Multifunctional nanoprobes based on upconverting lanthanide doped CaF2: towards biocompatible materials for biomedical imaging†
Irene Xochilt
Cantarelli
a,
Marco
Pedroni
a,
Fabio
Piccinelli
a,
Pasquina
Marzola
b,
Federico
Boschi
b,
Giamaica
Conti
c,
Andrea
Sbarbati
c,
Paolo
Bernardi
c,
Elisa
Mosconi
c,
Luigi
Perbellini
d,
Laura
Marongiu
e,
Marta
Donini
e,
Stefano
Dusi
e,
Lorenzo
Sorace
f,
Claudia
Innocenti
f,
Elvira
Fantechi
f,
Claudio
Sangregorio
g and
Adolfo
Speghini
*a
aDipartimento di Biotecnologie, Università di Verona and INSTM, UdR Verona, Strada le Grazie 15, 37134, Verona, Italy. E-mail: adolfo.speghini@univr.it
bDipartimento di Informatica, Università di Verona and INSTM, UdR Verona, Strada le Grazie 15, 37134, Verona, Italy
cDipartimento di Scienze Neurologiche e del Movimento, Università di Verona, Piazzale L.A. Scuro 10, 37134, Verona, Italy
dDipartimento di Sanità Pubblica e Medicina di Comunità, Università di Verona, Piazzale L.A. Scuro 10, 37134, Verona, Italy
eDipartimento di Patologia e Diagnostica, Sezione di Patologia Generale, Università di Verona, Strada Le Grazie, 8, 37134, Verona, Italy
fINSTM and Dipartimento di Chimica “U. Schiff”, Università degli Studi di Firenze, via della Lastruccia 3-13, Sesto Fiorentino, 50019, Firenze, Italy
gICCOM-CNR & INSTM, via Madonna del Piano 10, 50019 Sesto Fiorentino, Italy
First published on 24th June 2014
Abstract
Water dispersible Gd3+,Yb3+,Er3+ and Gd3+,Yb3+,Tm3+ doped CaF2 nanoparticles (NPs) were prepared by one-pot hydrothermal synthesis using citrate ions as capping agents without the need for any post-synthesis reaction. UC emissions are easily observed in the visible and infrared regions upon NIR diode laser excitation at 980 nm. EPR spectroscopy confirms the substitutional nature of the rare-earth doping, while magnetometric studies reveal that the NPs have a useful magnetization. MRI experiments conducted in vivo show that after 40 min from the injection, the NPs localize in the liver and spleen. Electron microscopy images of liver tissue reveal that the NPs are located in the Kupffer cells, although a small amount is also found in the hepatocytes. An excitation with a 980 nm emission on the excised liver and epithelial tissue induces clearly visible UC emission. The local temperature upon 980 nm irradiation was monitored in situ and it was found to increase slowly with the exposure time, maintaining under 1–2 °C for less than 60 second exposure. The NPs show a low toxicity towards cultured HeLa cells and human primary dendritic cells (DCs), and did not induce pro-inflammatory cytokine secretion by cultured human DCs, indicating that the NPs do not cause relevant adverse reactions in immune cells. Therefore, the present NPs are suitable candidates to be efficiently used in surgery applications, where spatial resolution and lack of harmful effects on human health are important issues.
1. Introduction
Multimodal bioimaging is a challenging research frontier in biology and medicine, and is becoming a technique of paramount importance in medical diagnostics,1 with particular relevance in the early diagnosis of tumors.2–5 In this respect, nanostructured materials have a very important role because of their intrinsic small size that allows their easy incorporation as probes into living cells or organisms.1,6,7 The possible functionalization of the nanostructured materials further opens the possibility of guiding them towards well-defined targets in the living body, such as tumors or other localized lesions.2–5,8,9 In recent years, important efforts have been made to develop new nanostructured materials able to combine more than one imaging modalities, such as optical and magnetic resonance imaging (MRI), X-ray computed tomography (CT) and positron emission tomography (PET).10–13 In this context, multimodal probes combining magnetic and optical properties are particularly appealing. The MRI technique presents not only superb soft tissue contrast and good spatial resolution, but also high penetration depth since biological tissues are essentially transparent to magnetic fields.14 Moreover, the MRI tool can provide important information for clinical surgery in terms of images with excellent anatomical details that can be acquired in short time intervals, such as minutes or less. Nevertheless, drawbacks of MRI are related to limited sensitivity and poor resolutions for imaging at cellular levels. In contrast, optical imaging is characterized by high sensitivity and sub-micrometer resolution. Hence, nanostructured materials with both optical and MRI abilities can combine the advantages of each technique, in terms of imaging resolution and penetration depth.In this scenario, lanthanide based or doped nanostructured inorganic materials represent very interesting probes in medical diagnostics for their well-known luminescence and magnetic properties,9,15–21 deriving from their peculiar electronic structure. In the last few years, lanthanide doped oxide and fluoride nanoparticles (NPs) have been the subject of many investigations.9,19–25 Among these materials, lanthanide doped upconverting (UC) NPs (UCNPs) have gained attention in biomedical imaging due to their peculiar properties.26 The UC process, relevant to the Ln3+ ions, involves the conversion of low energy excitation radiation (typically near-infrared, NIR) to higher energies and can be obtained using inexpensive continuous wave diode NIR lasers (e.g. at 980 nm) as the excitation source. NIR-to-NIR or NIR-to-red UC is particularly suitable for biomedical imaging as both the exciting and emitted radiation lie on the so-called first biological window of tissue transparency (650–1000 nm), in which the absorption of the biological tissues is very low, therefore ensuring a high degree of optical penetration.27,28
Lanthanide ions are also useful as paramagnetic contrast agents, due to unpaired electrons in their 4f orbitals. Gd-based molecular complexes, such as Gd-DOTA or Gd-DTPA are among the most diffuse MRI contrast agents.29,30 On the other hand, fluoride and oxide nanostructures containing Gd3+ ions, such as Gd2O3,31–33 GdPO4,34,35 GdVO4,36 GdF3,11,12,37–39 NaGdF4,20,40 KGdF4,41,42 and also Gd3+ doped NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19,21,25,43 or NaLuF4
19,21,25,43 or NaLuF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44–46 are reported to have significant positive or negative MRI capabilities. UC properties can be added to such MRI active nanostructures using suitable combinations and concentrations of luminescent lanthanide ions (e.g. Er/Yb or Tm/Yb).26,44,47–55 We denote hereafter these nanostructured materials as MRI/UCNPs.
44–46 are reported to have significant positive or negative MRI capabilities. UC properties can be added to such MRI active nanostructures using suitable combinations and concentrations of luminescent lanthanide ions (e.g. Er/Yb or Tm/Yb).26,44,47–55 We denote hereafter these nanostructured materials as MRI/UCNPs.
Taking advantage of these optical/magnetic multimodal bioprobes, the “real time evolution therapy” could be in principle possible. In the case of cancer surgery, the MRI/UCNPs, possibly conjugated with tumor-specific antibodies, could be injected intravenous or locally in order to target the tumor. At first stage, the bimodal NPs can localize the tumor, within a few millimeters, through an in vivo fast and non-invasive MRI scan. Then, the MRI/UCNPs could be supervised during the surgery, in the cancerous region, using a common NIR diode laser with sub-millimeter spatial resolution, guiding exactly the surgeon to excise the mass. This technique based on “see-treat-see” the injury opens the way to a fast surgical treatment of the tumor and to its precise and complete physical elimination, a result obtained combining the two imaging modalities and thus overcoming the limitations of single modal optical and MRI techniques.
From the design strategy point of view, multimodal NPs can be generally classified into two categories based on their structures: core/shell and single phase NPs. Important efforts have been recently dedicated to the synthesis of core/shell nanomaterials, such as NaYbF4:Tm/NaGdF4,56 NaGdF4:Yb,Er/SiO2,57,58 NaYF4:Yb,Tm/NaGdF4,59 NaYF4:Er,Yb/NaGdF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ,53,60 GdVO4:Yb,Er(Ho,Tm)/SiO2
,53,60 GdVO4:Yb,Er(Ho,Tm)/SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36 and NaLuF4:Yb,Tm@NaGdF4(153Sm)20 (NaYF4:20%Yb/2%Tm/15%Gd@NaGdF4).21 The preparation of the core/shell structures typically occurs through a multi-step sequence, which could be complicated and time-consuming. By contrast, single-phase multimodal NPs can be prepared by facile one-step routes, by co-doping magnetic and luminescent lanthanide ions in single hosts by solvothermal or coprecipitation methods. Examples of incorporation of lanthanide ions into single phase MRI/UCNPs are SrF2:Yb,Er,Gd,61 GdF3:Yb,Er,Gd and GdF3:Yb,Tm,Gd,13 NaYF4:Yb,Er,Gd,19,25,60 NaLuF4:Yb,Tm,Gd,44 NaGdF4:Yb,Tm,9,62 NaGdF4:Yb,Tm,Er,48 KGdF4:Yb,Tm51,63 and GdPO4:Yb,Tb.35
36 and NaLuF4:Yb,Tm@NaGdF4(153Sm)20 (NaYF4:20%Yb/2%Tm/15%Gd@NaGdF4).21 The preparation of the core/shell structures typically occurs through a multi-step sequence, which could be complicated and time-consuming. By contrast, single-phase multimodal NPs can be prepared by facile one-step routes, by co-doping magnetic and luminescent lanthanide ions in single hosts by solvothermal or coprecipitation methods. Examples of incorporation of lanthanide ions into single phase MRI/UCNPs are SrF2:Yb,Er,Gd,61 GdF3:Yb,Er,Gd and GdF3:Yb,Tm,Gd,13 NaYF4:Yb,Er,Gd,19,25,60 NaLuF4:Yb,Tm,Gd,44 NaGdF4:Yb,Tm,9,62 NaGdF4:Yb,Tm,Er,48 KGdF4:Yb,Tm51,63 and GdPO4:Yb,Tb.35
It is worth remarking that simple, low-cost, environmentally friendly synthetic pathways for the synthesis of the nanostructured materials, while maintaining efficient imaging capabilities, are of paramount importance for environmental sustainability. Moreover, biocompatibility, toxicity and long-term stability are also very important features that need to be fulfilled for in vivo biomedical imaging application of the NPs. In fact, a major problem hampering the use of NPs for medical applications is the ability of some nanostructures to stimulate immune cells, such as dendritic cells (DCs), to release proinflammatory cytokines that trigger hypersensitivity reactions and inflammation leading to harmful effects on human health.64–67 DCs are a heterogeneous cell population that capture antigens in peripheral tissues and then migrate to lymph nodes to present antigens to lymphocytes and to activate the specific T cell immunity.68 DCs produce various cytokines that orchestrate the inflammatory and immune response, such as IL-12 that plays a key role in the activation of natural killer cells and T lymphocytes, which in turn release immune regulatory mediators69 and IL-23 that induces T cells to produce pro-inflammatory chemical mediators.70 Moreover DCs release IL-6, IL-1β, and TNF-α that show pro-inflammatory and immune stimulatory effects and play a prominent role in the induction of systemic acute phase reaction, characterized by fever, headache, changes in the sleep–wake cycle, anorexia, inactivity, nausea and emesis.71–73 Therefore to be suitable candidates for biomedical purposes NPs should not induce DCs to produce cytokines leading to inflammation and/or to immune system derangement promoting an increased incidence of autoimmune and allergic diseases.74,75
The present investigation involves saline dispersible CaF2:Gd,Yb,Er and CaF2:Gd,Yb,Tm UCNPs, with the aim of achieving efficient, biologically compatible nanostructured platforms for fast multimodal imaging in medical diagnostics.
2. Experimental section
2.1 Synthesis details
Lanthanide tri-doped CaF2 NPs were synthesized by a hydrothermal method developed by our group.27 Briefly, stoichiometric amounts of the metal chlorides GdCl3·6H2O (Aldrich, 99.99%), CaCl2·2H2O, ErCl3·6H2O, YbCl3·6H2O and TmCl3·6H2O (Aldrich, 99.9%) were dissolved in 7 ml of deionized water (total metal amount equal to 3.5 mmol) and Ca![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gd
Gd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb
Yb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er = 70
Er = 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 or Ca
3 or Ca![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gd
Gd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb
Yb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Tm = 70
Tm = 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratios. To this solution, 20.0 ml of potassium citrate (1 M) and 3.0 ml of NH4F (3.5 M, Aldrich, 99.9%) solutions were added. The resulting clear solution was heated in a 50 ml stainless steel Teflon lined digestion Pressure Vessel (DAB-2, Berghof) at 190 °C for 6 h. After washing with acetone and drying at room temperature, the obtained NPs were directly dispersed in water. The dispersions remained stable for several weeks. Samples with lower concentration of Gd3+ ions (Ca
1 molar ratios. To this solution, 20.0 ml of potassium citrate (1 M) and 3.0 ml of NH4F (3.5 M, Aldrich, 99.9%) solutions were added. The resulting clear solution was heated in a 50 ml stainless steel Teflon lined digestion Pressure Vessel (DAB-2, Berghof) at 190 °C for 6 h. After washing with acetone and drying at room temperature, the obtained NPs were directly dispersed in water. The dispersions remained stable for several weeks. Samples with lower concentration of Gd3+ ions (Ca![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gd
Gd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb
Yb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er = 70
Er = 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) have been prepared for further EPR measurements.
2) have been prepared for further EPR measurements.
2.2 X-Ray powder diffraction setup
X-Ray powder diffraction (XRPD) patterns were recorded with a powder diffractometer (Thermo, ARL XTRA), operating in Bragg-Brentano geometry, equipped with a Cu-anode X-ray source (Kα = 1.5418 Å) and using a Peltier Si(Li) cooled solid state detector. The patterns were collected at a scan rate of 0.04° s−1 in the 15°–90° 2θ range. The powdered samples were finely ground in a mortar and then deposited in a low-background sample stage for the measurements.2.3 TEM images
The size and morphology of the NPs were analyzed by transmission electron microscopy (TEM) technique, using a CM12 Philips microscope operating at 100 kV. The samples were prepared by drop drying a dilute suspension of the NPs onto 200 mesh carbon-coated copper grids. The recorded micrographs were further analyzed with the Image Pro-Plus© software. The mean diameter and size distribution of each sample were obtained from a statistical analysis over ca. 800 NPs.A portion of the liver, excised after MRI experiments, was fixed with glutaraldehyde 2.5% in 0.1 M phosphate buffer, pH 7.4 at 4 °C for 3 h. Hence, they were postfixed in osmium tetroxide 1% for 1 hour in the same buffer, dehydrated in graded acetones (60–100%), embedded in Epon-Araldite and cut with an Ultracut ultramicrotome (Reichert, Wien, Austria). Ultrathin unstained sections were observed with a Philips Morgagni transmission electron microscope (FEI Company Italia Srl, Milano, Italy) operating at 80 kV and equipped with a Megaview II camera for digital image acquisition.
2.4 ICP-MS analysis
Mineralization was carried out with a microwave digestion system MARS XPRESS (CEM Corporation, North Carolina, USA) equipped with 10 ml Teflon PFA by adding 0.8 ml of nitric acid (Optima grade for ultra-low trace metal analysis from Fisher Scientific) and 1.1 ml high-purity water (Milli-Q apparatus, Millipore, Milan) to 0.1 ml of NP solution. The samples were heated for 10 min at 80 °C, for 10 min at 120 °C and then for 20 min at 180 °C. After cooling at room temperature the obtained samples were transferred into polypropylene tubes and stored at −20 °C until analysis. For ICP-MS analysis 0.1 ml of the digested sample was diluted by adding 4.90 ml Milli-Q water. External calibration solutions (10 to 400 μg L−1) were prepared by diluting with 10 mg L−1 multi-elemental standard solution. 2 μg L−1 rhodium was used as an internal standard. ICP-MS measurements were performed using a Thermo Fisher Scientific model X Series II equipped with a technology collision/reaction cell (CCT) and an ASX 520 autosampler (Cetac Technologies, Omaha, NB).2.5 Dynamic light scattering measurements
Dynamic light scattering (DLS) measurements were carried out using a Malvern Zetasizer Nano ZS90 operating with a He–Ne laser at 633 nm. Measurements were carried out in water colloidal dispersions of the CaF2 NPs with a 0.5 g L−1 concentration.2.6 Magnetic measurements
Magnetic measurements were performed using a Cryogenic S600 SQUID magnetometer operating in the 1.8–300 K temperature range with an applied field up to 6.5 T. All data were corrected for the diamagnetic contribution of the sample holder. Powder X-band electron paramagnetic resonance (EPR) spectra were recorded at variable temperatures using a Bruker Elexsys E500 spectrometer with a 100 kHz frequency field modulation.2.7 Upconversion spectra and temperature measurements
UC spectra were measured upon continuous wave laser excitation at 980 nm using a diode laser (1 W, MDL-III-980, CNI Optoelectronics Tech). The laser power was measured with a thermal power meter (S302C sensor, Thorlab). The emission signal was analyzed using a half-meter monochromator (HR460, Jobin Yvon) equipped with 1200 lines mm−1 grating and detected with a CCD detector (Spectrum One, Jobin Yvon). The spectral resolution of the UC spectra is 0.1 nm. The UC luminescence pictures under ambient light illumination were acquired using a Canon CCD camera. A microscope objective (mod. 4608, Leika) was used to focus the 980 nm laser radiation. An optical chopper (SR540, Stanford Research Systems) with a 5/6 slot chopper blade was used for some UC measurements. The focused laser spot size was 0.5 mm. Temperature measurements were carried out using the UC emissions (see below) and an infrared camera (FLIR i7).2.8 Cytotoxicity investigations
Cytotoxicity was studied by the MTT test performed on HeLa cells cultured on a 96 well plate. Cells were cultured with Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% of antibiotic mix (penicillin–streptomycin 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v). The plate was incubated for 24 hours at 37 °C and 5% of CO2. When at confluence, the growth medium was discarded and substituted with medium containing 1
1 v/v). The plate was incubated for 24 hours at 37 °C and 5% of CO2. When at confluence, the growth medium was discarded and substituted with medium containing 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 and 1
20 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 diluted NPs (stock colloidal dispersion were 1 wt%). The viability of cells was determined with the addition of 100 μl of 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) in the growth medium, which was internalized by living cells and converted into insoluble formazan resulting from the cellular ATP production. The concentration of living cells was determined by absorption measurements at 590 nm, after 6 hours of incubation of the HeLa cells with the NPs.
50 diluted NPs (stock colloidal dispersion were 1 wt%). The viability of cells was determined with the addition of 100 μl of 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) in the growth medium, which was internalized by living cells and converted into insoluble formazan resulting from the cellular ATP production. The concentration of living cells was determined by absorption measurements at 590 nm, after 6 hours of incubation of the HeLa cells with the NPs.
2.9 MRI setup and methods
MR imaging experiments were carried out using a Biospec Tomograph System (Bruker, Karlsruhe, Germany) equipped with a 4.7 Tesla, 33 cm bore horizontal magnet (Oxford Ltd, UK). A 72 mm internal diameter birdcage volume coil was used. Longitudinal (r1) and transversal (r2) relaxivities were measured in vitro using imaging sequences according to Passuello et al.13 For in vivo experiments, two Balb/c, male mice (6–8 week old) were intravenously injected with the CaF2 NPs at the dosage of 6 mg of Gd per kg, corresponding to 38 μmol kg−1, lower than the typical clinical dosage value for Gd-DTPA. For MRI acquisitions, animals were anesthetized with gas (a mixture of O2 and air containing 1–1.5% of isoflurane), placed in a heated animal bed and inserted in a 3.5 cm internal diameter bird-cage coil. T2-weighted images of the mouse body were acquired at 4.7 Tesla using a T2-weighted sequence with the following parameters: FOV = 6 × 3 cm2, MTX = 128 × 128 pixels, slice thickness = 0.2 cm, TE = 5.9 ms, TR = 2000 ms, NEX = 2, Echo = 16. T*2 weighted images were also acquired using a Gradient-Echo sequence with the following parameters: TE = 4.4 ms, TR = 1000 ms, FOV = 6 × 3 cm2, NEX = 2, MTX = 128 × 128 pixels, slice thickness = 0.2 cm, angle = 30°. The images were acquired before, 20 and 40 min after the NP injection. Furthermore, a volume of 250 μl of a colloidal dispersion of CaF2 NPs was subcutaneously injected into a region of the mouse left posterior leg. After the MRI investigation, the mice were sacrificed by CO2 and the liver and a section of the skin in the region of the subcutaneous injection of the NPs were excised. Both surgical samples were illuminated using the beam of a 980 nm diode laser, and images were obtained with a digital camera under natural light. A spectral filter (cut-off at 900 nm) was used to reject the diffused laser light. A further group of five mice were injected intravenously with the same dosage of the NPs under investigation and housed in a temperature and humidity controlled environment for one month in order to monitor the health status for prolonged periods. This group of mice was not monitored by the MRI technique. In vivo studies were conducted according to the guidelines of the Committee for Animal Research at the University of Verona.2.10 Two-photon imaging
Two-photon UC images were acquired with a confocal Leica TCS SP5 System using a Coherent Chameleon-Ultra Ti:sapphire pulsed laser. The NPs were excited at 975 nm and visualized with a 20× objective (water immersed, numerical aperture 1, gain = 59% and filter wheel (FW) = 40) in the range of 500–600 nm (first channel) and 600–700 nm (second channel). The LAS AF (Leica Application Suite Advance Fluorescence) (Leica) software was used to process, archive and analyze the image data. For the two-photon measurements, excised liver samples were fixed in a 2% water solution of glutaraldehyde and paraffin embedded for histological section preparation. Histological slices were dehydrated in xylene for 5 min and dried at 37 °C overnight.2.11 Preparation and culture of monocytes and DCs
After written informed consent and upon approval of the ethical committee, human blood was collected from healthy volunteers at the blood bank of the University of Verona. Monocytes were isolated from buffy coats by Ficoll-Hypaque and Percoll (GE Healthcare Life Science) density gradients and purified using the human monocyte isolation kit II (Miltenyi Biotec). The final monocyte population was 99% pure, as measured by FACS analysis. To generate DCs, monocytes were incubated at 37 °C in 5% CO2 for 5–6 days at 1 × 106 ml−1 in 6-well tissue culture plates (Greiner, Nürtingen, Germany) in RPMI 1640, supplemented with heat-inactivated 10% low endotoxin FBS, 2 mM L-glutamine, 50 ng ml−1 GM-CSF, and 20 ng ml−1 IL-4. The final DC population was 98% CD1a+, as measured by FACS analysis.2.12 Evaluation of cell viability and analysis of cytokine production
The percentage of live cells was determined using the Annexin V staining kit (Miltenyi Biotec) in DCs and monocytes according to the manufacturer's instructions. The samples were acquired on a seven-color MACSQuant Analyzer (Miltenyi Biotec) and analysis was performed using the FlowJo software (TreeStar, Ashland, OR, USA).Cytokine production in culture supernatants was determined by ELISA kits according to the manufacturer's instructions: IL-6 (range 8–800 pg ml−1), IL-12 (range 4–500 pg ml−1), IL-1β (range 4–500 pg ml−1), IL-23 (range 15–2000 pg ml−1), TNF-α (range 4–500 pg ml−1), purchased from eBioscience (San Diego, CA).
Ultrapure lipopolysaccharide (LPS, 0111: B4 strain) from E. coli was purchased from InvivoGen (San Diego, CA) and was used as a positive control.
3. Results and discussion
Fig. 1 shows the XRPD patterns for the tri-doped CaF2:Gd,Er,Yb and CaF2:Gd,Tm,Yb NPs, revealing that all the calcium fluoride NPs are single phase. The peak positions and intensities closely match with those of the XRPD patterns for cubic CaF2 powders (space group no. 225, Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m), shown in Fig. 1.
m), shown in Fig. 1.
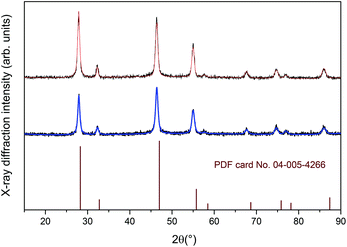 | ||
Fig. 1 XRPD patterns for the CaF2:Gd,Yb,Tm (upper graph) and CaF2:Gd,Yb,Er (lower graph) NPs. Vertical lines: PDF card pattern no. 04-005-4266 (cubic CaF2, space group no. 225, Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m). m). | ||
From a Rietveld refinement under the isotropic crystallite shape assumption for the two samples, crystal lattice parameters were obtained, reported in Table 1. As already observed for lanthanide doped CaF2 NPs, the expansion of the unit cell volume for the present samples with respect to undoped CaF2 hosts is due to the repulsion of the fluoride anions which play the role of charge compensators and which are also distributed in interstitial sites.76 Nonetheless, the dopant lanthanide ions substituting for Ca2+ ions in the fluorite structure can form clusters such as hexamers.76,77
| Sample | a (Å) | Unit cell volume (Å3) | Particle size (nm) |
|---|---|---|---|
CaF2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 78 78 |
5.463(1) | 163.04(1) | — |
| CaF2:Gd,Er,Yb | 5.539(1) | 169.94(1) | 18(1) |
| CaF2:Gd,Tm,Yb | 5.537(1) | 169.75(1) | 19(1) |
In Fig. 2 a typical TEM micrograph of the CaF2 NPs is shown, together with the particle size distribution obtained from a statistical analysis over ca. 800 NPs. The NPs have a quite regular spherical shape, with an average particle size of 15.6 ± 3.7 nm. The size distribution can be nicely fitted to a log-normal function, as commonly observed for NPs prepared with wet-chemical techniques. The best-fit parameters give a mean diameter of 14.6 nm (σ = 0.21), in agreement with the value obtained from direct statistics (see Fig. 2, inset).
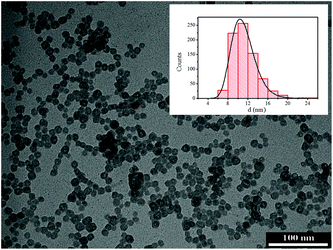 | ||
| Fig. 2 TEM image of the CaF2:Gd,Yb,Er NPs. Inset: particle size distribution histogram obtained by statistical analysis over ca. 800 NPs. Solid line: best fit curve using a log-normal distribution. | ||
The hydrodynamic sizes of the citrate capped NPs have been measured by the DLS technique (see Fig. S1, ESI†) and they are 18 ± 3 nm for the CaF2:Gd,Yb,Er NPs and 19 ± 3 nm for the CaF2:Gd,Yb,Tm NPs, in perfect agreement with the average particle size and dispersion obtained by TEM images. The DLS measurements confirm that the citrate capped NPs have a very narrow size dispersion, as observed for similar NPs.79 The zeta-potential is −19 ± 1 mV for both the NPs.
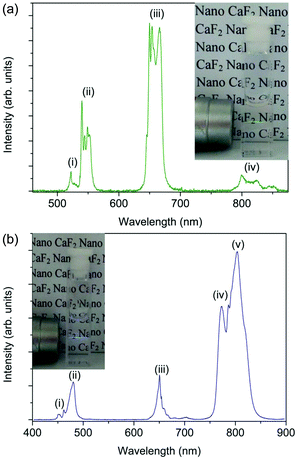
A water colloidal dispersion of the CaF2:Gd,Er,Yb NPs shows UC emission bands due to the Er3+ ion in the green (520–560 nm), red (640–680 nm) and infrared (790–860 nm) regions of the spectrum (Fig. 3a). The red emission is much stronger than the green UC emission (the red to green intensity ratio is about 2.5). The UC spectrum of a water colloidal dispersion of the CaF2:Gd,Yb,Tm NPs (Fig. 3b) shows three groups of bands in the blue (450–500 nm), red (630–670 nm) and in the NIR region around 800 nm. The infrared emission band is much stronger than the other emissions (the integrated intensity ratio is about blue![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) red
red![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NIR = 1
NIR = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4). Power studies are similar to those found in previous investigations on Er3+,Yb3+ and Tm3+,Yb3+ doped calcium fluoride NPs.27,76 In particular, the UC intensity as a function of the laser intensity increases with a power higher than one, depending on the number of photons partaking the UC process. We found that the UC emissions for the water colloidal dispersions (NPs concentration of 1 wt%) are easily detectable with our spectroscopic setup for excitation intensities as low as 20 and 10 W cm−2 for the Gd,Yb,Er and Gd,Yb,Tm doped CaF2 NPs, respectively.
4). Power studies are similar to those found in previous investigations on Er3+,Yb3+ and Tm3+,Yb3+ doped calcium fluoride NPs.27,76 In particular, the UC intensity as a function of the laser intensity increases with a power higher than one, depending on the number of photons partaking the UC process. We found that the UC emissions for the water colloidal dispersions (NPs concentration of 1 wt%) are easily detectable with our spectroscopic setup for excitation intensities as low as 20 and 10 W cm−2 for the Gd,Yb,Er and Gd,Yb,Tm doped CaF2 NPs, respectively.
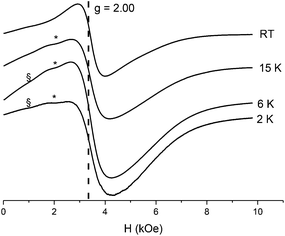
In order to obtain information on the magnetic properties of the NPs under investigation, EPR spectra and magnetic susceptibilities were measured. EPR spectra for CaF2:Gd,Yb,Er NPs at different temperatures are dominated by a signal centered at g = 2.00 which behaves as a superparamagnetic one (Fig. 4). Indeed, on decreasing temperatures the peak shifts to low field and becomes progressively broader,80,81 suggesting clustering of Gd3+ ions in the NPs, in agreement with the X-ray analysis. At low temperature, low field shoulders can be attributed to signals of Er3+ and Yb3+ ions in cubic symmetry occurring at about g = 6.7 and 3.4, respectively. These signals are more evident in samples containing lower amounts of Gd3+ ions (see Fig. S2, ESI†) and confirm that these ions are in the substitutional position in the CaF2 lattice.82–84
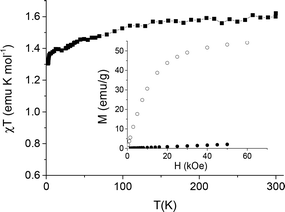
The temperature dependence of the χT product (shown in Fig. 5) follows the expected behavior for a system containing orbitally degenerate lanthanide ions, slowly decreasing with decreasing temperature due to the crystal field effect. The room temperature value, 1.60 emu K mol−1, is close to that expected on the basis of the Curie constant of the rare earth ions weighted for their molar ratios (1.8 emu K mol−1). Accordingly, the field dependent magnetization measured at 2.5 K, at 6 Tesla reaches 54.2 emu g−1 (Fig. 5) which is only slightly lower than the value calculated assuming an isotropic g = 2.00 with S = 7/2 for Gd3+ and an effective spin S = 1/2 with g = 3.43 and 6.7 for Yb3+ and Er3+, respectively, as obtained by EPR spectroscopy.
At room temperature the magnetization increases linearly with the magnetic field, as indeed expected for an assembly of paramagnetic ions, reaching 2.1 emu g−1 at 50 kOe. The CaF2:Gd,Yb,Tm NPs display a similar field dependent magnetization (at 50 kOe it reaches 45.1 and 1.7 emu g−1 at 2.5 and 300 K, respectively, Fig. S3, ESI†). All these data confirm the incorporation of the lanthanide ion into the fluoride lattice, producing a magnetization that makes the NPs suitable for being used as contrast agents for MRI.
In vitro MRI experiments were performed to measure T1 and T2 relaxation times of colloidal dispersions of the CaF2:Gd,Yb,Er and CaF2:Gd,Yb,Tm NPs vs. the Gd3+ concentration. Fig. 6 shows the 1/T1 and 1/T2 relaxation rates as a function of molar concentration of Gd3+ in the prepared dispersions. The slopes of the best fitting straight lines represent the longitudinal (r1) and transversal (r2) relaxivities of the NPs and the results are reported in Table 2. The obtained values of r2 are similar to those found for Gd-DTPA and for other Gd-containing NPs.85 The r2/r1 ratio is about 4, conferring to these substances the properties of T2-relaxing contrast agents.
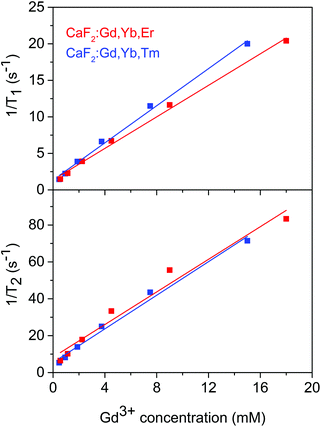 | ||
| Fig. 6 Longitudinal and transverse relaxation times as a function of the Gd3+ concentration for the CaF2:Gd,Yb,Er and CaF2:Gd,Yb,Tm NPs. | ||
| Sample | r 1 (mM−1 s−1) | r 2 (mM−1 s−1) |
|---|---|---|
| CaF2:Gd,Er,Yb | 1.1 ± 0.2 | 4.4 ± 0.2 |
| CaF2:Gd,Er,Tm | 1.3 ± 0.2 | 4.6 ± 0.2 |
For in vivo experiments, MRI images of mice before and at different times after NP intravenous administration were acquired. For this experiment, we used the CaF2:Gd,Yb,Tm NPs which shows a slightly higher r2 relaxivity (see Table 2).
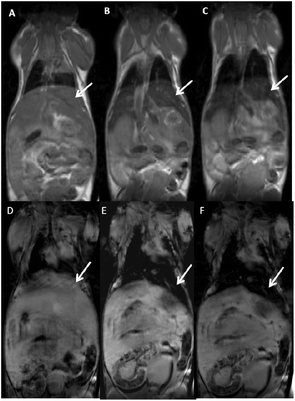
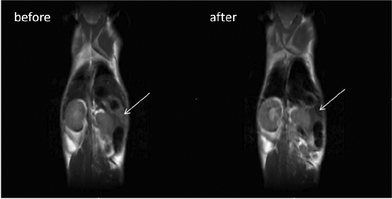
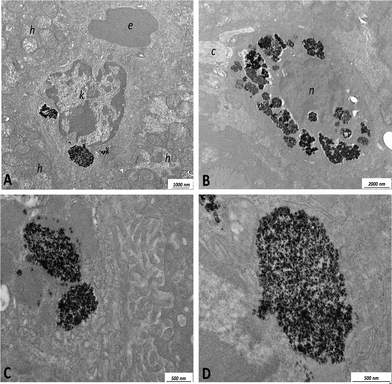
Representative T2 and T*2 weighted images in the coronal plane are shown in Fig. 7. It is clearly apparent that the signal intensity of the liver strongly decreases after the NP injection indicating that the NPs are accumulating in this organ and therefore indicating that the present NPs could be used as a T2-relaxing MRI contrast agent. Moreover, significant amounts of NPs are also found in the spleen (see Fig. 8), as also reported in literature for different NPs.9,19,20,86–88
It is worth remarking that the group of five animals housed in a controlled environment does not show any sign of mortality or modification of the daily life behavior for at least one month after the intravenous injection of the present NPs.
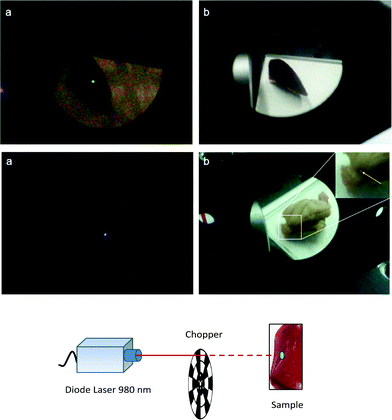
In order to demonstrate the suitability of these materials for optical imaging at high spatial resolution, experiments were carried out using the liver and a piece of epithelial tissue of the mouse previously investigated by MRI. Fig. 9a and 9b (upper figures) show images of excised mouse liver tissue upon illumination with a 980 nm diode laser beam. As revealed by these images, a green-yellow spot is clearly visible in the dark, due to the UC emission of the CaF2:Gd,Yb,Er NPs which have been accumulated in the liver after the intravenous injection.
In order to maximize the UC signal while maintaining a sufficiently low laser power to avoid the damage of the tissue, we used a laser beam modulated by a chopper. In fact, Zhan et al.89 demonstrated that with a modulated laser beam in the microsecond or millisecond time region it is possible to drastically reduce the average laser intensity on the sample, while maintaining the same UC detectability.
In the present setup, we used a chopper modulated at 100 Hz. Although in ambient light illumination the UC green spot appears not very bright (see Fig. 9b), under dark conditions we were able to clearly observe the spot with the naked eye for quite low laser intensities around 46 W cm−2 (with chopped laser exciting radiation, see Fig. 9a, upper figure). Therefore, this behavior suggests the use of the present calcium fluoride NPs in clinical surgery, with the aid of a cheap 980 nm laser pointer.
Moreover, the CaF2:Gd,Yb,Tm NPs were subcutaneously injected (about 3 mm in depth) into mice and the excised epithelial tissue was illuminated by a 980 nm diode laser. Pictures in the dark and in ambient light illumination for the epithelial tissue are shown in Fig. 9a and 9b (lower figures), respectively.
A bluish spot is clearly visible in both cases with the naked eye for a laser intensity as low as 16 W cm−2 (with chopped laser exciting radiation, see Fig. 9a, lower figure). Even if the NIR emission at 800 nm is not visible with the digital camera, this behavior demonstrates the practical possibility of real time optical imaging of the Tm3+ doped NPs with a low-power NIR laser for the clinical treatment of subtissue local lesions and tumors. A brighter spot, due to the 800 nm emission of Tm3+ ions in the NPs, would be much more visible using an infrared camera.
Recent investigations have demonstrated that the monitoring of the temperature is an important issue in the use of therapeutic materials, also in nanometer size, which base their effect on hyperthermia.90,91 For this reason, investigation in nanothermometry has been rapidly increased in the last few years for its importance in biomedicine.92–94 In this respect, lanthanide doped (for instance with Er3+, Tm3+, Nd3+, Gd3+) NPs have been demonstrated to be in principle very useful to monitor the local temperature both in vitro and in vivo.27,95,96 In particular, Er3+/Yb3+ doped UCNPs have been demonstrated to be particularly useful for nanothermometry.97 For the Er3+ lanthanide ion, the mechanism for thermometry is based on the thermalization of the two closely spaced 2H11/2 and 4S3/2 energy levels of the Er3+ ion, and emissions from these two levels can be used for measuring the local temperature. The thermal sensitivity S for the optical temperature sensor is calculated as the rate at which the experimental variable R changes with the temperature of the host matrix, as indicated by the equation:94,98,99
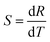 | (1) |
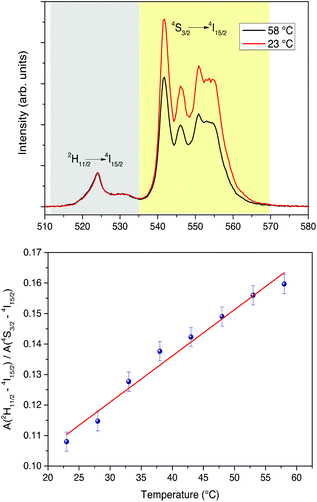
The possible use of near infrared radiation at 980 nm as an exciting source to generate UC emission for in vivo optical bioimaging is still under discussion. In fact, water exhibits an optical absorption band at a wavelength around 1000 nm. The absorbed light energy would induce local heating and eventually cell and tissue damage under continuous irradiation.100 In order to shed light into this issue, we carried out an analysis of the temperature changes induced by the 980 nm diode laser radiation directly to the liver tissue. First, we measured the temperature calibration curve for our present CaF2:Gd,Er,Yb NPs by monitoring the UC integrated intensities of the 2H11/2→4I15/2 and 4S3/2→4I15/2 transitions of the Er3+ ions, as shown in Fig. 10. The relative changes of the UC intensity as a function of the temperature for the two groups of bands located around 550 nm and 525 nm are clearly observable, and from a linear fit of the integrated UC intensities vs. temperature (in the 25–60 °C range), we obtain a thermal sensitivity (as defined in eqn (1)) of (15.2 ± 1.0) × 10−4 K−1. This value is in general similar to those observed for several Er3+ doped glass and glass-ceramics and it is even better than the RT value found for a fluorozirconate glass host, as reported recently by Lavín et al.101
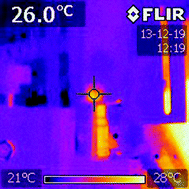
The UC spectra for the CaF2:Gd,Er,Yb NPs accumulated in the mouse excised liver are clearly visible upon excitation with a 100 Hz modulated diode laser 980 nm radiation beam, with an average power of 46 W cm−2 (see Fig. 11). A slight but significant change of the relative intensities of the UC bands is observed as a function of the irradiation time, as shown in Fig. 11. Using the temperature calibration curve, a local heating is observed. Nonetheless, the variation of the local temperature is around 2–3 °C after 1 minute and around 10–12 °C after 4 minutes of continuous laser irradiation, shown in Fig. 11, starting from a room temperature value of 22 °C. This temperature behavior is also confirmed by temperature measurements of the same biological tissue with comparable experimental conditions using an infrared camera (see Fig. 12 as an example). All images are shown in Fig. S4 (ESI†). Using the infrared camera, it was observed that the temperature on the surface of the biological tissue is similar to that induced by the 980 nm radiation, monitored by the UC emission, therefore corroborating the spectroscopic results. It was observed that the temperature does not increase linearly with the irradiation time but rather tends to a limiting value (see Fig. 11).
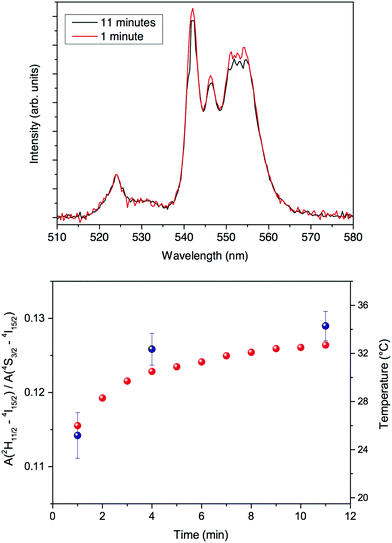 | ||
| Fig. 11 Upper: UC spectra for the CaF2:Gd,Yb,Er NPs in the excised liver (excitation with a 980 nm laser) for two different irradiation times. Lower: blue solid dots: time dependence of the ratio of the integrated 2H11/2→4I15/2 and 4S3/2→4I15/2 UC and the temperature calculated by the calibration curve shown in Fig. 10; red solid dots: time dependence of the temperature as measured by the IR camera on the same sample under the same excitation conditions. | ||
After 11 minutes of continuous irradiation, the temperature has raised to 32–33 °C, around 12 °C higher than the starting temperature before the irradiation (room temperature of 22 °C).
These results highlighted that although a local heating is observed, it can be limited to a rise of 1–2 °C in few seconds of laser irradiation at relatively low power, a time which is surely enough to monitor the presence of the NPs during a clinical surgery, for instance with the aid of a cheap 980 nm laser pointer.
Moreover, through careful working conditions, it would be possible to induce (and possibly monitor) a local heating by controlling the time of direct exposure of a focused laser radiation at 980 nm on the biological tissue, with a very high spatial resolution (around 500 μm2), for instance to induce cancer cell death or cauterize the tissue during a clinical surgery.
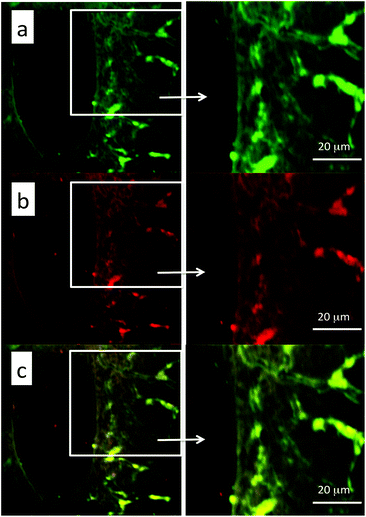
Two-photon images of surgical samples of the mouse liver upon excitation at 975 nm are shown in Fig. 13 (first channel: Fig. 13a; second channel: Fig. 13b). The merging of the two channels highlights the co-localization of the two signals, revealing the presence of the NPs in the liver (Fig. 13c).
From the pictures it is possible to observe the localization of the NPs mainly in the blood vessels, although a small amount of NPs is also visible in the interstitial spaces, suggesting the initial process of extravasation.
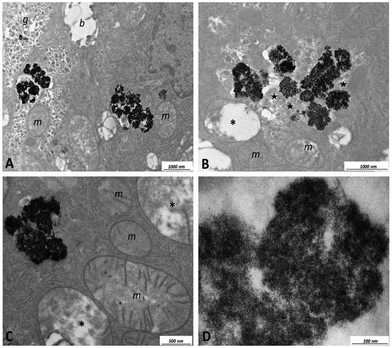
TEM images of the mouse liver tissue are shown in Fig. 14 and 15. Fig. 14 (panel C) shows preferential localization of NPs at the level of the sinusoids within the Kupffer cells. Fig. 14 (panel A) shows scarce uptake of NPs to hepatocytes probably due to a relatively short time interval elapsing after the intravenous injection. These findings suggest that a time interval of 40 min is enough for the NPs to reach the liver but not for appreciable internalization in the hepatocytes. Ultrastructural data shown in Fig. 14a suggest that NPs are internalized in the Kupffer cells after their adhesion to the membrane and subsequent transport into the cytoplasm where they are enveloped by membranes. Within the Kupffer cells, NPs are preferentially organized in clusters having sizes of the order of few microns. Within these clusters, it is possible to detect materials with an intermediate-electron density, which could correspond to polysaccharides. It is important to underline that hepatocytes did not show degenerative aspects or atypical phenotype.
Careful examination of TEM images indicated that within the present experimental conditions, the NPs are characterized by low hepatotoxicity since they do not cause cellular suffering with the exception of a modest appearance of swelling in the mitochondria when NP clusters are considerably large (see Fig. 15, panel C). Toxicity tests using the CaF2:Gd,Yb,Tm NPs on HeLa cells are shown in Fig. S5† for different dilutions of the NPs. The obtained results indicate that the NPs are characterized by low toxicity on the HeLa cultured cells. In fact, for a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 diluted dispersion, the percentage of living cells is of 77%, while for a 1
20 diluted dispersion, the percentage of living cells is of 77%, while for a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 diluted dispersion the percentage increased reaching 85% of living cells after 6 hours of incubation.
50 diluted dispersion the percentage increased reaching 85% of living cells after 6 hours of incubation.
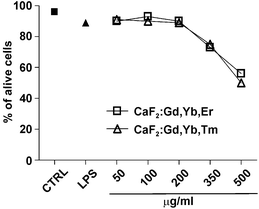
To investigate whether NPs induce the activation of the immune system, we analyzed their effects on human dendritic cells, which play an essential role in regulation of immunity.68 First of all we determined whether NPs exert toxic effects on these cells. For this purpose, human blood monocytes were cultured for 5 days with GM-CSF and IL-4 to obtain DCs that were then challenged with NPs or with 100 ng ml−1 lipopolysaccharide (LPS), a well-known bacterial immune cells activator, as a control. The results, shown in Fig. 16, clearly indicate that only extremely high doses of NPs affected DC viability, as assessed by Annexin V staining. Similar results have been obtained upon human monocytes challenge with NPs (results not shown). It is worth noting that NP doses higher than 200 μg ml−1 greatly exceed the ones commonly used for in vitro studies. Moreover, it is very unlikely that such high doses of NPs could reach the cells upon in vivo administration and have a chance to induce toxic effects. However, it is possible that several injections in the patients could lead to NP accumulation in tissues, causing unwanted effects. This possibility remains to be investigated, but in this case our NPs should be preferentially used for diagnostic purposes, requiring the administration of limited amounts of NPs rather than for other medical treatments requiring the injection of several NP doses. Future studies would also be undertaken to modify the NP surface (for instance by means of a different surface coating) in order to reduce their cytotoxicity.108
The activation of DCs results in the production of various cytokines that stimulate the inflammatory process and the immune response.75 Therefore it is important to assess whether nanostructures that have to be used for medical applications stimulate these cells causing inflammation and/or adverse immune reactions once injected into patients.64 We then examined whether our NPs induce pro-inflammatory cytokine secretion by cultured human DCs by the ELISA assay. We found that NPs did not elicit the release of IL-12, IL-23, IL-1β, IL-6 or TNF-α by DCs, as shown in Fig. 17. Similar results have been obtained by treating human monocytes with NPs (results not shown). It has been reported that the blockade of the above mentioned cytokine signaling is effective in treating experimental models of autoimmune and chronic inflammatory diseases such as inflammatory bowel diseases, diabetes, multiple sclerosis, asthma and rheumatoid arthritis.102–106 Therefore, the result that NPs do not induce the secretion of these cytokines is relevant because it indicates that the NPs are most probably immunologically inert and unable to cause harmful inflammatory responses. Moreover, this finding demonstrates that NPs are not contaminated by microorganisms or their derivatives able to induce unwanted immune reactions.107
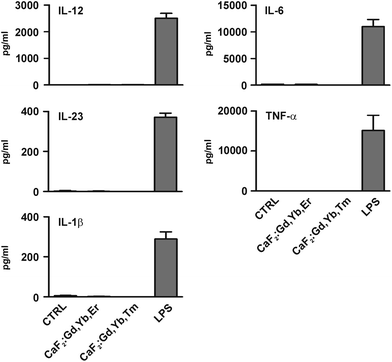 | ||
| Fig. 17 Effect of CaF2 NPs on dendritic cell (DC) cytokine secretion. DCs obtained as described in Fig. 11 were cultured for 24 hours in the absence (CTRL) or presence of 50 μg ml−1 of NPs. DCs were also cultured with 100 ng ml−1 LPS as a positive control. The release of the indicated cytokines in culture supernatants was evaluated by the ELISA assay. Results are expressed as the mean value + SD of three independent experiments. | ||
4. Conclusions
Calcium fluoride NPs doped with Gd3+,Er3+,Yb3+ or Gd3+,Tm3+,Yb3+ have been prepared for the first time in colloidal form in water solutions. The hydrophilic citrate moiety bound on the particle surface guarantees easy dispersibility in water. UC emission in the visible and infrared regions is clearly visible to the naked eye for excitation intensities as low as 10 W cm−2. Magnetic measurements suggest clustering of the Gd3+ ions in the crystal structure. In vivo MRI experiments with mice show that after 40 minutes from the intravenous injection, the CaF2 NPs are localized in the liver and in the spleen as demonstrated by the observed substantial decrease of the MRI signal in these organs. Two-photon microscopy images of surgical samples of mouse liver confirm the presence of the NPs mainly in the blood vessels. Transmission electron microscopy images of the liver tissue confirm that the NPs have preferential localization at the level of sinusoids within the Kupffer cells. Within the Kupffer cells, the NPs are organized in clusters with sizes of few microns. The NPs are clearly observable with the naked eye in the excised liver of the mouse using with a low power diode laser at 980 nm, suggesting that the present NPs can be used in clinical surgery.It has been demonstrated that the local heating induced by a diode laser at 980 nm at 46 W cm−2 increases the local temperature of the biological tissue of around 1–2 °C if the radiation exposure time is limited up to one minute, a time that could be enough to guide the surgery. These results clearly indicate that the NPs under investigation are suitable candidates to be efficiently used as bimodal probes for in vivo optical and magnetic resonance imaging and medical surgery. Moreover, it is worth remarking that biocompatibility is a very important feature that needs to be fulfilled for possible in vitro and in vivo biomedical applications, such as multimodal imaging. In this respect, the present NPs are not toxic to both HeLa cells and immune cells. In particular, the NPs did not activate human dendritic cell and monocyte response, indicating that these nanostructures do not elicit unwanted immune and inflammatory reactions. It is worth remarking that no sign of mortality or modification of the daily life status (body temperature, weight, nutritional state) of at least one month after an intravenous injection of the present NPs in a group of five mice has been observed. We are therefore confident that the investigated NPs could be considered as biocompatible multimodal imaging contrast agents.
Acknowledgements
Marzia Cerpelloni (Università di Verona, Verona, Italy) is acknowledged for expert technical support. Stefano Tambalo (Università di Verona, Verona, Italy) is gratefully acknowledged for help with measurements with the infrared camera. This work was supported by Fondazione Cariverona (Verona, Italy), project “Verona Nanomedicine Initiative” and by MIUR through FIRB project RBAP114AMK – RI.NA.ME. “Rete Integrata per la Nanomedicina”. C. S., C. I. and E. F. thank the INSTM-Regione Lombardia (Italy) MAG-NANO project for partly supporting the research.References
- A. Louie, Chem. Rev., 2010, 110, 3146–3195 CrossRef CAS PubMed.
- W. Wu, J. Shen, P. Banerjee and S. Zhou, Adv. Funct. Mater., 2011, 21, 2830–2839 CrossRef CAS.
- L. Cheng, K. Yang, Y. Li, J. Chen, C. Wang, M. Shao, S.-T. Lee and Z. Liu, Angew. Chem., Int. Ed., 2011, 123, 7523–7528 CrossRef.
- M. Perfézou, A. Turner and A. Merkoçi, Chem. Soc. Rev., 2012, 41, 2606–2622 RSC.
- H. Kumagai, W. Pham, M. Kataoka, K.-I. Hiwatari, J. McBride, K. J. Wilson, H. Tachikawa, R. Kimura, K. Nakamura, E. H. Liu, J. C. Gore and S. Sakuma, Int. J. Cancer, 2013, 132, 2107–2117 CrossRef CAS PubMed.
- M. Asadian-Birjand, A. Sousa-Herves, D. Steinhilber, J. C. Cuggino and M. Calderon, Curr. Med. Chem., 2012, 19, 5029–5043 CrossRef CAS.
- L. Wang, H. Xing, S. Zhang, Q. Ren, L. Pan, K. Zhang, W. Bu, X. Zheng, L. Zhou, W. Peng, Y. Hua and J. Shi, Biomaterials, 2013, 34, 3390–3401 CrossRef CAS PubMed.
- Y. Li, G. H. Gao and D. S. Lee, Adv. Healthcare Mater., 2012, 2, 388–417 CrossRef PubMed.
- C. Liu, Z. Gao, J. Zeng, Y. Hou, F. Fang, Y. Li, R. Qiao, L. Shen, H. Lei, W. Yang and M. Gao, ACS Nano, 2013, 7, 7227–7240 CrossRef CAS PubMed.
- L. M. Maestro, E. M. Rodríguez, F. Vetrone, R. Naccache, H. L. Ramirez, D. Jaque, J. A. Capobianco and J. G. Solé, Opt. Express, 2010, 18, 23544–23553 CrossRef CAS PubMed.
- H. B. Na, I. C. Song and T. Hyeon, Adv. Mater., 2009, 21, 2133–2148 CrossRef CAS.
- F. Evanics, P. R. Diamente, F. C. J. M. van Veggel, G. J. Stanisz and R. S. Prosser, Chem. Mater., 2006, 18, 2499–2505 CrossRef CAS.
- T. Passuello, M. Pedroni, F. Piccinelli, S. Polizzi, P. Marzola, S. Tambalo, G. Conti, D. Benati, F. Vetrone, M. Bettinelli and A. Speghini, Nanoscale, 2012, 4, 7682–7689 RSC.
- P. Marzola, F. Osculati and A. Sbarbati, Eur. J. Radiol., 2003, 48, 165–170 CrossRef PubMed.
- S. V. Eliseeva and J.-C. G. Bünzli, Chem. Soc. Rev., 2009, 39, 189–227 RSC.
- M. D'Onofrio, E. Gianolio, A. Ceccon, F. Arena, S. Zanzoni, D. Fushman, S. Aime, H. Molinari and M. Assfalg, Chem. – Eur. J., 2012, 18, 9919–9928 CrossRef PubMed.
- F. Wang and X. Liu, Chem. Soc. Rev., 2009, 38, 976–989 RSC.
- F. Wang, Y. Han, C. S. Lim, Y. Lu, J. Wang, J. Xu, H. Chen, C. Zhang, M. Hong and X. Liu, Nature, 2010, 463, 1061–1065 CrossRef CAS PubMed.
- Q. Liu, Y. Sun, C. Li, J. Zhou, C. Li, T. Yang, X. Zhang, T. Yi, D. Wu and F. Li, ACS Nano, 2011, 5, 3146–3157 CrossRef CAS PubMed.
- Y. Sun, X. Zhu, J. Peng and F. Li, ACS Nano, 2013, 7, 11290–11300 CrossRef CAS PubMed.
- D. Ni, J. Zhang, W. Bu, H. Xing, F. Han, Q. Xiao, Z. Yao, F. Chen, Q. He, J. Liu, S. Zhang, W. Fan, L. Zhou, W. Peng and J. Shi, ACS Nano, 2014, 8, 1231–1242 CrossRef CAS PubMed.
- D. Tu, Y. Liu, H. Zhu and X. Chen, Chem. – Eur. J., 2013, 19, 5516–5527 CrossRef CAS PubMed.
- F. Vetrone and J. A. Capobianco, Int. J. Nanotechnol., 2008, 5, 1306–1339 CrossRef CAS.
- F.-F. Chen, Z.-Q. Chen, Z.-Q. Bian and C.-H. Huang, Coord. Chem. Rev., 2010, 254, 991–1010 CrossRef CAS PubMed.
- R. Kumar, M. Nyk, T. Y. Ohulchanskyy, C. A. Flask and P. N. Prasad, Adv. Funct. Mater., 2009, 19, 853–859 CrossRef CAS.
- L. Cheng, C. Wang and Z. Liu, Nanoscale, 2012, 5, 23–37 RSC.
- N.-N. Dong, M. Pedroni, F. Piccinelli, G. Conti, A. Sbarbati, J. E. Ramírez-Hernández, L. M. Maestro, M. C. Iglesias-de la Cruz, F. Sanz-Rodriguez, A. Juarranz, F. Chen, F. Vetrone, J. A. Capobianco, J. G. Solé, M. Bettinelli, D. Jaque and A. Speghini, ACS Nano, 2011, 5, 8665–8671 CrossRef CAS PubMed.
- F. Vetrone, R. Naccache, A. Juarranz de la Fuente, F. Sanz-Rodriguez, A. Blazquez-Castro, E. Martin Rodriguez, D. Jaque, J. Garcia Sole and J. A. Capobianco, Nanoscale, 2010, 2, 495–498 RSC.
- S. Aime, F. Fedeli, A. Sanino and E. Terreno, J. Am. Chem. Soc., 2006, 128, 11326–11327 CrossRef CAS PubMed.
- J. L. Major and T. J. Meade, Acc. Chem. Res., 2009, 42, 893–903 CrossRef CAS PubMed.
- M. Ahren, L. Selegard, A. Klasson, F. Soderlind, N. Abrikossova, C. Skoglund, T. Bengtsson, M. Engstrom, P.-O. Kall and K. Uvdal, Langmuir, 2010, 26, 5753–5762 CrossRef CAS PubMed.
- G. K. Das, B. C. Heng, S.-C. Ng, T. White, J. S. C. Loo, L. D'Silva, P. Padmanabhan, K. K. Bhakoo, S. T. Selvan and T. T. Y. Tan, Langmuir, 2010, 26, 8959–8965 CrossRef CAS PubMed.
- T. Paik, T. R. Gordon, A. M. Prantner, H. Yun and C. B. Murray, ACS Nano, 2013, 7, 2850–2859 CrossRef CAS PubMed.
- W. Ren, G. Tian, L. Zhou, W. Yin, L. Yan, S. Jin, Y. Zu, S. Li, Z. Gu and Y. Zhao, Nanoscale, 2012, 4, 3754–3760 RSC.
- T. Grzyb, A. Gruszeczka, R. J. Wiglusz, Z. Śniadecki, B. Idzikowski and S. Lis, J. Mater. Chem., 2012, 22, 22989–22997 RSC.
- W. Yin, L. Zhou, Z. Gu, G. Tian, S. Jin, L. Yan, X. Liu, G. Xing, W. Ren, F. Liu, Z. Pan and Y. Zhao, J. Mater. Chem., 2012, 22, 6974–6981 RSC.
- M. Botta, L. Tei, F. Carniato and T. Kalaivani, J. Mater. Chem., 2013, 1, 2442–2446 RSC.
- S. Rodriguez-Liviano, N. O. Nuñez, S. Rivera-Fernández, J. M. de la Fuente and M. Ocaña, Langmuir, 2013, 29, 3411–3418 CrossRef CAS PubMed.
- Y. Tian, H.-Y. Yang, K. Li and X. Jin, J. Mater. Chem., 2012, 22, 22510–22516 RSC.
- N. J. J. Johnson, W. Oakden, G. J. Stanisz, R. Scott Prosser and F. C. J. M. van Veggel, Chem. Mater., 2011, 23, 3714–3722 CrossRef CAS.
- H.-T. Wong, M.-K. Tsang, C.-F. Chan, K.-L. Wong, B. Fei and J. Hao, Nanoscale, 2013, 5, 3465–3473 RSC.
- Q. Ju, D. Tu, Y. Liu, R. Li, H. Zhu, J. Chen, Z. Chen, M. Huang and X. Chen, J. Am. Chem. Soc., 2012, 134, 1323–1330 CrossRef CAS PubMed.
- J. Zhou, M. Yu, Y. Sun, X. Zhang, X. Zhu, Z. Wu, D. Wu and F. Li, Biomaterials, 2011, 32, 1148–1156 CrossRef CAS PubMed.
- S. Zeng, J. Xiao, Q. Yang and J. Hao, J. Mater. Chem., 2012, 22, 9870–9874 RSC.
- Q. Liu, Y. Sun, T. Yang, W. Feng, C. Li and F. Li, J. Am. Chem. Soc., 2011, 133, 17122–17125 CrossRef CAS PubMed.
- J. Zhou, X. Zhu, M. Chen, Y. Sun and F. Li, Biomaterials, 2012, 33, 6201–6210 CrossRef CAS PubMed.
- W. Yin, L. Zhao, L. Zhou, Z. Gu, X. Liu, G. Tian, S. Jin, L. Yan, W. Ren, G. Xing and Y. Zhao, Chem. – Eur. J., 2012, 18, 9239–9245 CrossRef CAS PubMed.
- J. Zhou, Y. Sun, X. Du, L. Xiong, H. Hu and F. Li, Biomaterials, 2010, 31, 3287–3295 CrossRef CAS PubMed.
- F. Liu, X. He, L. Liu, H. You, H. Zhang and Z. Wang, Biomaterials, 2013, 34, 5218–5225 CrossRef CAS PubMed.
- N. Bogdan, F. Vetrone, R. Roy and J. A. Capobianco, J. Mater. Chem., 2010, 20, 7543–7550 RSC.
- H.-T. Wong, F. Vetrone, R. Naccache, H. L. W. Chan, J. Hao and J. A. Capobianco, J. Mater. Chem., 2011, 21, 16589–16596 RSC.
- M. He, P. Huang, C. Zhang, H. Hu, C. Bao, G. Gao, R. He and D. Cui, Adv. Funct. Mater., 2011, 21, 4470–4477 CrossRef CAS.
- F. Chen, W. Bu, S. Zhang, J. Liu, W. Fan, L. Zhou, W. Peng and J. Shi, Adv. Funct. Mater., 2012, 23, 298–307 CrossRef.
- H.-T. Wong, H. L. W. Chan and J. Hao, Opt. Express, 2010, 18, 6123–6130 CrossRef CAS PubMed.
- K. A. Abel, J.-C. Boyer, C. M. Andrei and F. C. J. M. van Veggel, J. Phys. Chem. Lett., 2011, 2, 185–189 CrossRef CAS.
- G. Chen, T. Y. Ohulchanskyy, W.-C. Law, H. Agren and P. N. Prasad, Nanoscale, 2011, 3, 2003–2008 RSC.
- X.-F. Qiao, J.-C. Zhou, J.-W. Xiao, Y.-F. Wang, L.-D. Sun and C.-H. Yan, Nanoscale, 2012, 4, 4611–4623 RSC.
- F. Li, C. Li, X. Liu, Y. Chen, T. Bai, L. Wang, Z. Shi and S. Feng, Chem. – Eur. J., 2012, 18, 11641–11646 CrossRef CAS PubMed.
- C. Dong, A. Korinek, B. Blasiak, B. Tomanek and F. C. J. M. van Veggel, Chem. Mater., 2012, 24, 1297–1305 CrossRef CAS.
- F. Chen, W. Bu, S. Zhang, X. Liu, J. Liu, H. Xing, Q. Xiao, L. Zhou, W. Peng, L. Wang and J. Shi, Adv. Funct. Mater., 2011, 21, 4285–4294 CrossRef CAS.
- D. Q. Chen, Y. L. Yu, F. Huang, P. Huang, A. P. Yang and Y. S. Wang, J. Am. Chem. Soc., 2010, 132, 9976–9978 CrossRef CAS PubMed.
- F. Wang, R. Deng, J. Wang, Q. Wang, Y. Han, H. Zhu, X. Chen and X. Liu, Nat. Mater., 2011, 10, 968–973 CrossRef CAS PubMed.
- L. W. Yang, Y. Y. Zhang, J. J. Li, Y. Li, J. X. Zhong and P. K. Chu, Nanoscale, 2010, 2, 2805–2810 RSC.
- C. Chang, J. Autoimmun., 2010, 34, J234–J246 CrossRef CAS PubMed.
- D. Yang, Y. Zhao, H. Guo, Y. Li, P. Tewary, G. Xing, W. Hou, J. J. Oppenheim and N. Zhang, ACS Nano, 2010, 4, 1178–1186 CrossRef CAS PubMed.
- M. Di Gioacchino, C. Petrarca, F. Lazzarin, L. Di Giampaolo, E. Sabbioni, P. Boscolo, R. Mariani-Costantini and G. Bernardini, Int. J. Immunopathol. Pharmacol., 2011, 24, 65S–71S CAS.
- D. Segat, R. Tavano, M. Donini, F. Selvestrel, I. Rio-Echevarria, M. Rojnik, P. Kocbek, J. Kos, S. Iratni, D. Sheglmann, F. Mancin, S. Dusi and E. Papini, Nanomedicine, 2011, 6, 1027–1046 CrossRef CAS PubMed.
- F. Granucci, I. Zanoni and P. Ricciardi-Castagnoli, Cell. Mol. Life Sci., 2008, 65, 1683–1697 CrossRef CAS PubMed.
- D. A. A. Vignali and V. K. Kuchroo, Nat. Immunol., 2012, 13, 722–728 CrossRef CAS PubMed.
- M. Xu, I. Mizoguchi, N. Morishima, Y. Chiba, J. Mizuguchi and T. Yoshimoto, Clin. Dev. Immunol., 2010, 2010, 1–9 Search PubMed.
- J. K. Elmquist, T. E. Scammell and C. B. Saper, Trends Neurosci., 1997, 20, 565–570 CrossRef CAS.
- A. F. Suffredini, G. Fantuzzi, R. Badolato, J. J. Oppenheim and N. P. O'Grady, J. Clin. Immunol., 1999, 19, 203–214 CrossRef CAS.
- E. Gruys, M. J. M. Toussaint, T. A. Niewold and S. J. Koopmans, J. Zhejiang Univ. Sci., 2005, 6B, 1045–1056 CrossRef CAS PubMed.
- K. Schäkel, Exp. Dermatol., 2009, 18, 264–273 CrossRef PubMed.
- N. Cools, A. Petrizzo, E. Smits, F. M. Buonaguro, M. L. Tornesello, Z. Berneman and L. Buonaguro, Immunotherapy, 2011, 3, 1203–1222 CrossRef CAS PubMed.
- M. Pedroni, F. Piccinelli, T. Passuello, M. Giarola, G. Mariotto, S. Polizzi, M. Bettinelli and A. Speghini, Nanoscale, 2011, 3, 1456–1460 RSC.
- V. Petit, P. Camy, J. L. Doualan, X. Portier and R. Moncorgé, Phys. Rev. B: Condens. Matter, 2008, 78, 085131 CrossRef.
- V. A. Streltsov, V. G. Tsirelson, R. P. Ozerov and O. A. Golovanov, Kristallografiya, 1988, 33, 90–97 CAS.
- M. Pedroni, F. Piccinelli, T. Passuello, S. Polizzi, J. Ueda, P. Haro-Gonzalez, L. Martinez Maestro, D. Jaque, J. Garcia Sole, M. Bettinelli and A. Speghini, Cryst. Growth Des., 2013, 13, 4906–4913 CAS.
- Y. L. Raikher and V. I. Stepanov, Sov. Phys. JETP, 1992, 75, 764–771 Search PubMed.
- M. Fittipaldi, L. Sorace, A. L. Barra, C. Sangregorio, R. Sessoli and D. Gatteschi, Phys. Chem. Chem. Phys., 2009, 11, 6555–6568 RSC.
- U. Ranon and W. Low, Phys. Rev., 1963, 132, 1609–1611 CrossRef CAS.
- K. I. Gerasimov and M. L. Falin, Phys. Solid State, 2009, 51, 721–726 CrossRef CAS.
- I. A. Irisova, A. A. Rodionov, D. A. Tayurskii and R. V. Yusupov, J. Phys.: Conf. Ser., 2011, 324, 012026 CrossRef.
- M. Botta and L. Tei, Eur. J. Inorg. Chem., 2012, 2012, 1945–1960 CrossRef CAS.
- G. Liang, D. Ye, X. Zhang, F. Dong, H. Chen, S. Zhang, J. Li, X. Shen and J. Kong, J. Mater. Chem. B, 2013, 1, 3545–3552 RSC.
- E. Hemmer, H. Takeshita, T. Yamano, T. Fujiki, Y. Kohl, K. Löw, N. Venkatachalam, H. Hyodo, H. Kishimoto and K. Soga, J. Mater. Sci., Mater. Med., 2012, 23, 2399–2412 CrossRef CAS PubMed.
- C.-C. Huang, C.-Y. Tsai, H.-S. Sheu, K.-Y. Chuang, C.-H. Su, U.-S. Jeng, F.-Y. Cheng, C.-H. Su, H.-Y. Lei and C.-S. Yeh, ACS Nano, 2011, 5, 3905–3916 CrossRef CAS PubMed.
- Q. Zhan, S. He, J. Quian, H. Cheng and F. Cai, Theranostics, 2013, 3, 306–316 CrossRef PubMed.
- Y. Wang, K. C. L. Black, H. Luehmann, W. Li, Y. Zhang, X. Cai, D. Wan, S.-Y. Liu, M. Li, P. Kim, Z.-Y. Li, L. V. Wang, Y. Liu and Y. Xia, ACS Nano, 2013, 7, 2068–2077 CrossRef CAS PubMed.
- Y. Jin, J. Wang, H. Ke, S. Wang and Z. Dai, Biomaterials, 2013, 34, 4794–4802 CrossRef CAS PubMed.
- C. D. S. Brites, P. P. Lima, N. J. O. Silva, A. Millán, V. S. Amaral, F. Palacio and L. D. Carlos, Nanoscale, 2013, 5, 7572–7580 RSC.
- C. D. S. Brites, P. P. Lima, N. J. O. Silva, A. Millán, V. S. Amaral, F. Palacio and L. D. Carlos, New J. Chem., 2011, 35, 1177–1183 RSC.
- D. Jaque and F. Vetrone, Nanoscale, 2012, 4, 4301 RSC.
- U. Rocha, C. Jacinto da Silva, W. Ferreira Silva, I. Guedes, A. Benayas, L. Martinez Maestro, M. Acosta Elias, E. Bovero, F. C. J. M. van Veggel, J. A. García Solé and D. Jaque, ACS Nano, 2013, 7, 1188–1199 CrossRef CAS PubMed.
- K. Zheng, Z. Liu, C. Lv and W. Qin, J. Mater. Chem. C, 2013, 1, 5502–5507 RSC.
- F. Vetrone, R. Naccache, A. Zamarron, A. Juarranz de la Fuente, F. Sanz-Rodriguez, L. Martinez Maestro, E. Martin Rodriguez, D. Jaque, J. Garcia Sole and J. A. Capobianco, ACS Nano, 2010, 4, 3254–3258 CrossRef CAS PubMed.
- X.-D. Wang, O. S. Wolfbeis and R. J. Meier, Chem. Soc. Rev., 2013, 42, 7834–7869 RSC.
- C. D. S. Brites, P. P. Lima, N. J. O. Silva, A. Millán, V. S. Amaral, F. Palacio and L. D. Carlos, Nanoscale, 2012, 4, 4799–4829 RSC.
- Y. Zhong, G. Tian, Z. Gu, Y. Yang, L. Gu, Y. Zhao, Y. Ma and J. Yao, Adv. Mater., 2014, 26, 2831–2837 CrossRef CAS PubMed.
- S. F. León-Luis, U. R. Rodríguez-Mendoza, P. Haro-González, I. R. Martín and V. Lavín, Sens. Actuators, B, 2012, 174, 176–186 CrossRef PubMed.
- M. F. Neurath and S. Finotto, Cytokine Growth Factor Rev., 2011, 22, 83–89 CrossRef CAS PubMed.
- M. Wong, D. Ziring, Y. Korin, S. Desai, S. Kim, J. Lin, D. Gjertson, J. Braun, E. Reed and R. R. Singh, Clin. Immunol., 2008, 126, 121–136 CrossRef CAS PubMed.
- G. Bamias and F. Cominelli, Curr. Opin. Pharmacol., 2006, 6, 401–407 CrossRef CAS PubMed.
- A. So, A. Ives, L. A. B. Joosten and N. Busso, Nat. Rev. Rheumatol., 2013, 1–9 Search PubMed.
- D. De Nitto, M. Sarra, M. L. Cupi, F. Pallone and G. Monteleone, Curr. Pharm. Des., 2010, 16, 3656–3660 CrossRef CAS.
- B. Fadeel and A. E. Garcia-Bennett, Adv. Drug Delivery Rev., 2010, 62, 362–374 CrossRef CAS PubMed.
- J. Nam, N. Won, J. Bang, H. Jin, J. Park, S. Jung, S. Jung, Y. Park and S. Kim, Adv. Drug Delivery Rev., 2013, 65, 622–648 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: DLS measurements, EPR spectrum, magnetization curves, infrared camera pictures, toxicity tests on HeLa cells. See DOI: 10.1039/c4bm00119b |
| This journal is © The Royal Society of Chemistry 2014 |