Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Mechanistic investigation of the selective reduction of Meldrum's acids to β-hydroxy acids using SmI2 and H2O†
Michal
Szostak
*,
Sarah E.
Lyons
,
Malcolm
Spain
and
David J.
Procter
*
School of Chemistry, The University of Manchester, Oxford Road, Manchester, M13 9PL, UK. E-mail: michal.szostak@manchester.ac.uk; david.j.procter@manchester.ac.uk; Fax: +44 (0)161 2754939; Tel: +44 (0)161 2751425
First published on 5th June 2014
Abstract
The mechanism of a recently reported first mono-reduction of cyclic 1,3-diesters (Meldrum's acids) to β-hydroxy acids with SmI2–H2O has been studied using a combination of reactivity, deuteration, kinetic isotope and radical clock experiments. Most crucially, the data indicate that the reaction proceeds via reversible electron transfer and that water, as a ligand for SmI2, stabilizes the radical anion intermediate rather than only promoting the first electron transfer as originally proposed.
Reduction of carbonyl groups with low-valent metals to generate ketyl intermediates is a fundamental process in organic synthesis.1 For more polar carboxylic acid derivatives, single electron transfer from the metal centre to form acyl-type radicals is more challenging due to lower electrophilicity of the carbonyl group precursors, which prevents selective electron transfer under mild conditions.2 Moreover, the formed acyl-type radicals often undergo undesired decarbonylation to give carbon monoxide and alkyl radicals.3 Accordingly, successful examples of generation of acyl-type radicals from polar carbonyl groups remain rare.1–3
Over the past decade, samarium(II) iodide (SmI2, Kagan's reagent) has emerged as a valuable reagent for promoting electron transfer processes to carboxylic acid derivatives.4,5 In particular, the reagent formed by activation of SmI2 with Lewis basic ligands6 has enabled mild and modular synthesis of acyl-type radical intermediates from various carboxylic acid precursors.7,8 Perhaps most intriguing among these reagents are SmI2–H2O complexes6b formed via the addition of water to SmI2(THF)n due to their excellent chemoselectivity in diverse radical reactions.9
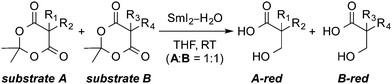
Recently, our laboratory has demonstrated that SmI2–H2O exhibits remarkable selectivity in the reduction of carbonyl groups of cyclic 1,3-diesters (Fig. 1)10 and lactones11 in that the system shows full selectivity for the electron transfer to cyclic esters over their acyclic analogues. The reduction of cyclic 1,3-diesters (Meldrum's acids) with SmI210 is of particular interest for the synthesis of β-hydroxy acids – important building blocks for the synthesis of pharmaceuticals and polymers12 – directly from Meldrum's acids.13 Furthermore, the easily assembled α,β-unsaturated Meldrum's acids13 undergo sequential reductions in the presence of SmI2–H2O,10a providing a general route for the synthesis of β-hydroxy acids, while other methods involve multiple steps.14 Finally, a large scale practical reduction of Meldrum's acids using SmI2–H2O has been developed,10d,e and the potential of acyl-type radical intermediates in cyclizations and reaction cascades has been demonstrated;10b,c however, at present the effect of the reaction components on the generation of acyl-type radicals from Meldrum's acids remains unclear.
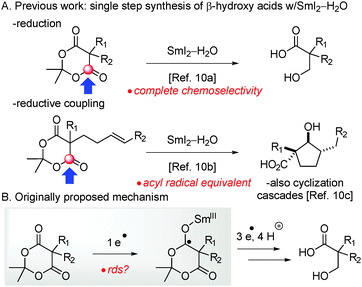 | ||
| Fig. 1 (A) Previous studies: chemoselective electron transfer to Meldrum's acids (reduction, cyclization and cyclization cascades). (B) This work: mechanistic investigation and role of water. | ||
Herein, we report the mechanistic investigation of the SmI2–H2O-promoted first mono-reduction of Meldrum's acids to β-hydroxy acids. The reaction proceeds via acyl-type radical intermediates generated by a direct electron transfer from Sm(II) to the ester carbonyl. The study represents one of the first mechanistic analyses of electron transfer processes to carbonyl groups mediated by SmI2.15 Importantly, this investigation outlines the interplay between SmI2 and H2O ligand,16 establishes the reversibility of the initial electron transfer and provides insights for the rational development of radical reactions based on the versatile Meldrum's acid template using Sm(II) complexes.
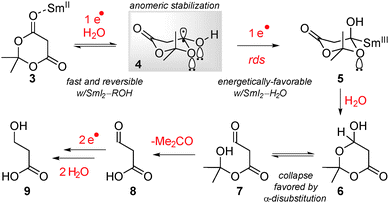
Initial studies suggested that selectivity in the mono-reduction of Meldrum's acids resulted from the rate of the initial electron transfer to the carbonyl group and anomeric stabilization of the radical anion intermediate.10a,b Moreover, water as the ligand to Sm(II) was critical for the observed reactivity. However, after continuous probing of the reaction pathway, several observations suggested that an alternative mechanism might be operative.
To elucidate the role of electronic and steric stabilization in the SmI2-mediated reduction of Meldrum's acids, a series of reactivity studies were performed (Table 1).17 Because of the heterogenicity of the reaction, kinetic studies proved not to be a viable tool in the present reaction. Remarkably, in the series of selected substrates (Table 1, entries 1–6) full selectivity for the reduction of alpha-disubstituted esters over alpha-monosubstituted esters was observed (Table 1, entries 4 and 5). Moreover, appreciable levels of selectivity were obtained depending on electronic properties of the α-carbon substituent at the ester group undergoing the reduction (Table 1, entries 1–3 and 6) consistent with electronic stabilization of ketyl-type radicals. The steric acceleration of the reduction is unexpected and contrasts with the previously observed steric inhibition of coordination of polar groups to Sm(II) in the reduction of lactones and acyclic esters.15a,b We hypothesize that the steric acceleration in the present case results from a torsional strain in the radical anion intermediate in a half-chair conformation.10a,b
| Entry | A | B | R1, R2 | R3, R4 | k A/kBb |
|---|---|---|---|---|---|
| a Conditions: Meldrum's acid (1 equiv.), SmI2 (2 equiv., THF), H2O (400 equiv.), 23 °C. Reaction time 30–60 s. b Determined by 1H NMR. c SmI2 (1 equiv.), H2O (200 equiv.). In all entries >95% yield based on reacted starting material. d Selective exo-cyclic reduction of olefin/cyclopropane to give the corresponding substituted Meldrum's acids. See ESI. | |||||
| 1 | 1a | 1b | Ph, H | Bn, H | 1.66 |
| 2 | 1b | 1c | Bn, H | i-Bu, H | 2.46 |
| 3 | 1a | 1c | Ph, H | i-Bu, H | 5.21 |
| 4 | 1d | 1a | Ph, Me | Ph, H | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 5 | 1e | 1c | i-Bu, Me | i-Bu, H | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 6 | 1d | 1e | Ph, Me | i-Bu, Me | 3.74 |
| 7c,d | 1f | 1e | ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHi-Pr CHi-Pr |
i-Bu, Me | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 8c,d | 1f | 1d | ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHi-Pr CHi-Pr |
Ph, Me | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 9d | 1h | 1e | –(CH2)2– | i-Bu, Me | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 10d | 1h | 1d | –(CH2)2– | Ph, Me | 5.13 |
| 11c,d | 1f | 1h | ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHi-Pr CHi-Pr |
–(CH2)2– | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 12c,d | 1f | 1g | ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHi-Pr CHi-Pr |
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHPh CHPh |
1.37 |
Evidence for the reaction pathway was further gained by examining the reactivity of substrates possessing two reactive sites (entries 7–12). These results indicate that α,β-unsaturated Meldrum's acids undergo chemoselective reduction in preference to their fully saturated counterparts (entries 7 and 8). Moreover, a cyclopropyl-clock containing substrate results in a faster reduction than for α,α-disubstituted Meldrum's acids (entries 9 and 10); however, its reduction is slower than that of an α,β-unsaturated ester (entry 11). Finally, electronically-differentiated α,β-unsaturated substrates result in a near statistical rate of reduction (entry 12). These results outline the order of reactivity of Meldrum's acids with SmI2: α,β-unsaturated ≫ cyclopropyl > α,α-disubstituted ≫ α-monosubstituted.18 Importantly, the data presented in Table 1 indicate high levels of chemoselectivity in the reduction of Meldrum's acids with SmI2–H2O.5f
To investigate the role of water as an activating ligand for Sm(II), a series of rate studies were performed (Fig. 2a and ESI†). Isobutyl-substituted ester was selected as a model substrate. The study was performed by monitoring the reduction of 1c with increasing concentrations of water and quenching the reaction at low conversions. Remarkably, a non-linear dependence on water concentration was found in the reduction of 1c (Fig. 2a). In agreement with the previous results, no reaction was observed without the water additive and at low concentration of water,10a,b (0–50 equiv.; see, ESI†). Next, at higher concentrations of water (100–400 equiv.) the rate was found to increase linearly with a slope. However, at high concentrations of water (>400 equiv.) the rate decreased dramatically, which is consistent with substrate dissociation from the inner coordination sphere of Sm(II).15 These results are in contrast to the thermodynamic redox potentials of SmI2–H2O complexes15d and other studies.11b Importantly, the rate of reduction of acyl-type radicals can be varied by simply changing the concentration of the water additive.19
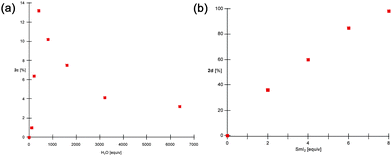 | ||
| Fig. 2 (a) Plot of conversion vs. concentration of H2O for reduction of 1c. (b) Plot of conversion vs. concentration of SmI2 for reduction of 1d. | ||
We likewise investigated the role of SmI2 in the reduction of Meldrum's acids (Fig. 2b and ESI†). The study was performed by monitoring the equivalents of SmI2 required for the reduction of 1d with a set concentration of water and quenching the reaction after disappearance of the active Sm(II) complex. As indicated in Fig. 2b, the reaction of 1d is linear in SmI2 and requires more than six equivalents of the reductant. This is consistent with previous mechanistic studies on the reduction of carbonyl groups with SmI215 and indicates that the collapse of acetonide occurs prior to the final electron transfer from Sm(II).
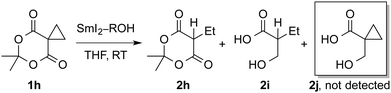
To gain independent evidence on the role of electron transfer steps, we carried out experiments employing cyclopropyl clocks (Table 2 and ESI†).20 The reaction of 1h (2 equiv. of SmI2) resulted in rapid cyclopropyl ring opening to give acyclic ester 2h. Cyclopropyl carbinol was not detected in the reaction. The reaction with excess of SmI2–H2O (10 equiv., rt, 2 h) resulted in a full reduction to the β-hydroxy acid. The reduction with SmI2–H2O (8 equiv., 15 min) gave approx. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of 2h to 2i. The reduction using SmI2–MeOH and SmI2–THF resulted in alcoholysis and slow, non-selective reaction. Overall, these findings strongly suggest that the reduction of Meldrum's acids with SmI2–H2O occurs via fast, reversible electron transfer and that water activates the reagent towards the electron transfer.
1 ratio of 2h to 2i. The reduction using SmI2–MeOH and SmI2–THF resulted in alcoholysis and slow, non-selective reaction. Overall, these findings strongly suggest that the reduction of Meldrum's acids with SmI2–H2O occurs via fast, reversible electron transfer and that water activates the reagent towards the electron transfer.
| Entry | SmI2 (equiv.) | ROH | ROH (equiv.) | Time | Conv.b (%) |
2h![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2ib (%) 2ib (%) |
|---|---|---|---|---|---|---|
a Conditions: Meldrum's acid (1 equiv.), SmI2 (in THF), ROH, 23 °C.
b Determined by 1H NMR. Combined yield of 2h and 2i. Entries 1–3, >85% yield based on reacted starting material.
c
Ref. 10a.
d Fragmentation product consists of 2h and its methanolysis product (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 85).
e Entry 4, 23% yield. See ESI for full experimental details. 85).
e Entry 4, 23% yield. See ESI for full experimental details.
|
||||||
| 1 | 2 | H2O | 200 | <1 min | 87 | >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| 2c | 10 | H2O | 1000 | 2 h | >95 | <5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) >95 >95 |
| 3d | 3 | MeOH | 200 | 1 h | >95 | >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| 4e | 3 | — | — | 3 h | 50 | >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
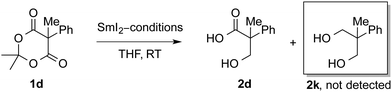
Additional experiments were performed to gain insight into the electron transfer steps (see ESI† and Table 3). (1) The reduction of a series of α-mono and α,α-disubstituted substrates with SmI2–D2O gives the β-hydroxy acid products with >98% D2 and D3 incorporation, suggesting that anions are protonated in a series of electron transfer steps (see ESI†). Control reactions demonstrate that α-proton exchange is faster than the reduction (see ESI†). (2) The secondary kinetic isotope effect in the reduction of 1d of 1.5 (intramolecular competition) suggests that proton transfer to carbon is not involved in the rate determining step of the reaction (see ESI†).21 (3) The reduction of α,β-unsaturated Meldrum's acids 1f and 1g (SmI2, 2 equiv.) proceeds with full selectivity for the 1,4-reduction to give saturated Meldrum's acid derivatives (see ESI†). (4) Evaluation of the reduction of the benchmark substrate using various Sm(II)–ROH systems indicates that other SmI2 reagents can be used to promote selective mono-reduction (Table 3). Importantly, under the optimized reaction conditions over-reduction to 1,3-diol is not observed. Note, however, that the reactivity trend is divergent from the ligand effect on the reduction of other carbonyl groups using Sm(II),15f which most likely results from a combination of lower kinetic reactivity of these substrates (entries 1–4), instability towards the reaction conditions (entries 5 and 6), and differential coordination of the sterically-encumbered reductants to the Meldrum's acid carbonyl groups (entries 7–12). Overall, these results demonstrate that Sm(II) reagents based on chelating ligands6 and multicomponent systems7 are new chemoselective reductants available for the reduction of cyclic 1,3-diesters.
| Entry | ROH/L.B. | ROH/L.B. (equiv.) | Time | Yieldb (%) | Selectivity 2d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2kb (%) 2kb (%) |
|---|---|---|---|---|---|
| a Conditions: Meldrum's acid (1 equiv.), SmI2 (4–6 equiv., THF), 23 °C. In all entries, preformed solution of SmI2–ROH/L.B. was used. b Determined by 1H NMR. In all entries, yield based on reacted starting material. ED = Ethylenediamine; DCH = trans-N,N′-dimethyl-1,2-cyclohexyldiamine; EG = ethylene glycol; TMEDA = tetramethylethylenediamine. | |||||
| 1 | MeOH | 4/1 v/v | 2 h | <5 | — |
| 2 | ED | 36 | 2 h | <5 | — |
| 3 | DCH | 36 | 2 h | <5 | — |
| 4 | EG | 36 | 2 h | 84 | >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| 5 | n-BuNH2/H2O | 12/18 | 5 min | <5 | — |
| 6 | Pyrrolidine/H2O | 12/18 | 5 min | <5 | — |
| 7 | Et3N/H2O | 12/18 | 5 min | 92 | >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| 8 | Et3N/EG | 12/18 | 5 min | 84 | >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| 9 | Et3N/MeOH | 12/18 | 2 h | <5 | — |
| 10 | TMEDA/H2O | 12/18 | 5 min | 46 | >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| 11 | N-Me-morpholine/H2O | 12/18 | 5 min | 80 | >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| 12 | DIPA/H2O | 12/18 | 5 min | 48 | >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
Scheme 1 shows a revised mechanism that is consistent with the kinetic and reactivity studies presented herein. The key features involve: (1) reversible initial electron transfer step; (2) non-linear rate dependence on water concentration; (3) rate determining second electron transfer step that is inhibited by large concentrations of water.
In conclusion, we have elucidated the mechanism of the selective mono-reduction of Meldrum's acids to β-hydroxy acids using the SmI2–H2O system. We hope that further research aimed at understanding processes involving activation of SmI2 by Lewis basic ligands will enable discovery of new radical reactions.
We are grateful to the EPRSC and GSK for financial support.
Notes and references
- Reviews on metal-mediated radical reactions: (a) B. M. Trost and I. Fleming, Comprehensive Organic Synthesis, Pergamon Press, 1991 Search PubMed; (b) A. Gansäuer and H. Bluhm, Chem. Rev., 2000, 100, 2771 CrossRef PubMed; (c) M. Szostak and D. J. Procter, Angew. Chem., Int. Ed., 2012, 51, 9238 CrossRef CAS PubMed; (d) J. Streuff, Synthesis, 2013, 45, 281 CrossRef CAS PubMed.
- For reviews see: (a) P. Renaud and M. Sibi, Radicals in Organic Synthesis, Wiley-VCH, 2001 Search PubMed; (b) C. Chatgilialoglu and A. Studer, Encyclopedia of Radicals in Chemistry, Biology and Materials, Wiley-Blackwell, 2012 Search PubMed; (c) T. Wirth, Angew. Chem., Int. Ed. Engl., 1996, 35, 61 CrossRef CAS; (d) J. Murphy, J. Org. Chem., 2014, 79, 3731 CrossRef CAS PubMed.
- For a comprehensive review, see: (a) C. Chatgilialoglu, D. Crich, M. Komatsu and I. Ryu, Chem. Rev., 1999, 99, 1991 CrossRef CAS PubMed ; For an elegant solution using SmI2, see: ; (b) C. M. Jensen, K. B. Lindsay, R. H. Taaning, J. Karaffa, A. M. Hansen and T. Skrydstrup, J. Am. Chem. Soc., 2005, 127, 6544 CrossRef CAS PubMed.
- D. J. Procter, R. A. Flowers, II and T. Skrydstrup, Organic Synthesis using Samarium Diiodide: A Practical Guide, RSC Publishing, Cambridge, 2009 Search PubMed.
- Recent reviews on SmI2: (a) H. B. Kagan, Tetrahedron, 2003, 59, 10351 CrossRef CAS PubMed; (b) D. J. Edmonds, D. Johnston and D. J. Procter, Chem. Rev., 2004, 104, 3371 CrossRef CAS PubMed; (c) K. C. Nicolaou, S. P. Ellery and J. S. Chen, Angew. Chem., Int. Ed., 2009, 48, 7140 CrossRef CAS PubMed; (d) C. Beemelmanns and H. U. Reissig, Chem. Soc. Rev., 2011, 40, 2199 RSC; (e) M. Szostak and D. J. Procter, Angew. Chem., Int. Ed., 2011, 50, 7737 CrossRef CAS PubMed; (f) M. Szostak, M. Spain and D. J. Procter, Chem. Soc. Rev., 2013, 42, 9155 RSC; (g) M. Szostak, N. J. Fazakerley, D. Parmar and D. J. Procter, Chem. Rev., 2014, 114 DOI:10.1021/cr400685r.
- For reviews on the influence of additives on properties of SmI2, see: (a) A. Dahlén and G. Hilmersson, Eur. J. Inorg. Chem., 2004, 3393 CrossRef; (b) M. Szostak, M. Spain, D. Parmar and D. J. Procter, Chem. Commun., 2012, 48, 330 RSC.
- For early studies, see: (a) Y. Kamochi and T. Kudo, Chem. Lett., 1993, 1495 CrossRef CAS; (b) Y. Kamochi and T. Kudo, Tetrahedron, 1992, 48, 4301 CrossRef CAS ; Recent examples: ; (c) M. Szostak, M. Spain and D. J. Procter, Chem. Commun., 2011, 47, 10254 RSC; (d) M. Szostak, M. Spain and D. J. Procter, Org. Lett., 2012, 14, 840 CrossRef CAS PubMed; (e) M. Szostak, K. D. Collins, N. J. Fazakerley, M. Spain and D. J. Procter, Org. Biomol. Chem., 2012, 10, 5820 RSC; (f) M. Szostak, M. Spain, A. J. Eberhart and D. J. Procter, J. Am. Chem. Soc., 2014, 136, 2268 CrossRef CAS PubMed; (g) M. Szostak, B. Sautier, M. Spain and D. J. Procter, Org. Lett., 2014, 16, 1092 CrossRef CAS PubMed.
- Representative examples of Sm(II)-Lewis base systems in other transformations: (a) A. Dahlén and G. Hilmersson, Tetrahedron Lett., 2002, 43, 7197 CrossRef; (b) A. Dahlén and G. Hilmersson, J. Am. Chem. Soc., 2005, 127, 8340 CrossRef PubMed; (c) C. Beemelmanns and H. U. Reissig, Angew. Chem., Int. Ed., 2010, 49, 8021 CrossRef CAS PubMed; (d) Z. Li, M. Nakashige and W. J. Chain, J. Am. Chem. Soc., 2011, 133, 6553 CrossRef CAS PubMed.
- Representative examples of SmI2–H2O systems: (a) E. Hasegawa and D. P. Curran, J. Org. Chem., 1993, 58, 5008 CrossRef CAS; (b) G. Masson, P. Cividino, S. Py and Y. Vallée, Angew. Chem., Int. Ed., 2003, 42, 2265 CrossRef CAS PubMed; (c) P. Gilles and S. Py, Org. Lett., 2012, 14, 1042 CrossRef CAS PubMed.
- (a) G. Guazzelli, S. De Grazia, K. D. Collins, H. Matsubara, M. Spain and D. J. Procter, J. Am. Chem. Soc., 2009, 131, 7214 CrossRef CAS PubMed; (b) K. D. Collins, J. M. Oliveira, G. Guazzelli, B. Sautier, S. De Grazia, H. Matsubara, M. Helliwell and D. J. Procter, Chem. – Eur. J., 2010, 16, 10240 CrossRef CAS PubMed; (c) B. Sautier, S. E. Lyons, M. R. Webb and D. J. Procter, Org. Lett., 2012, 14, 146 CrossRef CAS PubMed; (d) M. Szostak, M. Spain and D. J. Procter, Nat. Protoc., 2012, 7, 970 CrossRef CAS PubMed ; Detailed study on the preparation of SmI2: ; (e) M. Szostak, M. Spain and D. J. Procter, J. Org. Chem., 2012, 77, 3049 CrossRef CAS PubMed; (f) M. Szostak, B. Sautier, M. Spain, M. Behlendorf and D. J. Procter, Angew. Chem., Int. Ed., 2013, 52, 12559 CrossRef CAS PubMed.
- (a) L. A. Duffy, H. Matsubara and D. J. Procter, J. Am. Chem. Soc., 2008, 130, 1136 CrossRef CAS PubMed; (b) D. Parmar, L. A. Duffy, D. V. Sadasivam, H. Matsubara, P. A. Bradley, R. A. Flowers, II and D. J. Procter, J. Am. Chem. Soc., 2009, 131, 15467 CrossRef CAS PubMed; (c) D. Parmar, K. Price, M. Spain, H. Matsubara, P. A. Bradley and D. J. Procter, J. Am. Chem. Soc., 2011, 133, 2418 CrossRef CAS PubMed; (d) D. Parmar, H. Matsubara, K. Price, M. Spain and D. J. Procter, J. Am. Chem. Soc., 2012, 134, 12751 CrossRef CAS PubMed; (e) M. Szostak, M. Spain, K. A. Choquette, R. A. Flowers, II and D. J. Procter, J. Am. Chem. Soc., 2013, 135, 15702 CrossRef CAS PubMed ; Application to TmI2–H2O: ; (f) M. Szostak, M. Spain and D. J. Procter, Angew. Chem., Int. Ed., 2013, 52, 7237 CrossRef CAS PubMed . Application to Birch reductions: ; (g) M. Szostak, M. Spain and D. J. Procter, J. Org. Chem., 2014, 79, 2522 CrossRef CAS PubMed.
- Representative examples: (a) B. Hu, M. Prashad, D. Har, K. Prasad, O. Repič and T. J. Blacklock, Org. Process Res. Dev., 2007, 11, 90 CrossRef CAS; (b) H. S. Lee and D. H. Kim, Bioorg. Med. Chem., 2003, 11, 4685 CrossRef CAS PubMed; (c) P. M. Chaudhari, P. V. Kawade and S. M. Funne, Int. J. Pharm. Technol., 2011, 3, 774 Search PubMed; (d) D. Zhang, M. A. Hillmyer and W. B. Tolman, Macromolecules, 2004, 37, 8198 CrossRef CAS.
- Comprehensive reviews on Meldrum's acids: (a) H. McNab, Chem. Soc. Rev., 1978, 7, 345 RSC; (b) B. C. Chen, Heterocycles, 1991, 32, 529 CrossRef CAS PubMed; (c) A. M. Dumas and E. Fillion, Acc. Chem. Res., 2010, 43, 440 CrossRef CAS PubMed.
- M. O. Polla, L. Tottie, C. Nordén, M. Linschoten, D. Müsila, S. Trumpp-Kallmeyer, I. R. Aukrust, R. Ringom, K. H. Holm, S. M. Neset, M. Sandberg, J. Thurmond, P. Yu, G. Hategan and H. Anderson, Bioorg. Med. Chem., 2004, 12, 1151 CrossRef CAS PubMed.
- Relevant studies on mechanisms of SmI2-mediated reactions: (a) M. Szostak, M. Spain and D. J. Procter, Chem. – Eur. J., 2014, 20, 4222 CrossRef CAS PubMed; (b) M. Szostak, M. Spain and D. J. Procter, J. Am. Chem. Soc., 2014, 136 DOI:10.1021/ja503494b; (c) T. Ankner and G. Hilmersson, Tetrahedron, 2009, 65, 10856 CrossRef CAS PubMed; (d) P. R. Chopade, E. Prasad and R. A. Flowers, II, J. Am. Chem. Soc., 2004, 126, 44 CrossRef CAS PubMed; (e) E. Prasad and R. A. Flowers, II, J. Am. Chem. Soc., 2005, 127, 18093 CrossRef CAS PubMed; (f) D. V. Sadasivam, J. A. Teprovich, Jr., D. J. Procter and R. A. Flowers, II, Org. Lett., 2010, 12, 4140 CrossRef CAS PubMed.
- (a) A. Yacovan, I. Bilkis and S. Hoz, J. Am. Chem. Soc., 1996, 118, 261 CrossRef CAS; (b) A. Tarnopolsky and S. Hoz, J. Am. Chem. Soc., 2007, 129, 3402 CrossRef CAS PubMed; (c) M. Amiel-Levy and S. Hoz, J. Am. Chem. Soc., 2009, 131, 8280 CrossRef CAS PubMed; (d) S. K. Upadhyay and S. Hoz, J. Org. Chem., 2011, 76, 1355 CrossRef CAS PubMed.
- R. H. Taaning, K. B. Lindsay, B. Schiøtt, K. Daasbjerg and T. Skrydstrup, J. Am. Chem. Soc., 2009, 131, 10253 CrossRef CAS PubMed.
- Relative reactivity of Meldrum's acids with SmI2–H2O: Meldrum's acids ≫ barbituric acids ≫ lactones. See, ref. 10f.
- The onset of reactivity and maximum rate of the reduction of Meldrum's acids is shifted towards higher concentrations of water than the reduction of lactones using SmI2–H2O, despite the rate of lactone reduction being slower than the rate of Meldrum's acid reduction.18 See, also: H. Farran and S. Hoz, Org. Lett., 2008, 10, 4875 CrossRef CAS PubMed.
- M. Newcomb, Tetrahedron, 1993, 49, 1151 CrossRef CAS.
- E. M. Simmons and J. F. Hartwig, Angew. Chem., Int. Ed., 2012, 51, 3066 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4cc03216k |
| This journal is © The Royal Society of Chemistry 2014 |