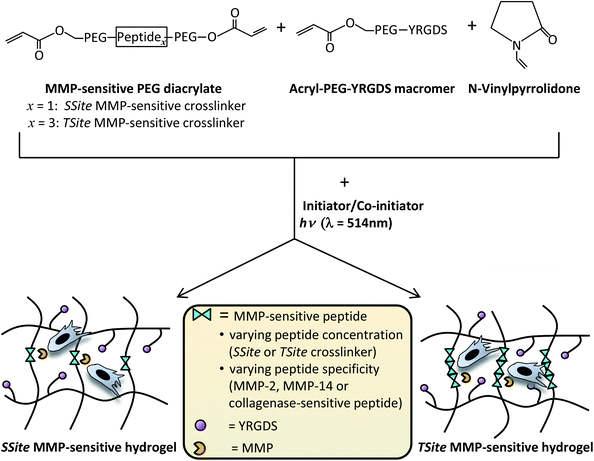
In our previously published study, we have shown that collagenase-sensitive peptide concentration in PEG hydrogels can be altered independent of changes in the degree of hydrogel crosslinking through the use of PEGDA crosslinking macromers of similar molecular weight and content, but with a variable number of protease-sensitive peptide repeats between the terminal acrylate groups of PEG.7 This methodology results in alterations in hydrogel degradation rate without inducing changes in the initial scaffold modulus, swelling ratio and network mesh size.7 The goal of the present study was to investigate the effects of MMP-sensitive peptide concentration and specificity on scaffold neovascularization and tissue remodeling. Peptide sequences previously optimized for cleavage by MMP-2 and MMP-14 enzymes were separately incorporated in single (SSite, one cleavable peptide sequence) or multiple (TSite, multiple cleavable peptide sequences) repeats between network crosslinks in PEG scaffolds in order to vary degradation rates independent of mechanical properties and evaluated for (1) sensitivity to degradation by MMP-2 and MMP-14, (2) neovascularization response in vitro, and (3) neovascularization and tissue remodeling in vivo (Fig. 1). Additionally, SSite and TSite PEG hydrogels containing a general collagenase-sensitive peptide sequence cleavable by various MMPs previously utilized in a variety of tissue engineering studies9–11,14 were used as comparative matrices.
Experimental
Peptide synthesis, design, and purification
The MMP-sensitive peptide sequences containing either one (SSite) or three protease-sensitive cleavage site repeats (TSite), previously identified for cleavage by MMP-2 (GDGIPVS↓LRSGGK; ↓ indicates cleavage site between amino acid residues), MMP-14 (GDIPES↓LRAGGK)15 and a general collagenase sensitive sequence (GGL↓GPAGGK) were synthesized by solid-phase peptide synthesis using a Focus Xi model (AAPPtec, Louisville, KY) with standard FMOC chemistry. Amino acids were coupled with a N,N-diisopropylethylamine (DIEA) and O-benzotriazole-N,N,N′,N′-tetramethyl-uronium-hexafluoro-phosphate (HBTU) mixture. The FMOC group was deprotected with 20% piperidine in N,N-dimethylformamide (DMF). Peptides were cleaved from the resin and deprotected with trifluoroacetic acid (TFA)–triisopropylsilane–ddH2O (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5
2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5). Peptides were precipitated in cold diethyl ether and desiccated overnight. The dried products were dissolved in ddH2O and lyophilized. Peptide molecular weights were confirmed by MALDI-TOF mass spectrometry and were purified by reverse-phase high-performance liquid chromatography to obtain purities of >95%. The final products were lyophilized and stored at −80 °C. All amino acids, HBTU, DMF, and TFA were purchased from AAPPtec (Louisville, KY). DIEA, triisopropylsilane, and diethyl ether were purchased from Fisher Scientific (Hanover Park, IL). Piperidine was purchased from Sigma (St. Louis, MO). The resulting SSite and TriSite peptide sequences were designed to yield comparable net charge and isoelectric point as indicated in Table 1.
2.5). Peptides were precipitated in cold diethyl ether and desiccated overnight. The dried products were dissolved in ddH2O and lyophilized. Peptide molecular weights were confirmed by MALDI-TOF mass spectrometry and were purified by reverse-phase high-performance liquid chromatography to obtain purities of >95%. The final products were lyophilized and stored at −80 °C. All amino acids, HBTU, DMF, and TFA were purchased from AAPPtec (Louisville, KY). DIEA, triisopropylsilane, and diethyl ether were purchased from Fisher Scientific (Hanover Park, IL). Piperidine was purchased from Sigma (St. Louis, MO). The resulting SSite and TriSite peptide sequences were designed to yield comparable net charge and isoelectric point as indicated in Table 1.
| MMP sensitivity | Cleavage site | Peptide sequence | Molecular weight (g mol−1) | Isoelectric point | Net charge (pH 7.0) |
|---|---|---|---|---|---|
| MMP-2 | SSite | GDFIPVS↓LRSGGK | 1242.4 | pH 10.09 | 1 |
| TSite | GDGIPVS↓LRSGDGIPVS↓LRSGDGIPVS↓LRSGGK | 3206.6 | pH 10.08 | 1 | |
| MMP-14 | SSite | GDGIPES↓LRAGGK | 1256.4 | pH 7.01 | 0 |
| TSite | GDGIPES↓LRA GDGIPES↓LRA GRGIPES↓LRAGGK | 3289.7 | pH 7.23 | 0 | |
| Collagenase | SSite | GGL↓GPAGGK | 712.8 | pH 10.01 | 1 |
| TSite | GGL↓GPAGDGL↓GPAGRGL↓GPAGGK | 1889.1 | pH 10.09 | 1 |
Synthesis of MMP-sensitive PEGDA macromers
PEGDA hydrogels were rendered degradable by the covalent incorporation of engineered peptides (Table 1) containing a single MMP-sensitive cleavage site repeat (SSite gels) or three MMP-sensitive cleavage site repeats (TSite gels) as described above. Peptides were conjugated to acrylate-PEG-succinimidyl-valerate (acrylate-PEG-SVA, molecular weight (MW) 5000 Da, Laysan Bio, Arab, AL) in 50 mM NaHCO3 (pH 8.0) in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PEG
1 PEG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) peptide molar ratio as previously described.7 The resulting macromer molecular weights and notation of the various MMP-sensitive PEGDA crosslinkers are presented in Table 2.
peptide molar ratio as previously described.7 The resulting macromer molecular weights and notation of the various MMP-sensitive PEGDA crosslinkers are presented in Table 2.
| MMP sensitivity | Cleavage site | PEGDA substrate notation | Molecular weight (g mol−1) |
|---|---|---|---|
| MMP-2 | SSite | M2S | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 242.4 242.4 |
| TSite | M2T | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 206.6 206.6 |
|
| MMP-14 | SSite | M14S | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 256.4 256.4 |
| TSite | M14T | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 289.6 289.6 |
|
| Collagenase | SSite | LGPAS | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 712.8 712.8 |
| TSite | LGPAT | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 889.1 889.1 |
PEGDA hydrogel formation
Hydrogel precursor solutions were prepared in 1× phosphate-buffered saline (PBS) (pH 7.4) with 37 mM N-vinylpyrrolidone (NVP), 0.05 mM of the photosensitive dye, eosin Y, 225 mM of the co-initiator, triethanolamine (TEA), with final MMP-2-sensitive, MMP-14-sensitive, or collagenase-sensitive SSite or TSite PEGDA crosslinker concentrations of 3% (weight/volume (w/vol)) and 15 mg mL−1 acrylate-PEG-YRGDS (MW = 3400 Da). Precursor solutions (100 μL per hydrogel) were photopolymerized by exposure to visible light (λ = 514 nm) for 0.25 min using an Argon Ion Laser (Coherent, Inc., Santa Clara, CA) at a laser flux of 100 mW cm−2. All chemicals were purchased from Sigma. Fig. 1 displays a schematic of the crosslinking chemistry used for synthesis of hydrogels containing varying MMP-sensitive peptide concentration and/or specificity.Evaluation of hydrogel mechanical properties
Compression experiments were conducted using a TA RSA3 mechanical tester (TA Instruments, New Castle, DE) controlled by TA Orchestrator software. PEGDA hydrogels were allowed to reach equilibrium swelling for 24 h prior to mechanical testing. Swollen hydrogels were compressed at a constant strain rate of 0.5 mm min−1 using a 10 N load cell. The compressive modulus was calculated from the slope of the linear region of the stress versus strain curve at less than 10% strain (r2 > 98%), as previously reported.17,18Quantification of hydrogel degradation kinetics
The various MMP-sensitive PEGDA substrates presented in Table 2 were evaluated for their sensitivity to degradation by MMP-2 and MMP-14 enzymes. Hydrogels were prepared as described above, allowed to reach equilibrium swelling for 24 hours prior to enzyme incubation. Enzyme solutions were prepared at a 1 nM enzyme concentration in degradation buffer (100 mM Tricine, 200 mM NaCl, 10 mM CaCl2, 0.05% Brij-35 at pH 7.5). Each hydrogel was incubated in 250 μL enzyme solution at 37 °C and the wet weight was monitored over time. Fresh enzyme solution was added every 24 hours. All enzymes were purchased from EMD Millipore (Billerica, MA).Cell maintenance
Human umbilical vein endothelial cells (HUVECs) were maintained in endothelial basal media supplemented with 2% fetal bovine serum, bovine brain extract, epidermal growth factor (EGF), hydrocortisone, ascorbic acid, and gentamicin. Human umbilical artery smooth muscle cells (HUASMCs) were maintained in smooth muscle basal media supplemented with 5% fetal bovine serum, insulin, fibroblast growth factor (FGF), EGF, and gentamicin. Cells were cultured in an incubator at 37 °C and 5% CO2. Both HUVECs and HUASMCs used in the experiments were between passages 5 and 10. Both cell lines and cell culture reagents were purchased from Lonza (Walkersville, MD).In vitro neovascularization model
A previously established in vitro assay consisting of a co-culture of HUVECs and HUASMCs was used to assess network invasion within PEGDA hydrogels.7,8,19 Co-culture cell spheroids were prepared with 2500 HUVECs and 2500 HUASMCs in endothelial basal media supplemented with 2% fetal bovine serum, bovine brain extract, EGF, hydrocortisone, ascorbic acid, and gentamicin containing 0.24% (w/v) carboxymethylcellulose (Sigma). Spheroids were seeded into a nonadherent 96-well round bottom plate at 37 °C and 5% CO2 for 24 hours. The resulting spheroids were encapsulated in the hydrogels by placing the spheroid in the prepolymer solution and polymerizing into the hydrogel network. Spheroids were imaged at week 0 (24 h post-encapsulation), 1, 2, and 3 with an Axiovert 200 inverted microscope using a 5× objective (1.255 μm per pixel) (Carl Zeiss MicroImaging, Inc., Thornwood, NY). Vessel invasion into the surrounding hydrogels was monitored by tracing the projected area of the spheroid using AxioVision 4.5 Image Analysis software.Sub-cutaneous rat implant model
All experiments were carried out at Edward Hines, Jr. VA hospital with procedures approved by the Institutional Animal Care and Use Committee (IACUC). SSite and TSite MMP-sensitive PEGDA hydrogels degradable by MMP-2 (M2S, M2T), MMP-14 (M14S, M14T), and collagenase enzyme (LGPAS, and LGPAT) were investigated for their ability to promote tissue invasion and vascularization in vivo. A subcutaneous rat implant model was used as previously published.20 Male Lewis rats (300–400 grams, n = 5, Charles River) were anesthetized with 5% isofluorane via a nose cone and maintained at 2–4% isofluorane/35% oxygen mixture during the procedure. Hydrogel implants were placed in a polypropylene shell in the shape of a top hat with a 4 mm height, 10.5 mm diameter, and a ring extending from the open end of the hat that was used for suturing. The polypropylene shells are biocompatible, induce a low inflammatory response and allow for unidirectional tissue ingrowth with the implanted biomaterial enabling quantification of depth of tissue invasion from the muscle wall. All shells were sterilized prior to animal surgeries. Hydrogels were prepared to fit the size of the shells and implanted subcutaneously in rats posterior to the shoulder blades along the dorsal muscle wall. Each rat received 6 hydrogel implants with a randomly determined implant location. Polypropylene shells containing the hydrogels were sutured with 4 sutures through the sewing ring extending from the top hat. Five animals were used per time point. All animals were housed in individual cages post-surgery. After 1 and 3 weeks post implantation, 100 μg Alexa Fluor 647-conjugated isolectin (Invitrogen) was injected through the tail vein of the animal. Animals were perfusion fixed with 4% paraformaldehyde and the implants were harvested.Harvested samples included the entire hydrogel and the underlying muscle. Samples were cut into 2 symmetric pieces. One half of the harvested tissue was embedded in Optimal Cutting Temperature (OCT), which was frozen, cut into 20 μm sections and used for imaging and analysis of vessel density. The other half was paraffin embedded, sectioned at 10 μm sections and used for histological staining of hematoxylin and eosin (H&E). Sections were cut through the center of the sample and contained a cross section of the harvested tissue, including the implanted hydrogel and underlying muscle wall interface.
Histology
To evaluate depth of tissue invasion and general histological structure, paraffin embedded tissue sections were stained for hematoxylin and eosin (H&E). Stained sections were imaged with a 5× objective using an Axiovert 200 inverted microscope (1.26 μm per pixel). To quantify depth of invasion, each tissue section was divided into three areas. The maximum depth of tissue ingrowth from the underlying muscle wall to the end of tissue ingrowth was measured in each area and used to calculate the average depth of invasion.Analysis of in vivo vasculature
For visualization and analysis of microvasculature, frozen sections that were previously perfused with isolectin before harvesting were imaged using a PASCAL Laser scanning microscope (LSM) system (633 nm excitation, 650 nm long-pass filter) from Carl Zeiss MicroImaging, Inc. (Thornwood, NY) with a 10× objective (1.8 μm per pixel). Isolectin is used to stain endothelial cells thus allowing for direct analysis of microvasculature. Vessel density was quantified from the captured images closest to the muscle hydrogel interface and calculated based on the following expression:Statistical analysis
All data are presented as mean ± standard deviation (S.D). To determine statistical significance between groups, analysis of variance (ANOVA) was performed followed by Tukey's HSD test for multiple comparisons (SigmaStat 3.5). In all cases, probability (p) values <0.05 were considered statistically significant.Results
Scaffold characterization
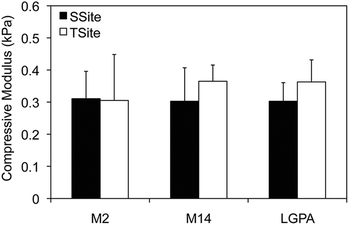
MMP-sensitive PEGDA hydrogels were engineered with peptide sequences identified for cleavage by MMP-2, MMP-14, and collagenase enzyme as identified in Table 1. Peptide substrates were chosen from a previously published study which optimized the cleavage site motifs of these peptide sequences for degradation by MMP-2 and MMP-14 enzymes.15 Specifically, these sequences have been previously tested for their susceptibility to degradation by multiple enzymes of the MMP family and showed increased catalytic activity for the targeted enzymes MMP-2 and MMP-14. This specificity, however, has only been evaluated for the peptide sequences themselves, but has not, to our knowledge, been investigated for its effect on scaffold degradability in response to these enzymes. The three peptide sequences were synthesized in a single cleavage site peptide form (SSite) and a multiple cleavage site peptide repeat form (TSite) as identified in Table 1. Each of these peptide sequences were conjugated to acrylate-PEG-SVA in a single step conjugation resulting in the synthesis of SSite and TSite PEGDA protease-sensitive crosslinking macromers identified in Table 2. The protease-sensitive crosslinking macromers were designed to be of similar molecular weight to allow for an equivalent degree of crosslinking at fixed crosslinker content which upon photopolymerization results in the formation of hydrogel networks that incorporate MMP-2, MMP-14, or collagenase-sensitive peptide sequences as well as varying protease-sensitive peptide concentrations (Fig. 1).To demonstrate that the concentration of cleavage sites and incorporation of the different MMP-sensitive peptide substrates does not result in changes in crosslinking and therefore in the resultant mechanical properties of the hydrogels, scaffolds were characterized using compression experiments (Fig. 2). No statistical significant differences in compressive modulus were found to occur between SSite and TSite hydrogel groups or between hydrogels modified with the different MMP-sensitive peptide sequences.
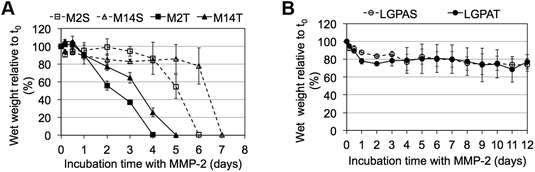
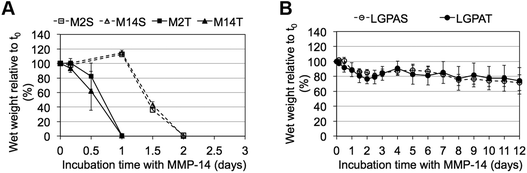
MMP-sensitive PEGDA hydrogels were further evaluated for their susceptibility to degradation by MMP-2 and MMP-14 enzymes. Hydrogels were incubated in 1 nM enzyme solution and the change in the wet weight was monitored over time with enzyme changes every 24 hours. Hydrogels synthesized with SSite MMP-substrates identified for cleavage by MMP-2 enzyme (MMP-2S) degraded within 6 days of MMP-2 enzyme incubation while the MMP-14 substrates (MMP-14S) demonstrated an increased degradation time of 7 days (Fig. 3). Hydrogels fabricated with TSite peptide substrates exhibited decreases in scaffold degradation time (more rapid degradation) as compared to their SSite counterparts while maintaining the overall sensitivity of the peptide substrates. TSite gels with substrates identified for cleavage by MMP-2 (M2T) underwent complete hydrogel degradation in 4 days while the MMP-14 (M14T) sensitive substrate had completely degraded by 5 days (Fig. 3A). Interestingly, in the case of the collagenase-sensitive PEGDA substrates, both SSite (LGPAS) and TSite (LGPAT) forms did not achieve complete hydrogel degradation, with ∼80% of the hydrogels remaining after 12 days of MMP-2 incubation (Fig. 3B). MMP-2 and MMP-14-sensitive hydrogels displayed relatively similar degradation profiles when incubated with MMP-14 enzyme regardless of incorporated peptide concentration (Fig. 4). The corresponding TSite hydrogels underwent complete degradation after 1 day while the SSite hydrogels underwent complete degradation in 2 days (Fig. 4A). Similar to results upon exposure to MMP-2, the LGPAS and LGPAT hydrogel groups degraded to ∼20% of the initial weight with 80% of the hydrogels remaining after 12 days of MMP-14 enzyme incubation. Estimates for times required to achieve 50% material degradation (t50%) for all hydrogel substrates are reported in Table 3.
| A | ||
|---|---|---|
| Substrate | t 50% SSite (days) | t 50% TSite (days) |
| M2 | 5.1 | 2.3 |
| M14 | 6.4 | 3.4 |
| LGPA | N.D. | N.D. |
| B | ||
|---|---|---|
| Substrate | t 50% SSite (days) | t 50% TSite (days) |
| N.D., not detected. | ||
| M2 | 1.4 | 0.7 |
| M14 | 1.5 | 0.6 |
| LGPA | N.D. | N.D. |
In vitro neovascularization within MMP-sensitive PEGDA hydrogels
The neovascularization assay utilized in this study involves a spheroidal co-culture of a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
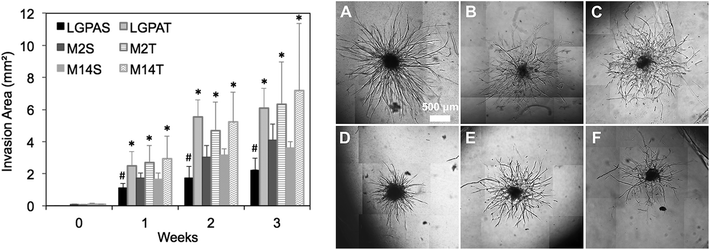
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 ratio of human umbilical vein endothelial cells (ECs) and human arterial smooth muscle cells (HUASMCs) in order to recapitulate EC–mural cell interactions occurring during neovascularization. In previous studies, we have confirmed that both cell types participate in the formation of vascular sprouts within MMP-sensitive hydrogels and in coordinated EC–SMC interactions.8 In the present study, hydrogels were formed using macromers that contained peptides with one (SSite) or three (TSite) MMP-sensitive cleavage site repeats between crosslinks and with different MMP enzyme sensitivities. MMP-2, MMP-14 and collagenase-sensitive peptide sequences were synthesized in SSite and TSite form, conjugated to PEGDA, copolymerized with 15 mg mL−1 acrylate-PEG-YRGDS, and investigated as matrices for inducing neovascularization. Cell co-culture spheroids were encapsulated in the MMP-sensitive PEGDA hydrogels during photopolymerization and cultured over a time course of 3 weeks. Vessel invasion was quantified as a function of cleavage site concentration and MMP substrate sensitivity. Quantitative analysis of vessel invasion areas of SSite and TSite MMP-sensitive PEGDA hydrogels as well as representative images of cell invasion within hydrogels formed with different MMP-sensitive peptide substrates are shown in Fig. 5. All hydrogel groups supported vessel invasion over the 3 week time course with significant increases over time. Within the SSite hydrogel groups, statistical significant decreases in vessel invasion area were noted in scaffolds containing the general collagenase-sensitive substrate (GGL↓GPAGGK) as compared to invasion occurring within scaffolds incorporating the more specific MMP-sensitive substrates. No statistical significant differences in invasion areas were noted among the TSite hydrogel groups. However, by week 3 an increase in invasion was noted in TSite scaffolds modified with the more specific peptide substrates (M2 and M14) as compared to TSite hydrogels containing the general LGPA peptide even though these increases were not found to be significant. In addition, there was a statistical significant increase in invasion in all TSite hydrogel groups as compared to SSite hydrogel groups.
50 ratio of human umbilical vein endothelial cells (ECs) and human arterial smooth muscle cells (HUASMCs) in order to recapitulate EC–mural cell interactions occurring during neovascularization. In previous studies, we have confirmed that both cell types participate in the formation of vascular sprouts within MMP-sensitive hydrogels and in coordinated EC–SMC interactions.8 In the present study, hydrogels were formed using macromers that contained peptides with one (SSite) or three (TSite) MMP-sensitive cleavage site repeats between crosslinks and with different MMP enzyme sensitivities. MMP-2, MMP-14 and collagenase-sensitive peptide sequences were synthesized in SSite and TSite form, conjugated to PEGDA, copolymerized with 15 mg mL−1 acrylate-PEG-YRGDS, and investigated as matrices for inducing neovascularization. Cell co-culture spheroids were encapsulated in the MMP-sensitive PEGDA hydrogels during photopolymerization and cultured over a time course of 3 weeks. Vessel invasion was quantified as a function of cleavage site concentration and MMP substrate sensitivity. Quantitative analysis of vessel invasion areas of SSite and TSite MMP-sensitive PEGDA hydrogels as well as representative images of cell invasion within hydrogels formed with different MMP-sensitive peptide substrates are shown in Fig. 5. All hydrogel groups supported vessel invasion over the 3 week time course with significant increases over time. Within the SSite hydrogel groups, statistical significant decreases in vessel invasion area were noted in scaffolds containing the general collagenase-sensitive substrate (GGL↓GPAGGK) as compared to invasion occurring within scaffolds incorporating the more specific MMP-sensitive substrates. No statistical significant differences in invasion areas were noted among the TSite hydrogel groups. However, by week 3 an increase in invasion was noted in TSite scaffolds modified with the more specific peptide substrates (M2 and M14) as compared to TSite hydrogels containing the general LGPA peptide even though these increases were not found to be significant. In addition, there was a statistical significant increase in invasion in all TSite hydrogel groups as compared to SSite hydrogel groups.
Neovascularization and tissue remodeling within MMP-sensitive PEGDA hydrogels in vivo
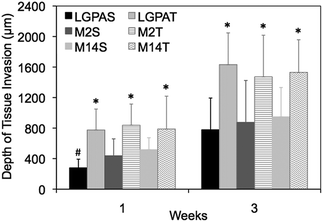
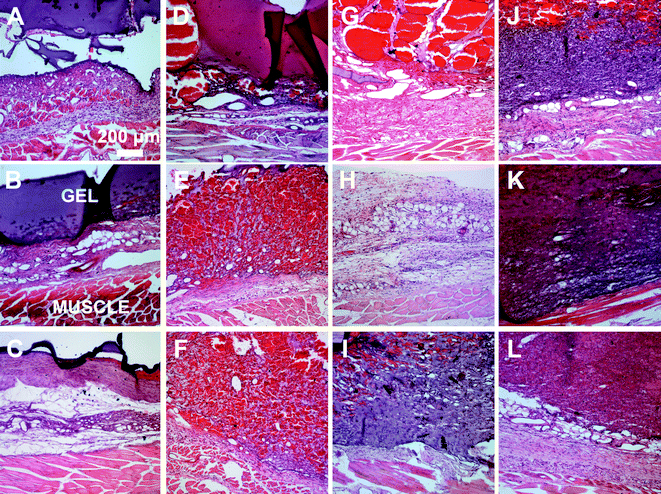
A subcutaneous rat implantation model was used to evaluate in vivo neovascularization and tissue remodeling within MMP-sensitive PEGDA hydrogels. For the in vivo studies, SSite and TSite MMP-sensitive PEGDA hydrogels were fabricated with MMP-2, 14 and collagenase sensitive peptide substrates. All hydrogels were formed with 15 mg mL−1 acrylate-PEG-YRGDS. Hydrogels were implanted in polypropylene shells subcutaneously in rats as previously described.20 At weeks 1 and 3, rats were injected with lectin for visualization of microvasculature upon which the hydrogel implants were harvested, sectioned and processed for histological staining.H&E staining was used for analysis of tissue invasion and general tissue histology. The greatest depth of tissue invasion from the underlying muscle was quantified for each sample (Fig. 6). Representative images of the H&E stained tissue sections are presented in Fig. 7. Similar to the in vitro findings, there was a statistical significant increase in tissue invasion in all TSite hydrogel groups as compared to SSite hydrogels. There were no statistical significant differences in invasion among the different MMP substrates within the TSite hydrogel group. The LGPAS peptide hydrogels had significantly lower tissue invasion as compared to the other more specific MMP-sensitive hydrogels at week 1 while there were no statistical significant differences in invasion noted by week 3. There were statistical significant increases in tissue invasion between weeks 1 and 3.
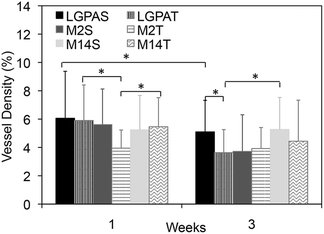
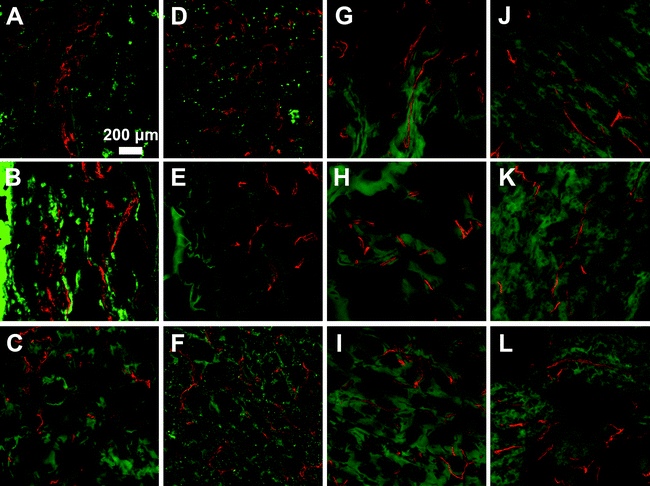
Microvasculature within the harvested tissue samples was visualized from tissue sections stained with lectin and imaged with confocal microscopy. For quantitative analysis of vessel density, images were captured close to the muscle interface and the lectin positive area was compared to the total tissue area (Fig. 8 and 9). In general, vessel density appeared to decrease from weeks 1 to 3, although this was not found to be statistically significant. There were no statistical significant differences in the vessel density between SSite and TSite hydrogel groups. At week 1, within the TSite hydrogel group, M2T hydrogels demonstrated significantly lower vessel density compared to the LGPAT and M14T hydrogel groups. There were no statistical differences noted at week 3. The MMP-14-sensitive hydrogels appeared to have the least decrease in vessel density from weeks 1 to 3 within both the SSite and TSite scaffold groups.
Discussion
Matrix metalloproteinases (MMPs) play an important role in regulating neovascularization and tissue remodelling. Their proteolytic activity is essential for the breakdown of ECM, cellular infiltration, lumen formation, and ECM synthesis. Among all enzymes of the MMP family, MMP-2 is primarily responsible for the degradation of the basement membrane and its activation is highly dependent on the presence of MMP-14 at the cell surface. The mechanism of action of MMP-14 is cleavage of ECM molecules, production of angiogenic factors, including VEGF, interaction with cell surface molecules, including CD44 and sphingosine-1-phosphate (S1P), and the degradation of decorin, an anti-angiogenic factor.21 Additionally, MMP-14 is known for its importance in regulating endothelial cell function including migration and lumen formation.3 The complex interaction of these MMPs is important in guiding vessel formation and tissue remodelling.PEG hydrogels are widely used as scaffolding materials because they can be easily modified to mimic key elements of the ECM. To render the hydrogels degradable by proteolytic mechanisms, collagen-based protease-sensitive substrates are commonly incorporated into the backbone of PEG. However, these peptide substrates exhibit slow cleavage rates and their incorporation into scaffolds results in longer rates of degradation thus limiting in vivo cell invasion, vascularization and neo-tissue formation. Recent biomaterial approaches have focused on enhancing the degradation rate of proteolytically degradable scaffolds. In a previous study, we have shown that the concentration of proteolytic cleavage sites incorporated into PEG hydrogel scaffolds has a profound impact on the rate of protease-mediated degradation and vascularization in vitro.7 This was achieved by synthesizing PEG diacrylate crosslinking macromers of similar molecular weight that contained either single (SSite) or multiple (TriSite) repeats of a collagenase-sensitive peptide sequence (GGLG↓PAGGK) between the terminal acrylate groups of PEG. Each of these protease-sensitive crosslinkers were subsequently polymerized with similar crosslinker content using free-radical photopolymerization to form hydrogel scaffolds that contained varying numbers of cleavable peptide sequences between network crosslinks but of similar crosslink density allowing for independent tuning of matrix mechanics and protease mediated degradation rate. Increases in proteolytic cleavage site concentration in hydrogels resulted in significant increases in the rate and extent of vascular network invasion in vitro over a broad range of biomaterial stiffness.7 Other strategies for enhancing the rates of proteolytic degradation in scaffolds have focused on incorporating peptide substrates with higher catalytic activity and specificity to cleavage by specific enzymes based on data available from combinatorial methods of peptide libraries that screened the sequence site specificity of MMPs.15 In one study, PEG hydrogels formed using Michael-type addition were engineered with peptides that had increased catalytic activity and substrate cleavage by MMP-1 and MMP-2 enzymes. These substrates led to enhanced degradation, cell spreading and proliferation in fibroblasts and sprout formation from implanted aortic ring segments.14 In a more recent study, use of specific MMP-sensitive peptides preferentially cleaved by MMP-14 and MMP-9 were crosslinked into synthetic scaffolds to control the in vitro invasion of different cell types.16 Despite the ability of these more specific peptide sequences to enhance scaffold degradation times as well as regulate matrix invasion of specific cell types in vitro, the in vivo translation of these substrates for the promotion of vascularized tissue formation requires further study due to the complex nature of this process which involves the interaction of multiple cell types with the ECM mimetic scaffold. To our knowledge, the role of peptide specificity and concentration on scaffold vascularization and tissue remodelling in vivo has not been previously explored.
In the present study, peptide substrates previously reported to be optimized for cleavage by MMP-2 and MMP-14 enzymes based on data obtained from combinatorial methods of peptide libraries were selected and incorporated into PEG scaffolds to determine the effects of peptide specificity and concentration on neovascularization and tissue remodelling in vivo. Variations in MMP-sensitive peptide concentration and/or specificity in scaffolds were achieved without inducing changes in mechanical properties of the biomaterial by synthesizing PEGDA macromers with one (SSite) or multiple (TSite) cleavage sites and with either MMP-2, MMP-14, or a general collagenase sensitive peptide sequence. Upon photopolymerization, these peptide substrates result in the formation of crosslinked hydrogel networks with different susceptibility to cleavage by MMP-2 or MMP-14, as well as varying peptide concentration as identified in Table 2.
Hydrogels were first characterized using compression experiments to ensure that the concentration of cleavage sites and incorporation of different MMP-sensitive peptide sequences did not result in alterations in the mechanical properties of the scaffolds. There were no statistical significant differences found in the compressive modulus of the SSite and TSite hydrogel groups or in scaffolds crosslinked with the different MMP-sensitive peptide substrates (Fig. 2).
Scaffolds were also characterized for their susceptibility to degradation by MMP-2 and MMP-14 enzymes. Interestingly, hydrogels containing the general collagenase-sensitive peptide sequence (GGL↓GPAGGK) had low sensitivity to degradation by MMP-2 and MMP-14 regardless of whether the sequence was presented in SSite or TSite form (Fig. 3B and 4B). This collagenase-sensitive peptide sequence has been designed based on the cleavage site in the α-1 chain of collagen type I and has been extensively used to promote protease-mediated degradation of PEG scaffolds to enable the invasion of multiple cell types.7,9,11,22 Degradation studies with hydrogel scaffolds incorporating this peptide sequence have been conducted in the presence of interstitial collagenase (MMP-1) which has been identified to cleave a variety of different types of ECM proteins also cleavable by MMP-2.23 Based on the results of the present study, hydrogels containing the collagenase-sensitive sequence were not capable of achieving more than 20% material degradation after 12 days of enzyme incubation in response to MMP-2 and MMP-14. Therefore, this peptide sequence has low catalytic activity and specificity to these enzymes; however, the susceptibility of this sequence to cleavage by other enzymes of the MMP family requires further exploration.
Incubation with MMP-2 or MMP-14 enzyme showed that the scaffolds that included the more specific MMP-sensitive substrates exhibited degradation kinetics that were dependent on both peptide concentration and specificity (Fig. 3A and 4A). TSite gels containing the MMP-2 sensitive peptide sequence exhibited faster degradation kinetics (M2T, 4 days) than those containing the MMP-14 sensitive peptide sequence (M14T, 5 days) in the presence of MMP-2 enzyme. The corresponding SSite M2S and M14S gels degraded more slowly with complete degradation achieved by 6 and 7 days, respectively (Fig. 3). Similar results in regards to SSite versus TSite hydrogels were found to occur with MMP-14 enzyme incubation. However, in this case, degradation kinetics depended only on peptide concentration rather than specificity with TSite gels reaching complete hydrogel degradation in 1 day as compared to 2 days in SSite gels (Fig. 4A). Table 3 displays the extrapolated times required to reach 50% scaffold degradation for all substrates. This further corroborates the fact that the in the case of the more specific substrates, peptide concentration has a substantial impact on scaffold degradation rate as compared to the general collagenase-sensitive peptide sequence.
Hydrogels were further evaluated for their vascular and tissue remodelling capabilities in vitro and in vivo. In vitro data showed no statistical significant differences in invasion between hydrogel groups synthesized using the different MMP-sensitive peptide substrates (Fig. 5). In vitro neovascularization, however, was found to increase with increases in cleavage site concentration (TSite gels), which may result from the enhancements in scaffold degradation rates achieved in TSite gels. Within the SSite hydrogel groups, the less specific LGPA peptide substrate had significantly lower vessel invasion as compared to the SSite hydrogel groups that incorporated the more specific MMP-sensitive substrates. In vivo tissue invasion results were consistent with in vitro vessel invasion (Fig. 5 and 6). Invasion increased from weeks 1 to 3 and increased with increasing cleavage site concentration (TSite gels). SSite hydrogels with the LGPA peptide substrate exhibited significantly lower tissue invasion as compared to gels made with the other MMP-sensitive peptide substrates at week 1 with no differences in invasion observed by week 3. This suggests that in vitro vessel invasion and in vivo tissue invasion and remodelling are dependent on the rates of scaffold degradation with decreases in scaffold degradation times leading to increases in cellular invasion. In vitro results also demonstrate that even though the collagenase-sensitive peptide substrates (LGPAS and LGPAT) had low sensitivity to degradation by MMP-2 and 14, these scaffolds resulted in invasion in vitro and in vivo. Furthermore, increasing collagenase-sensitive peptide concentration (LGPAT) in scaffolds resulted in similar invasion levels in vitro and in vivo as compared to scaffolds modified with the more specific peptide substrates, suggesting that cells, which facilitate invasion and tissue remodelling, express other enzymes that degrade this sequence. Identification of these enzymes during these processes requires further investigation.
Analysis of the microvasculature formed within the implanted hydrogels shows that all hydrogel groups supported vessel formation (Fig. 8 and 9) regardless of cleavage site concentration or the type of MMP-sensitive peptide substrate. From quantitative analysis, there were no significant differences in vessel density between the SSite and TSite hydrogel groups even though significant differences in tissue invasion were found to occur (Fig. 8). Higher vessel density at week 1 as compared to week 3 may be due to the presence of an inflammatory response occurring at week 1, although further studies are required to confirm this phenomenon. In addition, even though the LGPA peptide substrates had low sensitivity to degradation by MMP-2 and 14 enzymes, which are critical regulators of neovascularization, the inclusion of the collagenase substrates in hydrogels still supported the formation of vessels.
Previous studies have explored MMP-sensitive PEG hydrogels for their ability to induce vascularization.4,5,24 These hydrogel systems have induced neovascularization in the presence of growth factors, such as vascular endothelial growth factor (VEGF)4,6,25 or growth factor combinations.26 In these previous studies, however, the effect of cleavage site concentration or the specificity of the MMP-sensitive peptide substrates has not been explored in vitro or in vivo. In addition, PEG hydrogel systems have not been widely investigated for their ability to promote vascularization in the absence of growth factors in vivo. In this work, it was demonstrated that cleavage site concentration plays an important role in enhancing in vitro vascularization and in vivo tissue invasion and remodelling. Furthermore, the sensitivity and specificity of MMP-sensitive peptide substrates were screened for cleavage by MMP-2 and MMP-14 enzymes as well as for their role in neovascularization and tissue remodelling.
Conclusions
In this study, hydrogels synthesized to contain peptide substrates sensitive to cleavage by MMP-2, MMP-14, and collagenase enzyme were evaluated for their sensitivity and specificity to degradation by MMPs. The effects of peptide specificity and concentration were also investigated for their ability to induce scaffold neovascularization and tissue remodelling. The presented approach allows for the incorporation of varying cleavage site concentration and MMP-sensitive peptide substrates into PEG scaffolds for enhancing protease-mediated scaffold degradation without inducing alterations in the mechanical or physical properties of the hydrogels. In addition, it was demonstrated that in the absence of growth factors, cleavage site concentration has a profound impact in enhancing scaffold vascularization in vitro and tissue invasion in vivo. Regardless of the MMP-sensitive peptide sequence incorporated into the scaffold, vascularization and tissue invasion were supported in all MMP-sensitive hydrogel groups and were not found to be specific to a particular substrate. To our knowledge, this is the first study involved in screening MMP-sensitive peptide substrates for their sensitivity to degradation by MMP enzymes that play an important role in neovascularization and tissue remodelling inductive capabilities in vivo.Acknowledgements
This research was supported by the financial support of the National Heart, Lung, and Blood Institute of the National Institutes of Health (Award Number R21HL094916), the Veterans Administration and the Illinois Institute of Technology Educational Research Initiative Fund. The authors would like to thank the laboratory of Professor David Venerus in the Department of Chemical and Biological Engineering at the Illinois Institute of Technology for providing access for mechanical testing of our samples as well as the laboratory of Professor Joseph Orgel in the College of Science and Letters at the Illinois Institute of Technology for access to HPLC for peptide purification.Notes and references
- M. N. Nakatsu, R. C. A. Sainson, S. Pérez-del-Pulgar, J. N. Aoto, M. Aitkenhead and K. L. Taylor, et al., VEGF121 and VEGF165 Regulate Blood Vessel Diameter Through Vascular Endothelial Growth Factor Receptor 2 in an in vitro Angiogenesis Model, Lab. Invest., 2003, 83(12), 1873–1885 CrossRef CAS.
- H. Sung, C. Meredith, C. Johnson and Z. S. Galis, The effect of scaffold degradation rate on three-dimensional cell growth and angiogenesis, Biomaterials, 2004, 25(26), 5735–5742 CrossRef CAS PubMed.
- A. N. Stratman, W. B. Saunders, A. Sacharidou, W. Koh, K. E. Fisher and D. C. Zawieja, et al., Endothelial cell lumen and vascular guidance tunnel formation requires MT1-MMP-dependent proteolysis in 3-dimensional collagen matrices, Blood, 2009, 114(2), 237–247 CrossRef CAS PubMed.
- E. A. Phelps, N. Landazuri, P. M. Thule, W. R. Taylor and A. J. Garcia, Bioartificial matrices for therapeutic vascularization, Proc. Natl. Acad. Sci. U. S. A., 2010, 107(8), 3323–3328 CrossRef CAS PubMed.
- T. P. Kraehenbuehl, L. S. Ferreira, P. Zammaretti, J. A. Hubbell and R. Langer, Cell-responsive hydrogel for encapsulation of vascular cells, Biomaterials, 2009, 30(26), 4318–4324 CrossRef CAS PubMed.
- J. E. Leslie-Barbick, J. J. Moon and J. L. West, Covalently-immobilized vascular endothelial growth factor promotes endothelial cell tubulogenesis in poly(ethylene glycol) diacrylate hydrogels, J. Biomater. Sci., Polym. Ed., 2009, 20(12), 1763–1779 CrossRef CAS PubMed.
- S. Sokic and G. Papavasiliou, Controlled proteolytic cleavage site presentation in biomimetic PEGDA hydrogels enhances neovascularization in vitro, Tissue Eng., Part A, 2012, 18(23–24), 2477–2486 CrossRef CAS PubMed.
- M. V. Turturro, M. C. Christenson, J. C. Larson, D. A. Young, E. M. Brey and G. Papavasiliou, MMP-sensitive PEG diacrylate hydrogels with spatial variations in matrix properties stimulate directional vascular sprout formation, PLoS One, 2013, 8(3), e58897 CAS.
- J. L. West and J. A. Hubbell, Polymeric Biomaterials with Degradation Sites for Proteases Involved in Cell Migration, Macromolecules, 1998, 32(1), 241–244 CrossRef.
- S. H. Lee, J. S. Miller, J. J. Moon and J. L. West, Proteolytically degradable hydrogels with a fluorogenic substrate for studies of cellular proteolytic activity and migration, Biotechnol. Prog., 2005, 21(6), 1736–1741 CrossRef CAS PubMed.
- B. K. Mann, A. S. Gobin, A. T. Tsai, R. H. Schmedlen and J. L. West, Smooth muscle cell growth in photopolymerized hydrogels with cell adhesive and proteolytically degradable domains: synthetic ECM analogs for tissue engineering, Biomaterials, 2001, 22(22), 3045–3051 CrossRef CAS.
- M. P. Lutolf, J. L. Lauer-Fields, H. G. Schmoekel, A. T. Metters, F. E. Weber and G. B. Fields, et al., Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: engineering cell-invasion characteristics, Proc. Natl. Acad. Sci. U. S. A., 2003, 100(9), 5413–5418 CrossRef CAS PubMed.
- G. P. Raeber, M. P. Lutolf and J. A. Hubbell, Molecularly engineered PEG hydrogels: a novel model system for proteolytically mediated cell migration, Biophys. J., 2005, 89(2), 1374–1388 CrossRef CAS PubMed.
- J. Patterson and J. A. Hubbell, Enhanced proteolytic degradation of molecularly engineered PEG hydrogels in response to MMP-1 and MMP-2, Biomaterials, 2010, 31(30), 7836–7845 CrossRef CAS PubMed.
- B. E. Turk, L. L. Huang, E. T. Piro and L. C. Cantley, Determination of protease cleavage site motifs using mixture-based oriented peptide libraries, Nat. Biotechnol., 2001, 19(7), 661–667 CrossRef CAS PubMed.
- M. Bracher, D. Bezuidenhout, M. P. Lutolf, T. Franz, M. Sun and P. Zilla, et al. Cell specific ingrowth hydrogels, Biomaterials, 2013, 34(28), 6797–6803 CrossRef CAS PubMed.
- S. J. Bryant, T. T. Chowdhury, D. A. Lee, D. L. Bader and K. S. Anseth, Crosslinking density influences chondrocyte metabolism in dynamically loaded photocrosslinked poly(ethylene glycol) hydrogels, Ann. Biomed. Eng., 2004, 32(3), 407–417 CrossRef.
- B. J. DeKosky, N. H. Dormer, G. C. Ingavle, C. H. Roatch, J. Lomakin and M. S. Detamore, et al., Hierarchically designed agarose and poly(ethylene glycol) interpenetrating network hydrogels for cartilage tissue engineering, Tissue Eng., Part C, 2010, 16(6), 1533–1542 CrossRef CAS PubMed.
- Y. C. Chiu, M. H. Cheng, H. Engel, S. W. Kao, J. C. Larson and S. Gupta, et al., The role of pore size on vascularization and tissue remodeling in PEG hydrogels, Biomaterials, 2011, 32(26), 6045–6051 CAS.
- E. M. Brey, T. W. King, C. Johnston, L. V. McIntire, G. P. Reece and C. W. Patrick Jr., A technique for quantitative three-dimensional analysis of microvascular structure, Microvasc. Res., 2002, 63(3), 279–294 CrossRef PubMed.
- N. E. Sounni, A. Paye, L. Host and A. Noel, MT-MMPS as Regulators of Vessel Stability Associated with Angiogenesis, Front. Pharmacol., 2011, 2, 111 Search PubMed.
- S. Sokic and G. Papavasiliou, FGF-1 and proteolytically mediated cleavage site presentation influence three-dimensional fibroblast invasion in biomimetic PEGDA hydrogels, Acta Biomater., 2012, 8(6), 2213–2222 CrossRef CAS PubMed.
- N. Nagase and G. B. Fields, Human Matrix Metalloproteinase Specificity Studies Using Collagen Sequence-Based synthetic Peptides, Biopolymers, 1996, 40(4), 399–416 CrossRef.
- S. R. Peyton, C. B. Raub, V. P. Keschrumrus and A. J. Putnam, The use of poly(ethylene glycol) hydrogels to investigate the impact of ECM chemistry and mechanics on smooth muscle cells, Biomaterials, 2006, 27(28), 4881–4893 CrossRef CAS PubMed.
- S. R. Peyton, C. B. Raub, V. P. Keschrumrus and P. AJ, The use of poly(ethylene glycol) hydrogels to investigate the impact of ECM chemistry and mechanics on smooth muscle cells, Biomaterials, 2006, 27(28), 4881–4893 CrossRef CAS PubMed.
- J. E. Saik, D. J. Gould, E. M. Watkins, M. E. Dickinson and J. L. West, Covalently immobilized platelet-derived growth factor-BB promotes angiogenesis in biomimetic poly(ethylene glycol) hydrogels, Acta Biomater., 2011, 7(1), 133–143 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |